Neuroprotective mechanisms of rutin for spinal cord injury through anti-oxidation and anti-inflammation and inhibition of p38 mitogen activated protein kinase pathway
Hong-liang Song, Xiang Zhang, Wen-zhao Wang, Rong-han Liu, Kai Zhao, Ming-yuan Liu, Wei-ming Gong, Bin Ning,
1 Department of Spinal Surgery, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong Province, China
2 Hospital Pharmacy, Jinan Central Hospital Affiliated to Shandong University, Jinan, Shandong Province, China
3 Department of Gynecology and Obstetrics, Jinan Maternity and Child Care Hospital, Jinan, Shandong Province, China
Introduction
Spinal cord injury (SCI) is associated with high morbidity and severe complications. Effective treatments are lacking,resulting in an enormous financial and social burden on patients, their families and society (Phillips et al., 2015). SCI leads to severe neurological impairments, including motor,sensory and autonomic dysfunction. The secondary injury results in lesions extending out from the primary focus of damage, greatly worsening the damage caused by the initial trauma (Aslan et al., 2009; Elamin et al., 2013). The secondary injury includes oxidative stress and inflammatory response. Oxidative stress, which occurs when the production of reactive oxygen species exceeds the capacity of the antioxidant system, plays a major role in secondary SCI (Paterniti et al., 2009; Khayrullina et al., 2015). Along with an increase in the production of inflammatory factors (Didangelos et al.,2014), this results in a strong inflammatory response (Genovese et al., 2009; Ni et al., 2014). Inhibiting inflammation influences recovery from SCI (Ji, 2014; Machova et al., 2015;Chen and Jin, 2016).
Mitogen-activated protein kinase (MAPK) is involved in signal transduction for apoptosis (Pereira et al., 2013), and its levels predict whether the cell survives or dies, as they reflect damage to the cell (Yamaoka et al., 2012; Nafees et al.,2015). The expression of MAPK is upregulated during the apoptotic process in neurons and glial cells after SCI (Lee et al., 2010; Ha et al., 2011). Besides, the expression of MAPK could mediate the inflammatory response (Breton-Romero and Lamas, 2013).
Currently, there is no treatment for SCI that results in complete neurological or functional recovery (Varma et al.,2013). Rutin is a flavonoid of the flavonol type found widely in plants, including foods; it has a wide range of biological activities, and has a protective effect on experimental acute pancreatitis, and also inhibits cell proliferation and induces apoptosis (Zhang et al., 2015).
Rutin protects against liver and lung injury through antioxidative and anti-inflammatory actions and by modulating the MAPK pathway (Pan et al., 2014; Yeh et al., 2014).However, there is no report regarding the neuroprotective effects of rutin on SCI. In our study, wefirst investigated the neuroprotective effects of rutin in a rat model of SCI and examined the underlying mechanisms.
Materials and Methods
Animals
Totally 40 male Sprague-Dawley rats, at 8 weeks of age,weighing 200—230 g, were provided by the Animal Center of Shandong University of China (SCXK (Lu) 2013 0009). The rats were housed in individual cages under controlled conditions (22—24°C, relative humidity of 40—60%, with a 12-hour light-dark cycle and free access to food and water).
The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985). The study protocol was approved by the Animal Ethics Committee of Jinan Central Hospital Affiliated to Shandong University of China (2014-30).
Chemicals and reagents
Rutin (powder, purity > 98%) was purchased from Nanjing University of Traditional Chinese Medicine, Institute of Chinese Materia Medica (Nanjing, China), and prepared into solution 6 g/L with double distilled water, and its chemical structure is shown in Figure 1.
Generation of the rat SCI model
The SCI model was generated as described previously (Ravikumar et al., 2005). The rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg; Sigma-Aldrich, St. Louis, MO, USA) and a mixture of ketamine (44 mg/kg; Sangon Biotech, Shanghai, China),atropine (0.02633 mg/kg; Sintong Chemical Industrial Co.,Taoyuan, Taiwan, China) and xylazine (5 mg/kg; Sangon Biotech, Shanghai, China). The T8and T9vertebral peduncles were removed by laminectomy. After that, the moderate contusion injury was performed by a modified Allen’s weight drop apparatus (10 g weight at 50 mm, 10 g × 50 mm)on the exposed spinal cord. For the control rats, the same laminectomy was performed, but without SCI. The success of the compression can be confirmed by the spasm of the tail,the retraction-like flutter of legs and delayed paralysis.
