A growingfield: the regulation of axonal regeneration by Wnt signaling
Armando L. Garcia, Adanna Udeh, Karthik Kalahasty, Abigail S. Hackam*
Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA
Introduction
Wnt signaling pathways play essential roles in cellular proliferation, differentiation and cell migration during embryonic development. The importance of Wnt signaling is indicated by conservation of its molecular components across organisms ranging from nematodes to humans. Wnt pathways are classified into canonical Wnt/B-catenin or non-canonical(B-catenin-independent) pathways. Canonical Wnt/B-catenin signaling is the most studied, and is mediated by nuclear translocation of its central effector B-catenin. In the absence of Wnt ligands, cytoplasmic B-catenin is prevented from reaching its nuclear targets due to its constitutive degradation by a protein complex containing axin, adenomatous polyposis coli (APC), casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3B). Wnt signaling activation is initiated by binding of one of 19 Wnt ligands to one of 10 Frizzled (Fzd) receptors and the co-receptor low-density lipoprotein receptor related protein 5 or 6 (LRP5/6). Formation of this receptor complex and recruitment of the scaffolding protein Dishevelled (Dvl) leads to LRP5/6 phosphorylation and inhibits B-catenin degradation. Stabilized B-catenin then accumulates and translocates to the nucleus where it forms large protein complexes containing T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors,which leads to induction of Wnt target genes (MacDonald et al., 2009) (Figure 1).
Non-canonical Wnt signaling occurs independently of B-catenin—TCF/LEF and is stimulated by Wnt ligands that bind to a receptor complex of Fzd, Ror1/2 or Ryk (Figure 1)(Gomez-Orte et al., 2013). Binding to these receptors induces signaling through the Wnt/planar cell polarity (PCP) and Wnt/Ca2+pathways. The PCP pathway is initiated when Fzd receptors activate a cascade involving small GTPases RAC1,the Ras homolog gene family member A (RHOA) and c-Jun N-terminal-kinase (JNK). These downstream effectors induce cytoskeletal rearrangements and ultimately lead to morphogenetic changes during gastrulation in the developing embryo (Seifert and Mlodzik, 2007).
The other main non-canonical pathway, the Wnt/Ca2+pathway, shares several components with the PCP pathway.Wnt/Ca2+signaling is induced when Wnt ligands (primarily Wnt5a and 11) bind to Fzd receptors, which activates heterotrimeric G proteins and leads to phospholipase C activation and release of calcium from intracellular stores(Gomez-Orte et al., 2013). Elevated calcium levels activate protein kinase C and calcium/calmodulin-dependent kinase II, which regulate dorsal axis formation and promote ventral cell fate and tissue separation during gastrulation (Seifert and Mlodzik, 2007).
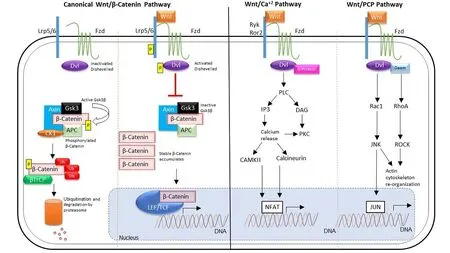
Figure 1 Overview of canonical and non-canonical Wnt signaling pathways.
Wnt Signaling Pathways Regulate Regeneration in the Central Nervous System (CNS)
In the developing optic nerve, brain and spinal cord, canonical Wnt signaling pathways regulate axonal growth and remodeling and act as critical axon guidance factors (Schmitt et al., 2006). Wnt ligands, such as Wnt3a, 4 and 7b, are target-derived signals during early and late CNS development and induce axon branching, growth cone formation, axonal outgrowth and synaptic assembly. The functional consequence of Wnt ligands on axons is complex due to their concentration-dependent effects that result in opposite activities when bound to different receptors. For example, Wnt3a is expressed in a medial-to-lateral gradient in the developing chick optic tectum and mouse superior colliculus. In the midbrain, it acts at low concentrations through Fzd receptors to attract growing retinal ganglion cell (RGC) axons, whereas at high Wnt ligand concentrations it acts through Ryk receptors to mediate axonal repulsion (Schmitt et al., 2006).
Recent studies in the adult CNS after injury demonstrated that canonical Wnt signaling induces axonal regeneration and neurite growth, suggesting that the developmental role for Wnt can be repurposed in adults by exogenous stimulation of the pathway. Intravitreal injections of the canonical Wnt/B-catenin signaling activator Wnt3a induced significant axonal growth past the axon lesion site in a murine optic nerve crush injury model (Patel et al., 2017). Additionally, Wnt3a activity led to increased RGC survival and higher function than was observed in controls (Patel et al.,2017). In another study, Wnt3a signaling promoted neurite outgrowth, increased neuronal function and induced repair after spinal cord contusion injury in adult rats (Yin et al.,2008). Wnt3a promoted the differentiation of endogenous neural stem cells into neurons and induced re-myelination of the lesion site, leading to improved axonal conduction and spinal cord function (Yin et al., 2008).
The zebrafish model of spinal cord injury has also been used to investigate the regenerative activity of Wnt signaling.Increased expression of B-catenin and Wnt4b were observed after spinal transection in adult zebrafish and correlated with axonal regeneration and functional recovery (Strand et al., 2016). The importance of endogenous Wnt/B-catenin signaling to spinal cord regeneration was shown by overexpressing the Wnt inhibitor Dkk1b, which inhibited axonal regeneration through the injury site and blocked recovery of swimming capability. In zebrafish, axon regeneration requires the formation of a glial bridge that serves as a scaffold for growing axons, and this glial bridge was also inhibited by blocking Wnt signaling (Strand et al., 2016). Elevated Wnt/B-catenin activity was observed in radial glia after spinal cord injury in zebrafish larvae, and Wnt signaling was necessary for neurogenesis and axonal regrowth (Briona et al., 2015).Furthermore, spinal cord injury in zebrafish caused increased Wnt/B-catenin signaling in fibroblast-like cells adjacent to regenerating axons (Wehner et al., 2017). Wnt signaling in these cells induced collagen XII expression and deposition at the lesion site, and the extracellular matrix changes facilitated axonal growth through the lesion. The authors also demonstrated that genetic and pharmacologic inhibition of Wnt/B-catenin signaling, or blocking collagen XII deposition, prevented axonal regeneration and functional recovery (Wehner et al., 2017). Experiments that manipulated the timing of Wnt inhibition indicated that Wnt signaling promoted regeneration only when axons were growing across the lesion site but not at later time-points (Wehner et al., 2017). Together, these studies demonstrate the influence that Wnt signaling exerts over the cellular environment that are permissive to axonal growth, and provide evidence for a regenerative role of canonical Wnt signaling within the CNS.
