Conundrums and confusions regarding how polyethylene glycol-fusion produces excellent behavioral recovery after peripheral nerve injuries
George D. Bittner, Dale R. Sengelaub, Cameron L. Ghergherehchi
1 Department of Neuroscience, University of Texas at Austin, Austin, TX, USA
2 Department of Psychological and Brain Sciences, Indiana University, Bloomington, IN, USA
3 Department of Molecular Biosciences, University of Texas at Austin, Austin, TX, USA
Current Expectations for Complete Transection or Ablated Segment of a Peripheral Nerve
Peripheral nerve injury (PNI) is the most common nerve trauma, the consequences of which significantly burden Health Care Systems in civilian and military populations.PNIs that occur clinically as either complete transections or ablations of a segment of a major nerve often exhibit very poor, if any, behavioral recovery with contemporary clinical practice. Immediately after these PNIs, motor and sensory function distal to the injury is completely lost due to interrupted axonal continuity distal to the lesion. Thereafter, the distal portions of axons always and irreversibly undergo Wallerian degeneration within 1–3 days. Protracted functional recovery can occur onlyviaslowly (1–2 mm/day) regenerating outgrowths from surviving proximal axons that often very inaccurately (non-specifically) reinnervate target tissues that may atrophy before reinnervation occurs, especially after ablation of a segment of a major nerve (Figure 1 and Table 1)(Brushart, 2011; Green and Wolfe, 2011; Kandel et al., 2013;Riley et al., 2015; Bittner et al., 2016)