Drug treatment
The rats were randomly divided into the following four groups: (1) control (n= 10), consisting of normal rats performed with laminectomy without spinal cord compression and treated with PBS; (2) SCI model (SCI,n= 10), consisting of rats with SCI treated with PBS; (3) methylprednisolone (MP,n= 10), consisting of rats with SCI treated with 100 mg/kg methylprednisolone once a day for 3 day. (intraperitoneally; Jinan Central Hospital Affiliated to Shandong University, China); (4) rutin (rutin,n= 10), consisting of rats with SCI treated with rutin 30 mg/kg, intraperitoneally)once a day for 3 days. All the treatments were performed at 6 hours after the injury happened.
Assessment of locomotor function
Locomotor function was evaluated using the Basso, Beattie and Bresnahan (BBB) score. A lower BBB score indicates more severe motor impairment. The rating scale ranges from 0 (no observable hindlimb movement) to 21 (normal locomotion) (Basso et al., 1996). BBB scores were assessed by four experienced experimenters who were blinded to the treatment conditions. BBB score was observed for 24, 48, 72 hours, 7 days after surgery.
Determination of spinal cord water content
After treatment with rutin for 3 days, the water content of the spinal cord was evaluated. All rats were killed by excess chloral hydrate, and the spinal cord samples were dried for 48 hours at 80°C for the determination of the dry weights.The water content of the spinal cord was calculated as follows: (wet weight − dry weight)/wet weight × 100%.
Measurement of oxidative stress
After treatment with rutin for 3 days, the peripheral blood was carefully collected from heart in each group.The blood samples were centrifuged at 12,000 ×gfor 10 minutes at 4°C. The supernatant was collected, and the concentrations of malon dialdehyde (MDA), superoxide dismutase (SOD) and catalase (CAT), as well as glutathione peroxidase (GSH-Px) activity were analyzed using commercial kits, following the manufacturer’s protocols(Beyotime, Nanjing, China).
Assessment of inflammation
After treatment with rutin for 3 days, approximately 5 mL peripheral blood was collected from heart directly of each group.The blood samples collected from heart were centrifuged at 12,000 ×gfor 10 minutes at 4°C. The supernatant was collected, and the serum levels of nuclear factor-κB (NF-κB) p65 unit, tumor necrosis factor alpha (TNF-α), interleukin (IL)-1B and IL-6 were measured using commercial kits, following the manufacturer’s protocols (Beyotime, Nanjing, China).
Western blot assay of p38MAPK protein expression

Figure 1 Chemical structure of rutin.
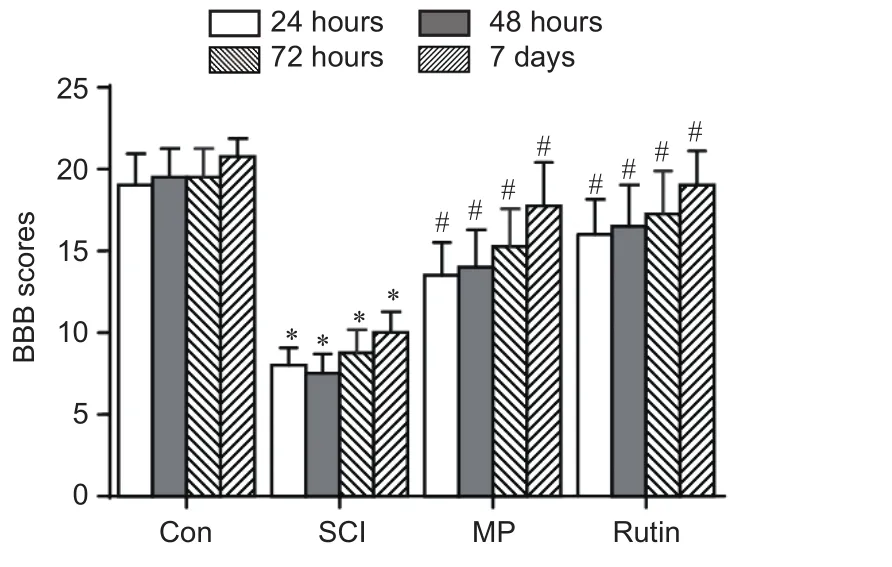
Figure 2 BBB scores for evaluating locomotor recovery 24, 48, 72 hours and 7 days after operations.