Although ligands that activate canonical Wnt signaling generally promote axonal growth, there is substantial evidence that ligands activating non-canonical Wnt-Ryk pathways inhibit axonal growth. In the developing cortical spinal tracts and corpus callosum, non-canonical ligands Wnt1 and 5a bind to Ryk receptors and repel growing axons (Yam and Charron, 2013). Also, the Wnt receptor Ryk was expressed at the lesion site in a rat spinal injury model, and addition of the Wnt inhibitors SFRP2 and WIF1 enhanced axonal regeneration, indicating a repulsive function of endogenous Wnt(Hollis and Zou, 2012). Furthermore, grafting bone marrow stem cells that over-expressed Wnt4 into spinal lesions of the dorsal column led to long-range retraction of the sensory nerve axons. Similarly, injection of function-blocking Ryk antibodies at the lesion site of the dorsal spinal cord in mice caused increased sprouting of corticospinal tract branches around the injury site (Liu et al., 2008). Miyashita et al. (2009)also demonstrated that blocking Wnt5a-Ryk signaling resulted in significant axonal growth of the corticospinal tract and enhanced functional recovery in a spinal cord contusion model. Therefore, expression of repulsive non-canonical Wnt ligands contributes to the lack of axon regeneration in the CNS in these models, and blocking non-canonical Wnt signaling promoted axonal growth and functional recovery(Salinas, 2012; Onishi et al., 2014).
The studies above demonstrate that different Wnt ligands have distinct effects on growing axons, and that canonical and non-canonical pathways can have opposite effects. Adding to the complexity is that the canonical ligand Wnt3a can activate both canonical and non-canonical signaling pathways in the same cell type (Nalesso et al., 2011), further increasing the possible effects of Wnt stimulation on axons.Finally, although the interaction between canonical and non-canonical Wnt signaling is complex, they generally have an inhibitory effect on each other, as demonstrated during development (Topol et al., 2003). Because non-canonical Wnt ligands and receptors are expressed in the adult retina and spinal cord, an interesting question is whether Wnt3a promotes axonal regeneration by antagonizing the anti-regenerative properties of non-canonical Wnt signaling.
Wnt Signaling and Neurite Growth
Neurite growth in neurons and explants grownin vitrois often used as a simple model of growth cone formation and axonal outgrowth. Many studies have analyzed the effect of Wnts on neuritogenesis in cultured neurons. Wnt ligands induce neurite extension in various neuronal types,including Wnt7a in cultured mouse cerebellar granule cells(Lucas and Salinas, 1997), Wnt3a in primary cultured RGCs(Udeh et al., submitted), Wnt5a and Wnt3b in chick RGCs(Rodriguez et al., 2005), and Wnt7b in mouse hippocampal neurons (Rosso et al., 2005). Also, Wnt3 and Wnt3a added to cultured spinal cord cell neural precursors increased neurogenesis and promoted neurite outgrowth through B-catenin/TCF4-dependent transcription (David et al., 2010).However, Wnt signaling had no effect in embryonic chick statoacoustic ganglion neurons (Fantetti et al., 2011), and inhibited neurite growth in other cells types, such as PC12 cells (Selvaraj et al., 2015). Furthermore, the Wnt antagonist secretedfizzled receptor protein 1 (SFRP1) induced neurite growth in chick RGC explants through the Fzd2 receptor,but was independent of Wnt/B-catenin signaling (Rodriguez et al., 2005). Wnt-dependent GSK3B inactivation also increased neurite formation (Lucas et al., 1998), and LiCl,which activates Wnt/B signaling by inhibiting GSK3B activity, enhanced neurite sprouting and branching, and altered microtubule organization in a dose-dependent manner in cultured adult spiral ganglion neurons (Shah et al., 2013).
Therefore, the activity of Wnt/B-catenin in promoting neurite formation in culture is consistent with its axonal growth role in the optic nerve and spinal cordin vivo, although its ability to promote neurite formation differs amongst neuronal types. Interestingly, Hur et al. (2011)demonstrated that the regulation of axonal growth is controlled by activity levels of GSK3B: high GSK3B activity impairs axonal growth by destabilizing microtubules, moderate GSK3B levels stabilize microtubules and promote axonal growth, whereas low GSK3B activity blocks microtubule extension and attenuates axon growth. This gradient effect may also occur downstream of Wnt/B-catenin activation and could explain the conflictingfindings of Wnt ligands on neurite outgrowth in different cell types.
Activation of Wnt/B-Signaling in Neurons and Glia
Canonical Wnt/B-catenin signaling reporter mice are strains of transgenic mice with aLacZtransgene controlled by TCF/LEF consensus DNA binding elements and a minimal promoter. The establishment of transgenic Wnt reporter mice and reliable antibodies allows researchers to identify cell types that contain functional Wnt signaling, express LRP and Fzd receptors and secrete Wnt ligands. Transgenic zebrafish with a fluorescent reporter of B-catenin/TCF-mediated transcription have also been used to follow Wnt signaling activation during injury and regeneration (Strand et al., 2016). Canonical Wnt pathway activation was shown to be induced dynamically in the developing retina (Liu et al., 2003), and Wnt signaling is constitutively activated in the ganglion cell layer and amacrine cells in adult wild-type mice (Liu et al., 2006; Yi et al., 2007). Transcript levels of various Wnt ligands, receptors and regulators show differential expression throughout retinal development and during RGC differentiation (Liu et al., 2006), and changes in several Wnt receptor genes were detected in degenerating retinas (Yi et al., 2007). Furthermore, activated endogenous Wnt/B-catenin signaling was localized in RGCs and adjacent Muller glia after optic nerve injury (Patel et al., 2017).
Multiple ligands in the retina have been identified in the adult retina, including Wnt1, Wnt2b, Wnt3a, Wnt4, Wnt5a,Wnt5b, and Wnt10a, and these ligands could potentially contribute to the regenerative response to axonal injury.Additionally, the identity of Wnt ligand-secreting cells has been examined by localizing Wnt ligands at the protein and transcript levels. Immunohistochemistry analyses demonstrated that cells within the GCL, as well as Muller glia and photoreceptors, express the canonical ligand Wnt3a (Patel et al., 2015), and in situ hybridization localized numerous transcripts for Wnt ligands to RGCs, amacrine cells, ciliary epithelium and retinal progenitor cells (Blackshaw et al.,2001; Liu et al., 2003, 2006). The signaling and regeneration mechanisms induced by the majority of these Wnt ligands remain to be characterized, with the exception of Wnt3a-induced optic nerve regeneration, which involves STAT3 (Patel et al., 2017), and Wnt10b, which stimulates robust axonal regeneration in mouse RGCs by activating mTOR (Tassew et al., 2017). These mechanisms are described in detail below.Finally, limited information is currently available regarding the identity of the Wnt receptors that are expressed in RGCs and regenerating axons. Because different combinations of ligand-receptors could have distinct outcomes to the cell, a focus of future studies should include characterizing the regenerative roles of different receptors.