The standard of care for a transection PNI is to reappose and microsuture the cut ends (Brushart, 2011; Green and Wolfe, 2011; Kandel et al., 2013). An ablation PNI is currently repaired by microsuturing surgically inserted 1) autografts harvested from other body regions; 2) non-viable(non-immunogenic) conduits; or 3) decellularized allograftnerve segments, each of which acts as a bridge to distal nerve tissue. These current techniques often do not prevent Wallerian degeneration and do not improve the speed nor quality of behavioral recovery, if any. Limb amputation is often an acceptable or better alternative after loss of a nerve segment(Brushart, 2011; Green and Wolfe, 2011; Kandel et al., 2013;Riley et al., 2015). Viable donor allografts as a possible alternative are rapidly rejected even with immunosuppression and major histocompatibility complex (MHC) matching,primarily because of T cell adaptive responses and secondarily because of innate antigen-independent pro-inflammatory events (Murphy and Weaver, 2016).
In contrast, we have recently published data showing that repair of transected nerves or ablated segments of nerve trunks by PEG-fusion produces dramatically better morphological, electrophysiological and (most relevant) behavioral recoveries than any other currently-available procedure(Bittner et al., 2012, 2016, 2017; Ghergherehchi et al., 2016).
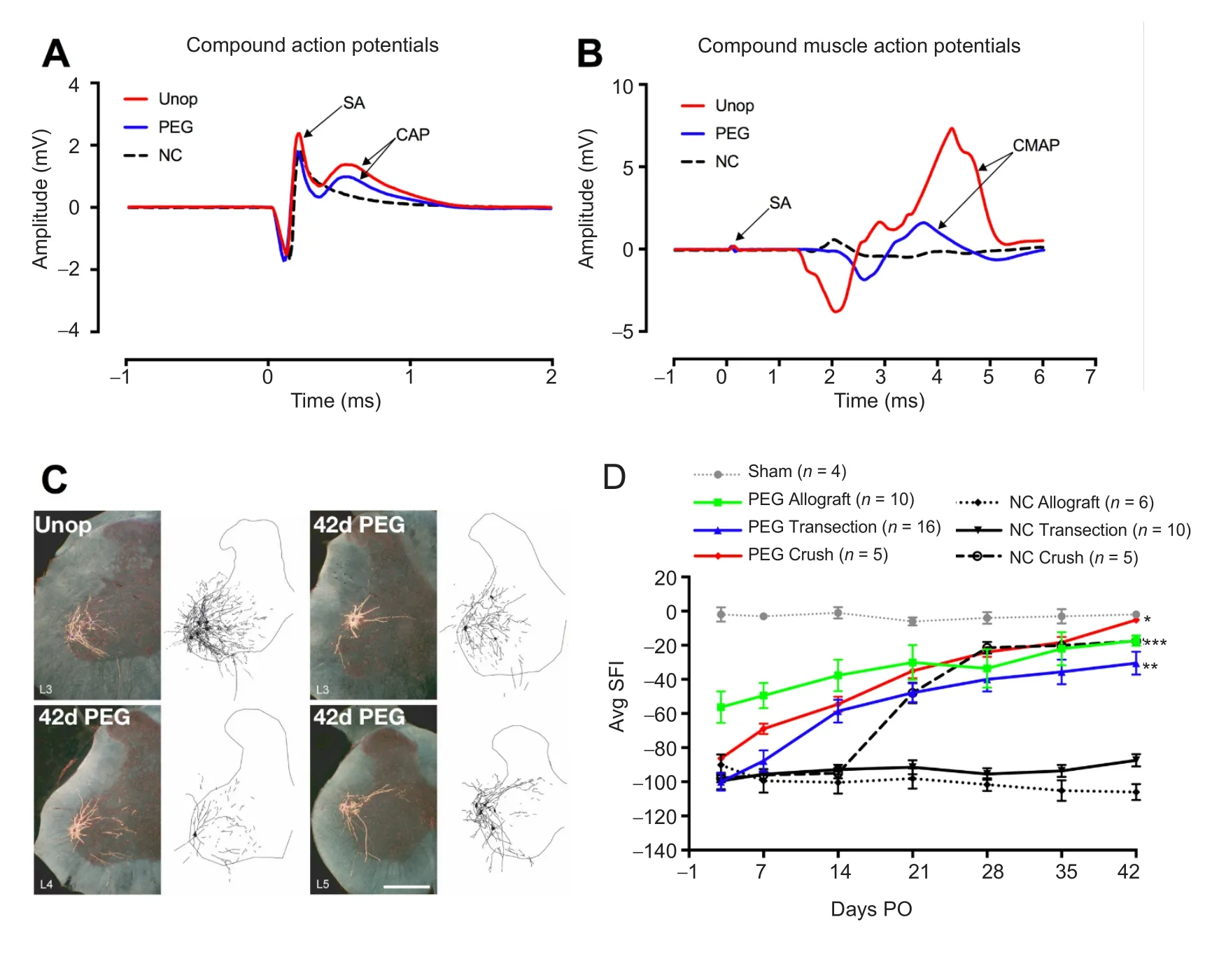
Figure 1 Electrophysiological, morphologicical and behavioral results of PEG-fusion.
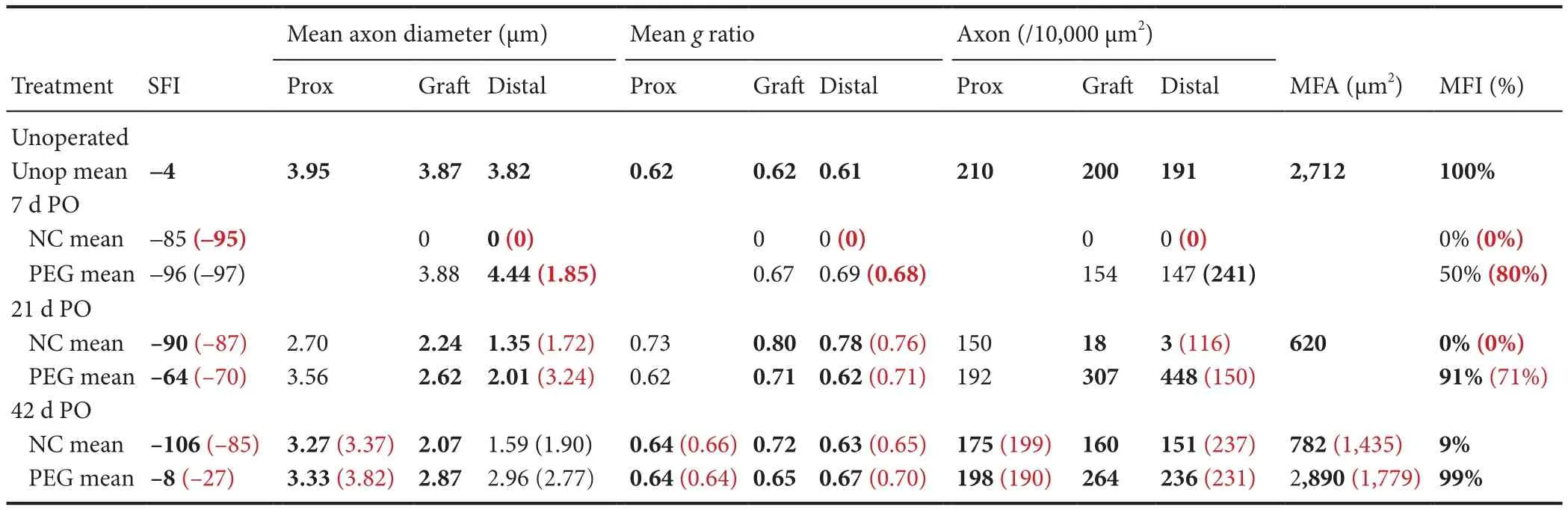
Table 1 Summary of means for single cut and allograft axonal morphometric data

Figure 2 PEG produces fusion of proximal and distal axons if their open, vesicle-free, cut ends are brought into close apposition by microsutures (“PEG-fusion”).