After treatment with rutin for 3 days, rats were scarified and approximately 15 cm long spinal cord containing T8and T9were taken out for further testing. 10-mg spinal cord tissue samples were incubated with 100 μL tissue lysis buffer (Beyotime) for 30 minutes on ice. Homogenates were centrifuged at 12,000 ×gfor 10 minutes at 4°C. The supernatants were collected, and the protein concentration was determined using a bicinchoninic acid kit (KeyGen Biotech). Equal amounts of protein were resolved on 12% sodium dodecyl sulfate-polyacrylamide gel electropheresis gels and then transferred to polyvinylidene fluoride membranes (0.22 μm). Membranes were blocked with PBS containing 5% non-fat milk to block nonspecific binding. Then, the membranes were incubated with anti-p38 MAPK mouse monoclonal antibody (1:2,000;sc-398305, Santa Cruz Biotechnology, Dallas, TX, USA) and anti-B-actin rabbit polyclonal antibody (1:500; D110007,Sangon Biotech, Shanghai, China) overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated anti-mouse IgG (1:1,000; sc-2005 and sc-2004, Santa Cruz Biotechnology) for 2 hours at room temperature (24—26°C).Signals were quantified using the Gel Doc XR+ Gel Documentation System (Bio-Rad, Hercules, CA, USA). Grayscale value (/internal reference) was detected by the image J soft-ware (NIH, Bethesda, Maryland, USA).
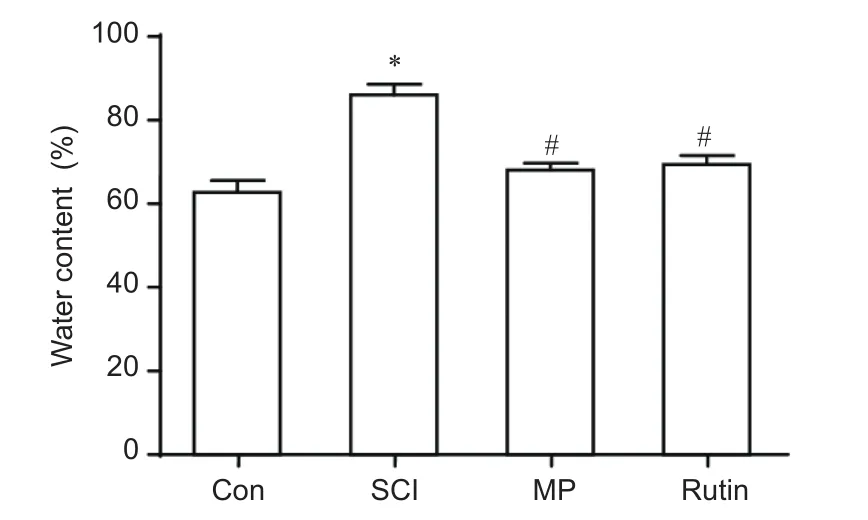
Figure 3 Rutin treatment for 3 days diminishes the SCI-induced increase in water content in the spinal cord.
Measurement of caspase-9 and caspase-3 activities
After treatment with rutin for 3 days, spinal cord protein samples were obtained as described above, and equal amounts of protein were mixed with reaction buffer (Ac-LEHD-pNA for caspase-9, Ac-DEVD-pNA for caspase-3) and incubated at 37°C for 2 hours in the dark. Caspase-9 and caspase-3 activities were measured at an absorbance of 405 nm on a spectrophotometer (Thermo Fisher Scientific, Waltham, NJ, USA).The results of each treatment groups were compared with the control group to obtain relative quantification (absorbance value) of the target proteins’ expression.
Statistical analysis
Data are presented as the mean ± SD. Statistics was performed using SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). Statistical analysis was conducted using one-way analysis of variance followed by Dunnett’spost hoctest. AP-value of less than 0.05 was considered statistically significant.
Results
Evaluation of locomotor recovery
We assessed whether rutin affected locomotor recovery in our rat SCI model using the BBB scoring system. As shown in Figure 2, BBB scores were reduced at 24, 48 and 72 hours post-surgery in the SCI group, compared with the control group. Treatment with rutin (30 mg/kg day per for 3 days after SCI) and MP resulted in an increase in the BBB score in comparison with the SCI group, and both showed significant difference compared with SCI group (P< 0.05; Figure 2).There was no significant difference between the rutin group(30 mg/kg) and MP group (P> 0.05; Figure 2).