Wnt ligands and B-catenin are also upregulated in the spinal cord following axonal injury (Lambert et al., 2016;Strand et al., 2016) and Wnt signaling was localized to various cell types, including fibroblast-like cells, radial glia,oligodendrocytes, microglia/macrophages, astrocytes and neurons (Lambert et al., 2016). Wnt receptors and ligands were down-regulated and Wnt inhibitors were upregulated at specific time-points after spinal cord injury in mice, and differential expression of the Fzd receptors was observed in neurons and glia (Gonzalez-Fernandez et al., 2014). In contrast, increased expression of Wnt ligands and activated B-catenin were observed in white matter in a rat spinal cord injury model (Fernandez-Martos et al., 2011). Additionally,the repulsive Ryk receptor was induced in injured cortico-spinal tract axons, and Wnt5a was induced in reactive astrocytes around the injury site (Liu et al., 2008).
Extrinsic and Intrinsic Effects of Wnt Signaling
Wnt ligands and receptors are expressed within neurons and non-neuronal cells, which raises questions about the relative roles of intrinsic and extrinsic influences on Wnt-dependent axonal regeneration. Indeed, the cell types that contain elevated Wnt signaling appear to be important to the overall effect of canonical Wnt signaling on neuronal damage. Activation of Wnt signaling in retinal Muller glia after optic nerve injury (Patel et al., 2017) and during retinal degeneration (Yi et al., 2007), suggests that radial glia cells influence the survival and regenerative activity of RGCs. Similarly,B-catenin accumulated in Muller glia nuclei after light injury in zebrafish (Meyers et al., 2012) and N-methyl-D-aspartic acid injury in rat (Osakada et al., 2007). Regenerative factors are expressed in various cell types in the retina in addition to RGCs, but the contribution of pro-regeneration signaling in non-RGCs to the overall regenerative capacity is mostly unknown.
Evidence is accumulating that activating regeneration pathways in uninjured non-RGC cell types may enhance axonal regeneration, compared with activating them in degenerating RGCs. As mentioned above, elevated Wnt/B-catenin activity was observed in radial glia and was required for axonal regeneration in a zebrafish spinal cord injury model(Briona et al., 2015). Also, activated Wnt/B-catenin signaling was localized tofibroblast-like cells adjacent to regenerating axons (Wehner et al., 2017). Similarly, elevated Wnt/B-catenin signaling within olfactory ensheathing cells (OECs),which are major glia cells in the olfactory system, promotes neurite growth and synaptogenesis of co-cultured neurons(Yang et al., 2013). Previous studies indicated the importance of retinal glia in mediating the regenerative activity of various growth factors. For example, viral delivery of ciliary neurotrophic factor (CNTF) to Muller glia promoted optic nerve axonal extension and sprouting (Pernet et al., 2013),and transplantation of astrocytes and Muller glia into the spinal cord led to axonal growth in DRG neurons (Lorber et al., 2015). Muller glia are generally protective to RGCs and induce RGC neuritogenesisin vitro(Garcia et al., 2002),suggesting that Wnt signaling in Muller glia could mediate protective and regenerative effects on RGCs. Therefore, the cellular localization of active Wnt signaling after injury raises the concept that the pro-regenerative effect of Wnt/B-catenin is the culmination of its activity in multiple cell types,including not only the injured neurons, but also radial glia,microglia and other inflammatory cells. Although the role of these cells with active Wnt is beginning to be understood after spinal cord injury, additional studies are needed to determine the contributions of Wnt signaling in the retina in non-RGC cells, such as Muller glia and microglia, to axonal regeneration after optic nerve injury.
The location of Wnt signaling relative to the injury site may be a key factor in whether axons regenerate. During spinal injury, formation of a glial scar around the lesion limits inflammation and reduces further tissue damage (Herrmann et al., 2008). While astrocytes are thought to be the primary source of the glial scar, oligodendrocyte precursor cells (OPCs) have been shown to regulate astrogliosis in a B-catenin dependent manner (Figure 2–(3)). OPCs express PDGFRα and NG2, and proliferate and mature during injury to aid in myelin repair (Tripathi and McTigue, 2007).Reduced gliosis was observed in conditional knock-out mice that lacked B-catenin specifically in PDGFRα-expressing OPCs after spinal cord injury and optic nerve crush (Duncan et al., 2014; Rodriguez et al., 2014). Additionally, knockout of B-catenin within OPCs reduced the accumulation and proliferation of these cells at the site of spinal injury, leading to reduced glial scar and increased RGC axonal regeneration(Rodriguez et al., 2014). Comparing between the studies of optic nerve and spinal cord regeneration described above,we hypothesize that the anti-regenerative effect of B-catenin in the Rodriguez study is due to Wnt signaling within glial-scar forming cells, in contrast to the regenerative effect in the optic nerve when Wnt3a signaling was activated within the retina in the affected neurons and adjacent radial glia.Therefore, studies that examine the axonal growth effect of Wnt signaling activators or inhibitors need to consider where the pathway is being stimulated relative to the injury site, in which cell types it is activated, and also potentially the dose used due to the differential concentration-dependent gradient effect of Wnt ligands.
Mechanisms of Wnt-Induced Regeneration
Molecular pathways that regulate axonal regrowth after optic nerve injury fall into several broad categories, including growth factors, such as Wnt, CNTF, brain derived neurotrophic factor (BDNF) and semaphorins, growth-suppressive transcription factors, such as KLF4, essential signaling mediators such as signal transducer and activator of transcription 3 (STAT3) and phosphatase and tensin homolog deleted on chromosome ten 2 (PTEN2), in flammatory factors, synaptic activity and visual stimuli (Pernet and Schwab,2014). The mechanisms by which Wnt signaling promotes axonal regeneration could involve induction of these axon growth-promoting genes, and/or Wnt signaling may directly regulate growth cone remodeling by altering microtubule stability at the axonal growth cone (Figure 2). Additional mechanisms may involve Wnt-dependent regulation of inflammation and neuronal survival. In the following sections,we summarize evidence for the contribution of these candidate mechanisms.