Rutin reduced spinal cord water content in rats with SCI
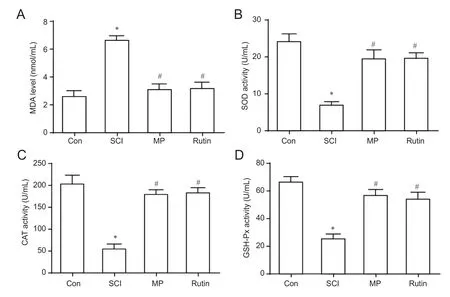
Figure 4 Antioxidative effects on SCI after 3 days of treatment with rutin.

Figure 5 Anti-inflammatory effects of 3-day treatment with rutin on SCI.

Figure 6 Rutin decreases p38 MAPK protein expression after 3-day treatment with rutin on SCI.
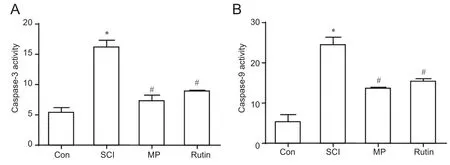
Figure 7 Rutin treatment for 3 days decreases caspase-9 and caspase-3 activities in the SCI rats.
We examined the water content of the spinal cord after 3-day treatment. As shown in Figure 3, spinal cord water content was increased in the SCI group compared with the control group. The water content of the spinal cord decreased in the rutin group (30 mg/kg/day for 3 days) compared with the SCI group (P< 0.05; Figure 3). Interestingly, the spinal cord water content in the rutin group was very similar to that in the MP group (P> 0.05; Figure 3).
Antioxidative effects of rutin in SCI
After 3 days of treatment, MDA concentration was increased in the SCI group compared with the control group (Figure 4A). MDA concentrations were reduced by treatment with rutin, compared to the SCI group (Figure 4A). The concentrations of SOD and catalase, and GSH-Px activity were lower in the SCI group, compared to the control group (Figure 4B—D). Rutin treatment in rats with SCI increased their levels compared with rats in the SCI group (Figure 4B—D;P< 0.05). We found no significant difference between the rutin and MP groups (P> 0.05; Figure 4A—D).
Anti-inflammatory effects of rutin on SCI
As shown in Figure 5A—D, SCI induced an inflammatory reaction and increased the levels of NF-κB p65, TNF-α, IL-1B and IL-6 in comparison to the control group. However,these inflammatory cytokines were reduced in the rutin group (30 mg/kg/day for 3 days) compared to the SCI group(P< 0.05; Figure 5A—D). No significant differences in NF-κB p65, TNF-α, IL-1B or IL-6 levels were observed between rutin and MP groups (P> 0.05; Figure 5A—D).
Rutin decreased p38MAPK protein expression in the rat models of SCI
There is growing evidence that the protective effects of rutin in SCI involve changes in p38 MAPK expression. p38 MAPK protein expression was substantially increased in the SCI group compared with the control group (Figure 6A, B). Treatment with rutin (30 mg/kg/day for 3 days)reduced p38 MAPK expression compared with the SCI group (Figure 6A, B). There was no significant difference in p38 MAPK protein expression between rutin and MP groups (P> 0.05; Figure 6A, B).
Rutin decreased caspase-9 and caspase-3 activities in rats with SCI
Caspase-9 and caspase-3 activities were increased in the SCI group, compared with the control group (P< 0.05).Rutin reduced the activities of these caspases in the animals with SCI (P< 0.05) (Figure 7A, B).
Discussion
With the rise in modern transportation, mining and industry,SCI has become increasingly common in China. SCI is a debilitating injury that negatively impacts human health and the quality of life. In this study, we demonstrate, for thefirst time,that rutin is neuroprotective in SCI. Rutin increased the BBB scores and reduced spinal cord water content in rats with SCI.
Oxidative stress after SCI plays a major pathogenetic role in secondary injury, and free radical levels are an indicator of the degree of injury (Song et al., 2015; Wang et al.,2015; Colón and Miranda, 2016). Strategies that prevent or reduce oxidative stress have shown therapeutic efficacy for SCI (Tian et al., 2016). Indeed, in the present study, rutin reduced MDA content and increased the concentrations of SOD and catalase and increased the activity of GSH-Px in the rat models of SCI, suggesting that the flavonoid protects cells of the spinal cord by reducing oxidative stress(Bhandary et al., 2012; Warford et al., 2014). The rutin reduced MDA concentrations and elevated SOD levels (Su et al., 2014). Scholars demonstrated that rutin improved spatial memory by attenuating oxidative stress and neuroin flammation in Alzheimer’s disease transgenic mice (Xu et al., 2014; Wu et al., 2016).
Inflammatory cytokines regulate the immune response and participate in cross-talk between immune cells and other cell types (Miller et al., 2013; Xie et al., 2014). NF-κB is a transcription complex that plays a key role in cytokine-mediated in flammatory reactions (Chen et al., 2014b; Kang et al., 2015; Zhang et al., 2015). NF-κB induces transcription of numerous in flammatory cytokines during the early stage,including TNF-α, IL-1B and IL-6, thereby increasing the inflammatory response (Shin et al., 2011; Du et al., 2013).
In this study, the levels of NF-κB p65, TNF-α, IL-1B and IL-6 were reduced by rutin treatment in the rat SCI model (Paniagua-Torija et al., 2015). This anti-inflammatory effect of rutin might contribute to the neuroprotection provided by the flavonoid. Our findings are consistent with previous studies. Previous studies showed that rutin decreased TNF-α and IL-1B generation in microglia (Wang et al., 2012; Zong et al., 2012). Furthermore, Abd-El-Fattah et al. (2010) reported that rutin exerted an anti-inflammatory effect in irradiated rats with cerebral ischemia/reperfusion injury.
Inactive p38 MAPK is mainly distributed in the cytoplasm,and translocates to the nucleus upon activation to regulate gene expression through phosphorylation of transcription factors (Tang et al., 2013; Chen et al., 2014a). Extracellular stimuli, such as inflammatory cytokines, induce the phosphorylation and activation of p38 MAPKviaa kinase cascade (Zhu et al., 2013; Park et al., 2015). Activated p38 MAPK induces the expression of enzymes, such as COX and iNOS, as well as numerous inflammatory-related molecules,which mediate the inflammatory response (Breton-Romero and Lamas, 2013).
In this study, we observed that p38 MAPK expression in the SCI model was reduced by rutin, suggesting that it may, in part, protect cells in the spinal cord by lowering the expression of pro-apoptotic proteins. Our results are in line with those of other studies. For example, Park et al.(2014) observed that rutin protected human dopaminergic cells against rotenone-induced injury by inhibiting the p38 MAPK signaling pathway. A previous study showed that rutin exerted anti-inflammatory effects, which were attributable to its suppression of p38 MAPK in UVB-irradiated mouse skin (Choi et al., 2014). Importantly, we found that reduction of caspase-9 and caspase-3 activities are the key to the neuroprotective action of rutin on spinal cord cells. This observation was in accordance with previous reports showing that rutin alleviated prion peptide-induced cell death by inhibiting caspase-3 activity in dopaminergic and hippocampal neurons (Na et al., 2014; Song et al., 2014).
In summary, ourfindings demonstrate that rutin protects spinal cord cells by reducing oxidative stress and inflammation, and by lowering the expression of pro-apoptotic proteinsviainhibition of the p38 MAPK pathway. Rutin plays an important role in the treatment of various disorders,and has been applied in health-care system due to its wide pharmacological activities, lower cost and high safety margins which is the most significant preponderance compare with the glucocorticoids (Sharma et al., 2013). In contrast,glucocorticoids have a wide foreseeable range of side effects;for instance, patients receiving high-dose MP after SCI had a significantly increased risk of major complications, in particular, gastrointestinal ulcer/bleeding and pulmonary embolism (Chikuda et al., 2014; Evaniew et al., 2015). However,the major problem associated with rutin is its poor solubility in aqueous media (0.8 mg/mL) (Lauro et al., 2002), which means poor bioavailability.
In the near future, enhancing its bioavailability using novel drug delivery methods and fully elucidating the neuroprotective effects and mechanisms of action will add evidence for use of traditional Chinese medicine for treatment of SCI.
Author contributions:BN and WMG designed this study. HLS, XZ,WZW and RHL performed experiments. RHL and KZ analyzed data.HLS, MYL and KZ wrote the paper. All authors approved thefinal ver-sion of the paper.
Conflicts of interest:The authors declare no competingfinancial interests.
Financial support:This work was supported in part by grants from the Young Scientists Awards Foundation of Shandong Province of China,No. BS2013YY049; and the China Postdoctoral Science Foundation, No.2012M511036. Funders had no involvement in the study design, expeirment conduction, data analysis, paper preparation, or decision to submit the paper for publication.
Research ethics:The study protocol was approved by the Animal Ethics Committee of Jinan Central Hospital Affiliated to Shandong University of China (2014-30). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Data sharing statement:The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Abd-El-Fattah AA, El-Sawalhi MM, Rashed ER, El-Ghazaly MA (2010)Possible role of vitamin E, coenzyme Q10 and rutin in protection against cerebral ischemia/reperfusion injury in irradiated rats. Int J Radiat Biol 86:1070-1078.