Transcriptional regulation of pro-survival and regenerative genes
Wnt signaling induces genes that promote neuronal survival in the retina after cellular injury and axonal damage, including CNTF, BDNF, nerve growth factor (NGF) and STAT3(Mansour-Robaey et al., 1994; Seitz et al., 2010; Patel et al.,2015, 2017). Many of these growth factors also induce axonal growth in the optic nerve when over-expressed. Similarly, neurotrophins and developmental growth factors, such as BDNF,fibroblast growth factor (FGF), sonic hedgehog (Shh)and bone morphogenetic proteins (BMPs) are implicated in spinal cord regeneration (Cardozo et al., 2017). These growth factors could contribute to Wnt-dependent neuronal survival and regeneration, or they may primarily promote survival such that the surviving neurons are able to respond to other axonal growth cues (Figure 2–(2)). Pro-survival activity is important for regeneration, although surviving cells need additional growth cues to extend their axons. Studies have not yet distinguished whether these growth pro-survival proteins are necessary for both Wnt-dependent survival and regeneration, or are only required for survival and that the surviving RGCs were able to respond to other axonal growth cues.
BDNF is a well-characterized growth factor that is induced by Wnt signaling in the retina. Wnt3a and the atypical Wnt ligand Norrin induced BDNF in several retinal cell types, including Muller glia and RGCs (Seitz et al., 2010; Yi et al., 2012). The upstream promoter region of the BDNF gene contains binding motifs for Wnt3a-dependent TCF/LEF transcription factors, indicating that Wnt directly regulates BDNF expression (Yi et al., 2012). Previous studies demonstrated that exogenous BDNF promotes the survival of axotomized RGCs following axonal injury (Sawai et al.,1996) and induces axonal regeneration when used in combination with other neurotrophic factors (Mansour-Robaey et al., 1994). BDNF and Wnt2 also synergized to regulate growth and maturation of dendritic spines in cultured cortical neurons (Hiester et al., 2013).
CNTF is another well-studied growth factor that is regulated by Wnt signaling (Seitz et al., 2010). Multiple lines of evidence demonstrate that CNTF protects axotomized RGCs and stimulates axonal regeneration when delivered by viral injections (Leaver et al., 2006; Muller et al., 2009) or injections of high doses of recombinant CNTF (Cui et al., 2003).Intraocular injection of CNTF induced signaling pathways in Muller glia that led to increased RGC survival and axonal growth (Pernet et al., 2013), similar to RGC axonal growth following Wnt3a-inducing signaling in Muller glia (Patel et al., 2017). Therefore, these studies support the idea that Wnt may promote RGC survival and regeneration by inducing BDNF or CNTF.
Additionally, Wnt signaling upregulates Stat3 expression and activation (Fragoso et al., 2012; Patel and Hackam,2012, 2014; Patel et al., 2015). Stat3 is a transcription factor that mediates signaling from the ligands CNTF, interleukin-6 (IL-6), leukemia inhibitory factor (LIF), oncostatin M and other cytokines. A direct role for Stat3 in axonal regeneration after optic nerve injury has been reported by several groups. For example, over-expression of an active variant of Stat3 led to robust neurite outgrowthin vitro, and transduction of Stat3 into RGCsin vivoresulted in substantial axonal regeneration of the optic nerve following injury (Mehta et al., 2016). Furthermore, knockdown of the endogenous Stat3 inhibitor SOCS3 induced axonal outgrowth and elongation in the optic nerve (Smith et al., 2009; Sun et al., 2011). Stat3 activation also contributes to Wnt3a-induced axonal regeneration and RGC survival after optic nerve crush (Patel et al.,2017). Elevated retinal Stat3 activation was associated with Wnt3a-induced axonal regeneration, and reducing Stat3 expression using a conditional Stat3 knock-out mouse line diminished Wnt3a-dependent axonal regeneration and reduced RGC survival (Patel et al., 2017). Notably, loss of Stat3 did not completely eliminate Wnt3a-dependent axonal regeneration,which suggests that additional target genes or mechanisms contribute to axon growth after Wnt3a stimulation.

Figure 2 Potential mechanisms of Wnt-induced regeneration.
Inflammation
Inflammatory signaling has complex effects on neuronal survival and axonal regeneration that range from beneficial to deleterious. For example, intravitreal injections of zymosan or lens injury stimulated axonal growth in the optic nerve (Yin et al., 2009). Consistent with this observation is that cytokines released during low levels of inflammation induced regeneration and increased the ability of NT-3 and other trophic factors to promote corticospinal tract sprouting (Chen et al., 2008; Benowitz and Popovich, 2011). Several molecules have dual functions in inflammatory signaling and axonal regeneration, including CNTF and LIF (Leibinger et al., 2009), which are both induced by Wnt/B-catenin signaling. Indeed, Wnt/B-catenin signaling has both positive and negative effects on local inflammatory pathways in the brain after neuronal damage induced by stroke, trauma and disease (Marchetti and Pluchino, 2013), suggesting that Wnt may have a complicated, and not yet understood, role in immune-stimulated axonal regeneration.
Macrophages and microglia are the primary inflammatory cells in the retina. They act as important mediators of neuronal damage and play roles in axonal regeneration following injury. Microglia produce proinflammatory cytokines and phagocytose injured cells and debris and are implicated in regulating neuronal survival and axonal growth. Activated canonical Wnt signaling was localized to retinal microglia during retinal degeneration in Wnt reporter mice (Yi et al.,2007) (Figure 2–(1)). Cultured microglia expressed multiple Wnt receptors and signaling components, and Wnt3a induces cytokine secretion (Halleskog et al., 2011). Additionally, ocular injections of Wnt3a increased the number of microglia in the retina, suggesting a role for microglia in Wnt3a-mediated axonal regeneration after optic nerve crush(Patel et al., 2017). However, microglia showing increased B-catenin expression, implying elevated Wnt signaling,was associated with their pathological pro-inflammatory transformation in Alzheimers disease tissue and in a mouse model of beta-amyloid-induced degeneration (Halleskog et al., 2011). Interestingly, a recent study found that depletion of microglial cells in the retina and optic nerve had no effect on its regenerative capacity (Hilla et al., 2017). Therefore,whether increased Wnt signaling within microglia is related to Wnt3a-dependent neuronal survival and regeneration in the optic nerve requires further investigation.
Another site of cross-talk between Wnt signaling and inflammation is through the intersection between Wnt/B-catenin signaling and nuclear factor kappaB (NF-κB) pathways(Du and Geller, 2010). Activation and translocation of the NF-κB transcription factor induces numerous pro-inflammatory genes that could modulate regeneration (Haenold et al., 2014). For example, NF-κB is activated in the retina and at the lesion site after optic nerve crush injury, and cell-type-specific deletion of the positive regulator RelA in neurons and oligodendrocytes led to robust axonal growth,indicating that abrogation of NF-κB may stimulate axonal growth (Haenold et al., 2014). Conversely, genetic loss of the NF-κB suppressor p50 promoted degeneration and inhibited axonal growth. In addition, intravitreal delivery of a p65 mutant increased RGC survival in optic nerve crush and transient ischemia by constitutively activating NF-κB(Dvoriantchikova et al., 2016).Therefore, the Wnt/B-catenin pathway may coordinate with the proin flammatory NF-κB pathway to modulate axonal regeneration in the optic nerve.
Growth inhibition
Intrinsic barriers limit the regenerative potential of injured neurons in the mammalian CNS, including expression of genes that reduce axonal growth, such as Kruppel-like factor-4 (KLF4) and ephrins (Schmitt et al., 2006; Cui et al.,2013). KLF4 suppresses axonal regeneration following injury to the mouse optic nerve (Moore et al., 2009). Evidence for an interaction between KLF4 and Wnt comes primarily from proliferating cells. For example, KLF4 directly antagonizes B-catenin/TCF binding at Wnt target promoters in cancer cells by reducing B-catenin binding to TCF4 (Sellak et al., 2012). Furthermore, KLF4 physically interacts with TCF4 and the C-terminal transactivation domain of B-catenin and blocks formation of a multiprotein complex required for promoter modification of Wnt target genes (Evans et al., 2010). The interaction of KLF4 with B-catenin, or to other members of the Wnt pathway, has not been examined in the retina, optic nerve or elsewhere in the CNS. KLF4 also blocks the DNA-binding activity of Stat3 in RGCs (Qin et al., 2013), raising the possibility that KLF4 may regulate Wnt3a-induced Stat3 levels and reduce the regenerative ability of endogenous Wnt3a. Further studies are needed to determine whether exogenous Wnt stimulation could overcome the inhibitory activity of KLF4 to induce axonal regeneration.
Ephs are a large family of receptor tyrosine kinases that play important roles in various cellular activities, including axonal guidance, and are stimulated by A and B subtypes of ephrin ligands (Pasquale, 2008). A well-studied function of ephrins is their role in topographic organization of the retinocollicular projections (Triplett and Feldheim, 2012).Through mechanisms not fully understood, Wnt signaling has been associated with Eph/Ephrin Bs to establish topography along the medial-lateral axis of the superior colliculus,which corresponds with the ventral-dorsal axis of the retina respectively. The expression pattern of Wnt3 in the mouse superior colliculus is similar to the region-specific EphrinB that guides axonal growth (Schmitt et al., 2006). Furthermore, canonical Wnt signaling upregulates EphB and downregulates ephrin B, and ephrin A3-EphA4 activation suppresses Wnt3a/B-catenin signaling within retinal stem cells, indicating cross-talk between the two signaling systems(Clevers and Batlle, 2006, Fang et al., 2013).
Several extracellular inhibitory molecules prevent axon regeneration in mammals after axonal injury. For example,deletion of phosphatase and tensin homolog (PTEN) in RGCs, which negatively regulates mammalian target of rapamycin (mTOR), resulted in significant axonal regeneration following nerve injury (Park et al., 2008). Although no studies have reported direct interaction between Wnt and the mTOR pathways in the context of nerve injury, GSK3B inhibits mTOR by phosphorylating TSC2, which is a negative regulator of mTOR activity. During neuronal development,GSK3B interacts with mTOR and inhibits mTOR-dependent cortical progenitor self-renewal (Ka et al., 2014). Inoki et al.(2006) demonstrated that Wnt activates mTOR in multiple cell lines, and blocking mTOR activity suppressed Wnt-induced cell growth in bone marrow stromal cells. Wnt may also promote optic nerve regeneration by blocking GSK-3B inactivation of CRMP2, which promotes axonal regrowth in the presence of inhibitory molecules (Leibinger et al., 2017).These data suggest a connection between Wnt and mTOR through GSK3B, which needs to be explored within the nervous system following injury.
B-catenin and microtubules
Microtubules and actin rapidly depolymerize after axonal injury, and subsequent stabilization and polymerization of these cytoskeleton elements is essential for growth cone formation, transport of molecules to the axon tip, axonal growth and regeneration. B-Catenin associates with the actin cytoskeleton through its interaction with α-catenin and cadherins. Several studies provide evidence that B-catenin plays a role in growth cone remodeling and microtubule stability. B-Catenin localizes to the growth cone in chick dorsal root ganglion (DRG) neurons, and alterations in B-catenin,GSK3B and Axin levels at the growth cone mediate semaphorin3A-induced effects on growth cone dynamics and cytoskeletal reorganization (Hida et al., 2012). Also, B-catenin associates with microtubule plus-ends in polarized epithelial cells and colocalizes with the microtubule plus-end tracking proteins EB1 and CLIP-170 (Bellett et al., 2009), which are involved in neuronal growth cone advancement and direction. Additionally, Hur et al. (2011) demonstrated that the activity level of GSK3B modifies actin-microtubule association and axon outgrowth in cortical neurons, as noted earlier. When GSK3B was highly active, the microtubule-associated protein CLASP dissociated from microtubule plus ends and axonal growth was reduced, whereas with moderate GSK3B activity, CLASP could bind to microtubules and promote axon elongation. With low GSK3B activity, CLASP promoted association with F-actin and caused microtubule looping instead of outgrowth (Hur et al., 2011). Although the Hur study used shRNA to regulate GSK3B activity, varying the dose of Wnt ligands could also regulate GSK3B activity levels and subsequent effects on microtubules, which provides another potential link between Wnt/B-catenin,microtubule stability and axonal growth (Figure 2-(4)).