Aslan A, Cemek M, Buyukokuroglu ME, Altunbas K, Bas O, Yurumez Y,Cosar M (2009) Dantrolene can reduce secondary damage after spinal cord injury. Eur Spine J 18:1442-1451.
Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, Holford TR, Hsu CY, Noble LJ, Nockels R, Perot PL, Salzman SK,Young W (1996) MASCIS evaluation of openfield locomotor scores:effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma 13:343-359.
Bhandary B, Piao CS, Kim DS, Lee GH, Chae SW, Kim HR, Chae HJ (2012)The protective effect of rutin against ischemia/reperfusion-associated hemodynamic alteration through antioxidant activity. Arch Pharm Res 35:1091-1097.
Breton-Romero R, Lamas S (2013) Hydrogen peroxide signaling mediator in the activation of p38 MAPK in vascular endothelial cells. Methods Enzymol 528:49-59.
Chen S, Jin ZS (2016) Local transplantation of human umbilical cord mesenchymal stem cells at different time after spinal cord injury in rats: a histological observation. Zhongguo Zuzhi Gongcheng Yanjiu 20:6714-6719.
Chen Z, Cai Y, Zhang W, Liu X, Liu S (2014a) Astragaloside IV inhibits platelet-derived growth factor-BB-stimulated proliferation and migration of vascular smooth muscle cells via the inhibition of p38 MAPK signaling. Exp Ther Med 8:1253-1258.
Chen Z, Fu Q, Shen B, Huang X, Wang K, He P, Li F, Zhang F (2014b) Enhanced p62 expression triggers concomitant autophagy and apoptosis in a rat chronic spinal cord compression model. Mol Med Rep 9:2091-2096.
Chikuda H, Yasunaga H, Takeshita K, Horiguchi H, Kawaguchi H, Ohe K, Fushimi K, Tanaka S (2014) Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg Med J 31:201-206.
Choi KS, Kundu JK, Chun KS, Na HK, Surh YJ (2014) Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch Biochem Biophys 559:38-45.
Colón JM, Miranda JD (2016) Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res 11:1208-1211.
Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ (2014) Regulation of IL-10 by chondroitinase ABC promotes a distinct immune response following spinal cord injury. J Neurosci 34:16424-16432.
Du JS, Zhao Q, Zhang YL, Wang Y, Ma M (2013) 7,8-dihydroxycoumarin may promote sciatic nerve regeneration by suppressing NF-kappaB expression in mice. Mol Med Rep 8:1525-1530.
Elamin A, Zhu H, Hassan AM, Xu N, Ibrahim ME (2013) Peroxiredoxin V: a candidate breast tumor marker of population specificity. Mol Clin Oncol 1:541-549.
Evaniew N, Noonan VK, Fallah N, Kwon BK, Rivers CS, Ahn H, Bailey CS, Christie SD, Fourney DR, Hurlbert RJ, Linassi AG, Fehlings MG, Dvorak MF; RHSCIR Network (2015) Methylprednisolone for the treatment of patients with acute spinal cord injuries: a propensity score-matched cohort study from a canadian multi-center spinal cord injury registry. J Neurotrauma 32:1674-1683.
Genovese T, Menegazzi M, Mazzon E, Crisafulli C, Di Paola R, Dal Bosco M, Zou Z, Suzuki H, Cuzzocrea S (2009) Glycyrrhizin reduces secondary in flammatory process after spinal cord compression injury in mice.Shock (Augusta, Ga) 31:367-375.
Ha KY, Carragee E, Cheng I, Kwon SE, Kim YH (2011) Pregabalin as a neuroprotector after spinal cord injury in rats: biochemical analysis and effect on glial cells. J Korean Med Sci 26:404-411.
Ji W (2014) Tissue engineering is a promising method for the repair of spinal cord injuries (Review). Exp Ther Med 7:523-528.
Kang N, Hai Y, Yang J, Liang F, Gao CJ (2015) Hyperbaric oxygen intervention reduces secondary spinal cord injury in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Int J Clin Exp Pathol 8:1141-1153.
Khayrullina G, Bermudez S, Byrnes KR (2015) Inhibition of NOX2 reduces locomotor impairment, in flammation, and oxidative stress after spinal cord injury. J Neuroin flammation 12:172.
Lauro MR, Torre ML, Maggi L, De Simone F, Conte U, Aquino RP (2002)Fast- and slow-release tablets for oral administration of flavonoids: rutin and quercetin. Drug Dev Ind Pharm 28:371-379.
Lee KM, Jeon SM, Cho HJ (2010) Interleukin-6 induces microglial CX3CR1 expression in the spinal cord after peripheral nerve injury through the activation of p38 MAPK. Eur J Pain 14:682.e682.e12.
Machova Urdzikova L, Karova K, Ruzicka J, Kloudova A, Shannon C,Dubisova J, Murali R, Kubinova S, Sykova E, Jhanwar-Uniyal M, Jendelova P (2015) The anti-in flammatory compound curcumin enhances locomotor and sensory recovery after spinal cord injury in rats by immunomodulation. Int J Mol Sci 17:E49.
Miller AH, Haroon E, Raison CL, Felger JC (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297-306.
Na JY, Kim S, Song K, Kwon J (2014) Rutin alleviates prion peptide-induced cell death through inhibiting apoptotic pathway activation in dopaminergic neuronal cells. Cell Mol Neurobiol 34:1071-1079.
Nafees S, Rashid S, Ali N, Hasan SK, Sultana S (2015) Rutin ameliorates cyclophosphamide induced oxidative stress and in flammation in Wistar rats: role of NFκB/MAPK pathway. Chem Biol Interact 231:98-107.
Ni H, Jin W, Yuan B, Zhu T, Wang J, Jiang J, Liang W, Ma Z (2014)Curcumin inhibits the increase of labile zinc and the expression of in flammatory cytokines after traumatic spinal cord injury in rats. J Surg Res 187:646-652.
Pan PH, Lin SY, Wang YY, Chen WY, Chuang YH, Wu CC, Chen CJ (2014)Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Radic Biol Med 73:106-116.
Paniagua-Torija B, Arevalo-Martin A, Molina-Holgado E, Molina-Holgado F, Garcia-Ovejero D (2015) Spinal cord injury induces a long-lasting upregulation of interleukin-1B in astrocytes around the central canal.Neuroscience 284:283-289.
Park J, Min JS, Kim B, Chae UB, Yun JW, Choi MS, Kong IK, Chang KT,Lee DS (2015) Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-κB pathways. Neurosci Lett 584:191-196.
Park SE, Sapkota K, Choi JH, Kim MK, Kim YH, Kim KM, Kim KJ, Oh HN, Kim SJ, Kim S (2014) Rutin from Dendropanax morbifera Leveille protects human dopaminergic cells against rotenone induced cell injury through inhibiting JNK and p38 MAPK signaling. Neurochem Res 39:707-718.
Paterniti I, Genovese T, Crisafulli C, Mazzon E, Di Paola R, Galuppo M,Bramanti P, Cuzzocrea S (2009) Treatment with green tea extract attenuates secondary in flammatory response in an experimental model of spinal cord trauma. Naunyn Schmiedebergs Arch Pharmacol 380:179-192.
Pereira L, Igea A, Canovas B, Dolado I, Nebreda AR (2013) Inhibition of p38 MAPK sensitizes tumour cells to cisplatin-induced apoptosis mediated by reactive oxygen species and JNK. EMBO Mol Med 5:1759-1774.Phillips AA, Krassioukov AV (2015) Contemporary cardiovascular concerns after spinal cord injury: mechanisms, maladaptations, and management. J Neurotrauma 32:1927-1942.
Ravikumar R, Fugaccia I, Scheff SW, Geddes JW, Srinivasan C, Toborek M(2005) Nicotine attenuates morphological deficits in a contusion model of spinal cord injury. J Neurotrauma 22:240-251.
Sharma S, Ali A, Ali J, Sahni JK, Baboota S (2013) Rutin: therapeutic potential and recent advances in drug delivery. Exp Opin Inves Drugs 22:1063-1079.
Shin JS, Noh YS, Lee YS, Cho YW, Baek NI, Choi MS, Jeong TS, Kang E,Chung HG, Lee KT (2011) Arvelexin from Brassica rapa suppresses NF-kappaB-regulated pro-in flammatory gene expression by inhibiting activation of IkappaB kinase. Br J Pharmacol 164:145-158.
Song K, Kim S, Na JY, Park JH, Kim JK, Kim JH, Kwon J (2014) Rutin attenuates ethanol-induced neurotoxicity in hippocampal neuronal cells by increasing aldehyde dehydrogenase 2. Food Chem Toxicol 72:228-233.