A study by Purro et al. (2008) used time-lapse recordings to show that Wnt3a regulates growth cone remodeling in DRG neurons by altering microtubule stability through differential binding of its downstream proteins APC and Dvl1 to the positive ends of microtubules. In the presence of Wnt3a, APC disassociated from the plus ends of microtubules,which blocked forward growth and caused the microtubules to loop back, suggesting that Wnt-APC regulates growth cone steering. The activity of APC on microtubules required B-catenin and GSK3B inhibition, although it was not dependent on B-catenin transcriptional activity. The authors concluded that the effect of Wnt3a on growth cone remodeling is consistent with a role for Wnt3a as a target-derived signal that regulates terminal arborization of axons as they arrive at their targets and form synapses (Purro et al., 2008).Interestingly, the amount of APC bound to growth cones of extending DRG neurites correlates with increased neurite growth (Zhou et al., 2004), and APC loss correlates with growth cone pausing and enlargement (Purro et al., 2008).Also, levels of phospho-B-catenin, which is influenced by Wnt signaling, regulate microtubule reorganization in other cellular regions (Ligon et al., 2001; Huang et al., 2007) and may be involved in neurite stabilization and growth. Therefore, these studies indicate several links between proteins in the canonical Wnt signaling pathway and growth cone/cytoskeleton changes, although studies that examine the effect of directly manipulating B-catenin have not yet been reported.The non-canonical Wnt pathway is associated with microtubule stability through its regulation of calcium influx,which is a major early event after injury and is essential for axonal sealing and growth cone formation. For example, in cultured hamster cortical neurons, Wnt5a increased axon outgrowth by redistributing microtubules to one side of the growth cone through calcium signaling and CaM kinase II(CaMKII) activation (Li et al., 2014). Also, Daam, a component of the non-canonical Wnt pathway, binds to F-actin and microtubules. Daam is essential for linking the actin and microtubule cytoskeletons, and loss of Daam disrupted microtubule stabilization, growth cone formation and axon growth (Szikora et al., 2017). Furthermore, Dishevelled,which connects Wnt receptors to its downstream effectors in both the canonical and non-canonical pathways, also plays a role in microtubule stability. In neurons, Dvl regulated microtubule stability and growth cone size by inhibiting GSK-3B-mediated MAP-1B phosphorylation (Ciani et al., 2004). Dvl1 interaction with the actin-binding protein Esp8 is essential for Wnt3a-mediated axonal remodeling and growth cone enlargement in rodent DRG neurons (Stamatakou et al., 2015). Therefore, stabilizing the microtubules of growing axons by Wnt signaling is another potential mechanism of Wnt-dependent axon growth.
Future Studies
Studies in mammalian andfish optic nerve and spinal cord injury models indicate the effect of Wnt ligands on axonal growth depends on the cell types with activated Wnt signaling, the type of ligand, the timing of activation relative to the injury and the site of activation. Further studies are needed to determine whether the extent of axonal regeneration is influenced by the dose of Wnt activator and the type of Wnt ligand/receptor combination, as shown in the developing CNS. It will also be important to determine how extrinsic Wnt signaling within glia affects axonal regeneration of adjacent neurons, for example, Muller glia and RGC interactions within the retina during optic nerve regeneration. Glia may be involved immediately after injury to promote neuronal survival, or they could also promote regeneration several days to weeks afterwards. Surviving RGCs do not necessarily grow axons if axonal growth cues are lacking (Pernet and Schwab, 2014). Also, successful axonal regeneration requires the assembly of a growth cone and sustained elongation.Whether signaling from Wnt ligands leads to growth cone assembly and changes in direction and movement, and whether it uses GSK3B/APC to integrate the complex series of events involving local molecular signaling and events in the cell, remains to be discovered. Also, several Wnt pathway components regulate neurite growth independently of Wnt ligands, such as SFRP1, but how these signals integrate to Wnt pathways is unknown.
Finally, off-target effects of Wnt signaling are possible because Wnt/B-catenin induces angiogenesis and tumorigenesis under certain conditions. Fortunately, negative effects from Wnt3a injections were not observed in our previous studies (Patel et al., 2015, 2017) or reported by others.Off-target effects from other regenerative therapeutic molecules are also expected, including Stat3, KLF4 and mTOR,due to their essential roles in the CNS, but should not detract from using these molecules in experimental systems to understand the fundamental mechanisms of axonal regeneration. In summary, accumulating evidence suggests that Wnt has therapeutic potential for axonal regeneration after injury, and future studies will help define its mechanism of action to maximize its regeneration efficacy.
Author contributions:All authors contributed to writing and editing the manuscript. AU also made thefigures.
Conflicts of interest:None declared.
Financial support:This study is provided by the NEI grant R01EY026546. AU is a recipient of a Research to Prevent Blindness Medical Student Eye Research Fellowship. Financial support from Fight for Sight (summer student fellowship to AU) is gratefully acknowledged. Institutional support is from an NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Open peer review report:
Reviewer:Fabrizia Cesca, The Italian Institute of Technology, Italy.
Comments to authors:This review addresses the role of canonical and non-canonical Wnt signaling in axon growth and regeneration. This work is very nicely written and the topic is dealt with exhaustively, taking into consideration several recently published papers. It covers both in vitro and in vivo evidence in a number of experimental models including zebrafish and transgenic mouse lines, and also details the complex signaling network involved in the various experimental systems.
Bellett G, Carter JM, Keynton J, Goldspink D, James C, Moss DK, Mogensen MM (2009) Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil Cytoskeleton 66:893-908.
Benowitz LI, Popovich PG (2011) Inflammation and axon regeneration. Curr Opin Neurol 24:577-583.
Blackshaw S, Fraioli RE, Furukawa T, Cepko CL (2001) Comprehensive analysis of photoreceptor gene expression and the identification of candidate retinal disease genes. Cell 107:579-589.
Briona LK, Poulain FE, Mosimann C, Dorsky RI (2015) Wnt/ß-catenin signaling is required for radial glial neurogenesis following spinal cord injury. Dev Biol 403:15-21.
Cardozo MJ, Mysiak KS, Becker T, Becker CG (2017) Reduce, reuse,recycle - Developmental signals in spinal cord regeneration. Dev Biol 432:53-62.
Chen Q, Smith GM, Shine HD (2008) Immune activation is required for NT-3-induced axonal plasticity in chronic spinal cord injury.Exp Neurol 209:497-509.
Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC (2004) A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol 164:243-253.
Clevers H, Batlle E (2006) EphB/EphrinB receptors and Wnt signaling in colorectal cancer. Cancer Res 66:2-5.
Cui J, Shi M, Quan M, Xie K (2013) Regulation of EMT by KLF4 in gastrointestinal cancer. Curr Cancer Drug Targets 13:986-995.
Cui Q, Yip HK, Zhao RC, So KF, Harvey AR (2003) Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci 22:49-61.
David MD, Canti C, Herreros J (2010) Wnt-3a and Wnt-3 differently stimulate proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth by canonical signaling. J Neurosci Res 88:3011-3023.
Du Q, Geller DA (2010) Cross-regulation between wnt and NF-kappaB signaling pathways. For Immunopathol Dis Therap 1:155-181.
Duncan GJ, Assinck P, Hilton BJ (2014) Canonical Wnt signalling in PDGFRalpha-expressing cells is a critical regulator of astrogliosis and axon regeneration following CNS injury. J Neurosci 34:16163-16165.
Dvoriantchikova G, Pappas S, Luo X, Ribeiro M, Danek D, Pelaez D,Park KK, Ivanov D (2016) Virally delivered, constitutively active NFkappaB improves survival of injured retinal ganglion cells. Eur J Neurosci 44:2935-2943.
Evans PM, Chen X, Zhang W, Liu C (2010) KLF4 interacts with beta-catenin/TCF4 and blocks p300/CBP recruitment by beta-catenin.Mol Cell Biol 30:372-381.
Fang Y, Cho KS, Tchedre K, Lee SW, Guo C, Kinouchi H, Fried S, Sun X, Chen DF (2013) Ephrin-A3 suppresses Wnt signaling to control retinal stem cell potency. Stem Cells 31:349-359.
Fantetti KN, Zou Y, Fekete DM (2011) Wnts and Wnt inhibitors do not influence axon outgrowth from chicken statoacoustic ganglion neurons. Hear Res 278:86-95.
Fernandez-Martos CM, Gonzalez-Fernandez C, Gonzalez P, Maqueda A, Arenas E, Rodriguez FJ (2011) Differential expression of Wnts after spinal cord contusion injury in adult rats. PLoS One 6:e27000.
Fragoso MA, Patel AK, Nakamura RE, Yi H, Surapaneni K, Hackam AS (2012) The Wnt/beta-catenin pathway cross-talks with STAT3 signaling to regulate survival of retinal pigment epithelium cells.PLoS One 7:e46892.
Garcia M, Forster V, Hicks D, Vecino E (2002) Effects of muller glia on cell survival and neuritogenesis in adult porcine retina in vitro.Invest Ophthalmol Vis Sci 43:3735-3743.
Gomez-Orte E, Saenz-Narciso B, Moreno S, Cabello J (2013) Multiple functions of the noncanonical Wnt pathway. Trends Genet 29:545-553.
Gonzalez-Fernandez C, Fernandez-Martos CM, Shields SD, Arenas E,Javier Rodriguez F (2014) Wnts are expressed in the spinal cord of adult mice and are differentially induced after injury. J Neurotrauma 31:565-581.
Haenold R, Weih F, Herrmann KH, Schmidt KF, Krempler K, Engelmann C, Nave KA, Reichenbach JR, Lowel S, Witte OW, Kretz A(2014) NF-kappaB controls axonal regeneration and degeneration through cell-specific balance of RelA and p50 in the adult CNS. J Cell Sci 127:3052-3065.
Halleskog C, Mulder J, Dahlstrom J, Mackie K, Hortobagyi T, Tanila H,Kumar Puli L, Farber K, Harkany T, Schulte G (2011) WNT signaling in activated microglia is proinflammatory. Glia 59:119-131.
Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA,Takeda K, Akira S, Sofroniew MV (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231-7243.
Hida T, Yamashita N, Usui H, Nakamura F, Sasaki Y, Kikuchi A,Goshima Y (2012) GSK3beta/axin-1/beta-catenin complex is involved in semaphorin3A signaling. J Neurosci 32:11905-11918.
Hiester BG, Galati DF, Salinas PC, Jones KR (2013) Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation. Mol Cell Neurosci 56:115-127.
Hilla AM, Diekmann H, Fischer D (2017) Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J Neurosci 37:6113-6124.
Hollis ER 2nd, Zou Y (2012) Reinduced Wnt signaling limits regenerative potential of sensory axons in the spinal cord following conditioning lesion. Proc Natl Acad Sci U S A 109:14663-14668.
Huang P, Senga T, Hamaguchi M (2007) A novel role of phospho-beta-catenin in microtubule regrowth at centrosome. Oncogene 26:4357-4371.
Hur EM, Saijilafu, Lee BD, Kim SJ, Xu WL, Zhou FQ (2011) GSK3 controls axon growth via CLASP-mediated regulation of growth cone microtubules. Genes Dev 25:1968-1981.
Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q,Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955-968.
Ka M, Condorelli G, Woodgett JR, Kim WY (2014) mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development 141:4076-4086.
Lambert C, Cisternas P, Inestrosa NC (2016) Role of wnt signaling in central nervous system injury. Mol Neurobiol 53:2297-2311.
Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR (2006) AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther 13:1328-1341.
Leibinger M, Andreadaki A, Golla R, Levin E, Hilla AM, Diekmann H, Fischer D (2017) Boosting CNS axon regeneration by harnessing antagonistic effects of GSK3 activity. Proc Natl Acad Sci U S A 114:E5454-5463.
Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D(2009) Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci 29:14334-14341.
Li L, Fothergill T, Hutchins BI, Dent EW, Kalil K (2014) Wnt5a evokes cortical axon outgrowth and repulsive guidance by tau mediated reorganization of dynamic microtubules. Dev Neurobiol 74:797-817.
Ligon LA, Karki S, Tokito M, Holzbaur EL (2001) Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol 3:913-917.
Liu H, Mohamed O, Dufort D, Wallace VA (2003) Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn 227:323-334.
Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA (2006) Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol Vis Sci 47:5088-5097.
Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y (2008)Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci 28:8376-8382.
Lorber B, Chew DJ, Hauck SM, Chong RS, Fawcett JW, Martin KR(2015) Retinal glia promote dorsal root ganglion axon regeneration.PLoS One 10:e0115996.
Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC (1998) Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci 111:1351-1361.
Lucas FR, Salinas PC (1997) WNT-7a induces axonal remodeling and increases synapsinilevels in cerebellar neurons. Dev Biol 192:31-44.
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling:components, mechanisms, and diseases. Dev Cell 17:9-26.
Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ(1994) Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc Natl Acad Sci U S A 91:1632-1636.
Marchetti B, Pluchino S (2013) Wnt your brain be inflamed? Yes, it Wnt! Trends Mol Med 19:144-156.
Mehta ST, Luo X, Park KK, Bixby JL, Lemmon VP (2016) Hyperactivated Stat3 boosts axon regeneration in the CNS. Exp Neurol 280:115-120.
Meyers JR, Hu L, Moses A, Kaboli K, Papandrea A, Raymond PA(2012) B-catenin/Wnt signaling controls progenitor fate in the developing and regenerating zebrafish retina. Neural Dev 7:30.
Miyashita T, Koda M, Kitajo K, Yamazaki M, Takahashi K, Kikuchi A, Yamashita T (2009) Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma 26:955-964.
Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL (2009) KLF family members regulate intrinsic axon regeneration ability. Science 326:298-301.
Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D (2009) Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci 41:233-246.
Nalesso G, Sherwood J, Bertrand J, Pap T, Ramachandran M, De Bari C,Pitzalis C, Dell’accio F (2011) WNT-3A modulates articular chondrocyte phenotype by activating both canonical and noncanonical pathways. J Cell Biol 193:551-564.
Onishi K, Hollis E, Zou Y (2014) Axon guidance and injury-lessons from Wnts and Wnt signaling. Curr Opin Neurobiol 27:232-240.
Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M (2007)Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci 27:4210-4219.
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L,Kramvis I, Sahin M, He Z (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963-966.
Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133:38-52.
Patel AK, Hackam AS (2012) Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol 54:122-131.
Patel AK, Hackam AS (2014) A novel protective role for the innate immunity Toll-Like Receptor 3 (TLR3) in the retina via Stat3. Mol Cell Neurosci 63:38-48.
Patel AK, Park KK, Hackam AS (2017) Wnt signaling promotes axonal regeneration following optic nerve injury in the mouse. Neuroscience 343:372-383.
Patel AK, Surapaneni K, Yi H, Nakamura RE, Karli SZ, Syeda S, Lee T, Hackam AS (2015) Activation of Wnt/beta-catenin signaling in Muller glia protects photoreceptors in a mouse model of inherited retinal degeneration. Neuropharmacology 91:1-12.
Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, Schaffer DV,Flannery JG, Schwab ME (2013) Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis 51:202-213.
Pernet V, Schwab ME (2014) Lost in the jungle: new hurdles for optic nerve axon regeneration. Trends Neurosci 37:381-387.
Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC (2008) Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J Neurosci 28:8644-8654.
Qin S, Zou Y, Zhang CL (2013) Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat Commun 4:2633.
Rodriguez J, Esteve P, Weinl C, Ruiz JM, Fermin Y, Trousse F,Dwivedy A, Holt C, Bovolenta P (2005) SFRP1 regulates the growth of retinal ganglion cell axons through the Fz2 receptor. Nat Neurosci 8:1301-1309.
Rodriguez JP, Coulter M, Miotke J, Meyer RL, Takemaru K, Levine JM(2014) Abrogation of beta-Catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J Neurosci 34:10285-10297.
Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC (2005) Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci 8:34-42.
Salinas PC (2012) Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol 4: a008003
Sawai H, Clarke DB, Kittlerova P, Bray GM, Aguayo AJ (1996)Brain-derived neurotrophic factor and neurotrophin-4/5 stimulate growth of axonal branches from regenerating retinal ganglion cells. J Neurosci 16:3887-3894.
Schmitt AM, Shi J, Wolf AM, Lu CC, King LA, Zou Y (2006) Wnt-Ryk signalling mediates medial-lateral retinotectal topographic mapping.Nature 439:31-37.
Seifert JR, Mlodzik M (2007) Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 8:126-138.
Seitz R, Hackl S, Seibuchner T, Tamm ER, Ohlmann A (2010) Norrin mediates neuroprotective effects on retinal ganglion cells via activation of the Wnt/beta-catenin signaling pathway and the induction of neuroprotective growth factors in Muller cells. J Neurosci 30:5998-6010.
Sellak H, Wu S, Lincoln TM (2012) KLF4 and SOX9 transcription factors antagonize beta-catenin and inhibit TCF-activity in cancer cells.Biochim Biophys Acta 1823:1666-1675.
Selvaraj P, Huang JS, Chen A, Skalka N, Rosin-Arbesfeld R, Loh YP(2015) Neurotrophic factor-alpha1 modulates NGF-induced neurite outgrowth through interaction with Wnt-3a and Wnt-5a in PC12 cells and cortical neurons. Mol Cell Neurosci 68:222-233.
Shah SM, Patel CH, Feng AS, Kollmar R (2013) Lithium alters the morphology of neurites regenerating from cultured adult spiral ganglion neurons. Hear Res 304:137-144.
Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z (2009) SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 64:617-623.
Stamatakou E, Hoyos-Flight M, Salinas PC (2015) Wnt signalling promotes actin dynamics during axon remodelling through the actin-binding protein Eps8. PLoS One 10:e0134976.
Strand NS, Hoi KK, Phan TMT, Ray CA, Berndt JD, Moon RT (2016)Wnt/beta-catenin signaling promotes regeneration after adult zebrafish spinal cord injury. Biochem Biophys Res Commun 477:952-956.
Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C,Feng G, Yankner BA, He Z (2011) Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 480:372-375.
Szikora S, Foldi I, Toth K, Migh E, Vig A, Bugyi B, Maleth J, Hegyi P, Kaltenecker P, Sanchez-Soriano N, Mihaly J (2017) The formin DAAM is required for coordination of the actin and microtubule cytoskeleton in axonal growth cones. J Cell Sci 130:2506-2519.
Tassew NG, Charish J, Shabanzadeh AP, Luga V, Harada H, Farhani N, D’Onofrio P, Choi B, Ellabban A, Nickerson PEB, Wallace VA,Koeberle PD, Wrana JL, Monnier PP (2017) Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep 20:99-111.
Tripathi R, McTigue DM (2007) Prominent oligodendrocyte genesis along the border of spinal contusion lesions. Glia 55:698-711.
Triplett JW, Feldheim DA (2012) Eph and ephrin signaling in the formation of topographic maps. Semin Cell Dev Biol 23:7-15.
Udeh A, Dvoriantchikova D, Carmy T, Ivanov D, Hackam AS (submitted) Neurite growth in retinal ganglion cells is induced by Wnt/B-catenin signaling and is modulated by Ripk1 signaling. Sci Rep.
Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG (2017) Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat Commun 8:126.
Yam PT, Charron F (2013) Signaling mechanisms of non-conventional axon guidance cues: the Shh, BMP and Wnt morphogens. Curr Opin Neurobiol 23:965-973.
Yang Z, Wu Y, Zheng L, Zhang C, Yang J, Shi M, Feng D, Wu Z, Wang YZ (2013) Conditioned medium of Wnt/beta-catenin signaling-activated olfactory ensheathing cells promotes synaptogenesis and neurite growth in vitro. Cell Mol Neurobiol 33:983-990.
Yi H, Hu J, Qian J, Hackam AS (2012) Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport 23:189-194.
Yi H, Nakamura RE, Mohamed O, Dufort D, Hackam AS (2007) Characterization of Wnt signaling during photoreceptor degeneration.Invest Ophthalmol Vis Sci 48:5733-5741.
Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, Zack DJ, Benowitz LI (2009)Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A 106:19587-19592.
Yin ZS, Zu B, Chang J, Zhang H (2008) Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol Res 30:480-486.
Zhou FQ, Zhou J, Dedhar S, Wu YH, Snider WD (2004) NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron 42:897-912.
- 中国神经再生研究(英文版)的其它文章
- Neural Regeneration Research: Information for Authors
- Conundrums and confusions regarding how polyethylene glycol-fusion produces excellent behavioral recovery after peripheral nerve injuries
- LETTER FROM THE EDITORS-IN-CHIEF
- Brain injury and neural stem cells
- Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease
- Tackling dipeptidyl peptidase IV in neurological disorders