Song K, Na JY, Kim S, Kwon J (2015) Rutin upregulates neurotrophic factors resulting in attenuation of ethanol-induced oxidative stress in HT22 hippocampal neuronal cells. J Sci Food Agric 95:2117-2123.
Su KY, Yu CY, Chen YW, Huang YT, Chen CT, Wu HF, Chen YL (2014)Rutin, a flavonoid and principal component of saussurea involucrata,attenuates physical fatigue in a forced swimming mouse model. Int J Med Sci 11:528-537.
Tang N, Zhang YP, Ying W, Yao XX (2013) Interleukin-1B upregulates matrix metalloproteinase-13 gene expression via c-Jun N-terminal kinase and p38 MAPK pathways in rat hepatic stellate cells. Mol Med Rep 8:1861-1865.
Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, Chen X (2016) Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol 771:84-92.
Varma AK, Das A, Wallace G 4th, Barry J, Vertegel AA, Ray SK, Banik NL (2013) Spinal cord injury: a review of current therapy, future treatments, and basic science frontiers. Neurochem Res 38:895-905.
Wang SW, Wang YJ, Su YJ, Zhou WW, Yang SG, Zhang R, Zhao M, Li YN, Zhang ZP, Zhan DW, Liu RT (2012) Rutin inhibits beta-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proin flammatory cytokines. Neurotoxicology 33:482-490.
Wang W, Shen H, Xie JJ, Ling J, Lu H (2015) Neuroprotective effect of ginseng against spinal cord injury induced oxidative stress and in flammatory responses. Int J Clin Exp Med 8:3514-3521.
Warford J, Jones QR, Nichols M, Sullivan V, Rupasinghe HP, Robertson GS (2014) The flavonoid-enriched fraction AF4 suppresses neuroinflammation and promotes restorative gene expression in a mouse model of experimental autoimmune encephalomyelitis. J Neuroimmunol 268:71-83.
Wu J, Maoqiang L, Fan H, Zhenyu B, Qifang H, Xuepeng W, Liulong Z(2016) Rutin attenuates neuroin flammation in spinal cord injury rats. J Surg Res 203:331-337.
Xie YG, Mu HJ, Li Z, Ma JH, Wang YL (2014) Supression of chronic central pain by superoxide dismutase in rats with spinal cord injury: Inhibition of the NMDA receptor implicated. Exp Ther Med 8:1137-1141.
Xu PX, Wang SW, Yu XL, Su YJ, Wang T, Zhou WW, Zhang H, Wang YJ,Liu RT (2014) Rutin improves spatial memory in Alzheimer’s disease transgenic mice by reducing Abeta oligomer level and attenuating oxidative stress and neuroin flammation. Behav Brain Res 264:173-180.
Yamaoka G, Morino T, Morizane K, Horiuchi H, Miura H, Ogata T (2012)p38 mitogen-activated protein kinase inhibitor reduces neurocan production in cultured spinal cord astrocytes. Neuroreport 23:546-550.
Yeh CH, Yang JJ, Yang ML, Li YC, Kuan YH (2014) Rutin decreases lipopolysaccharide-induced acute lung injury via inhibition of oxidative stress and the MAPK-NF-kappaB pathway. Free Radic Biol Med 69:249-257.
Zhang P, Ma X (2015) Effect of rutin on spinal cord injury through inhibition of the expression of MIP-2 and activation of MMP-9, and downregulation of Akt phosphorylation. Mol Med Rep 12:7554-7560.
Zhang Z, Sun T, Niu JG, He ZQ, Liu Y, Wang F (2015) Amentoflavone protects hippocampal neurons: anti-in flammatory, antioxidative, and antiapoptotic effects. Neural Regen Res 10:1125-1133.
Zhu J, Luo C, Wang P, He Q, Zhou J, Peng H (2013) Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-kappaB pathways in LPS-stimulated RAW 264.7 cells. Exp Ther Med 5:1345-1350.
Zong S, Zeng G, Wei B, Xiong C, Zhao Y (2012) Beneficial effect of interleukin-1 receptor antagonist protein on spinal cord injury recovery in the rat. In flammation 35:520-526.
- 中国神经再生研究(英文版)的其它文章
- Neural Regeneration Research: Information for Authors
- A growingfield: the regulation of axonal regeneration by Wnt signaling
- LETTER FROM THE EDITORS-IN-CHIEF
- Brain injury and neural stem cells
- Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease
- Tackling dipeptidyl peptidase IV in neurological disorders