PEG-Fusion Results for Complete Transection or Ablated Segment of a Peripheral Nerve
PEG-fusion protocols consist of a well-specified sequence of solutions directly applied to well-trimmed, open axonal cut ends that are closely apposed by microsutures through the connective-tissue epineurium to provide mechanical strength to PEG-fused axons (see Figure 4 from Bittner et al., 2016). In brief, 50% w/w 2–5 kDa PEG/distilled water removes plasmalemmal-bound water to induce lipids in the apposed axolemmas of open axonal ends to produce axolemmal continuity(fuse) across the lesion site. Intact and repaired axolemmas have very low tensile strength/resistance to stretching.
Our laboratories have much published and other data documented in part in Figure 1 and Table 1 showing that after PEG-fusion axolemmal and axoplasmic continuity is rapidly (within minutes) restored as assessed by conduction of extracellularly-recorded compound action potentials(CAPs) (Figure 1A) and intracellular dye diffusion in both directions across the lesion site(s). Compound muscle action potentials (CMAPs) also confirm such continuity (Figure 1B). Proximal and distal ends of motor or sensory axons are non-specifically connected (Figure 1C) and motor axons can be fused to sensory axons. Nevertheless, the following morphological, cell biological, and functional events are consistently obtained (Bittner et al., 2012, 2016, 2017; Riley et al., 2015; Ghergherehchi et al., 2016):
1) Fast axonal transport between cell bodies and distal motor nerve junctions or sensory nerve endings is restored days (single cuts) or weeks (ablations) after PEG-fusing single cuts and/or allografts, thereby supplying host proteins to all regions of the axon, as demonstrated by retrograde transport of tracers (Figure 1C). 2) Survival of distal segments of many transected axons or donor graft axons is continuously maintained from 0–150 days PO for single cuts and allografts,i.e.,reducing or preventing axonal Wallerian degeneration, as assessed by photon microscopy and transmission electron microscope (TEM) of cross and longitudinal sections (Table 1).3) Neuromuscular junctions (NMJs) are continuously maintained, as measured by confocal immunohistochemistry, TEM and counts of innervated musclefibers (Table 1). 4) Muscle fibers are continuously maintained and often undergo very little atrophy, as assessed by TEM (Table 1). 5) Function/behavioral recovery is restored within days to weeks and approaches or equals that of unoperated animals as measured by the Sciatic Functional Index (SFI), especially for PEG-fused allografts.The SFI is a commonly used behavioral measure primarily determined byfine control of distal muscle masses responsible for toe spread and foot placement (Figure 1D and Table 1). Note that we always analyze the structure and function of nerves, muscles, myelin, and NMJs. However,we always defi ne successful PEG-fusion repair by behavioral measures, not axon counts or any other morphological or electrophysiological measure, as Brushart (2011) has emphasized.
Remarkably, PEG-fused allografts are not rejected as assessed by photon microscopy, TEM, histological or immuno-histochemical methods: This is important because unlike brain or spinal nerves, mammalian peripheral nerves are in a non-immunoprivileged environment as are hearts, livers and kidneys (Murphy and Weaver, 2016).
Conundrums and Confusions Regarding PEG- Fusion
Some results of PEG-fusion present unexpected conundrums and/or are contrary to neuroscience (Kandel et al.,2013) or immunology (Murphy and Weaver, 2016) textbook dogma and hence lead to confusion (e.g., Robinson and Madison, 2016). As one example, some do not recognize or understand that when used in different circumstances or formulations, PEG can produce either membrane fusion(Bittner et al., 2016, 2017), membrane sealing (Spaeth et al.,2012) or cell protection (Kwon et al., 2009). In particular, our 2–5 kDa PEG dissolved in distilled water in a 50% w/w solution causes the closely apposed membranes of open cut axon ends to flow into each other (fuse) to join (repair) the cut,aka “PEG-fusion” (Figure 2; also see Figure 4 in Bittner et al.,2016). However, if the cut ends are not closely apposed, the PEG solution causes the axolemma at the open axonal ends to collapse, fuse, and seal-off (“PEG-sealing”) (Figure 2; Spaeth et al., 2012). These collapsed and sealed cut ends are very difficult to PEG-fuse unless recut and opened (Ghergherehchi et al., 2016). Alternatively, PEG used in synthetic hydrogels or in high (e.g., 15 kDa) molecular weight polymers may have some neuroprotective effects by unknown mechanisms (Kwon et al., 2009). Lower kDa PEG polymers may have some neuroprotective effects due to PEG-sealing, but if so, not by PEG-fusion. PEG applied to crushed segments of peripheral nerves produces a slight increase in recovery compared to recovery if nothing is done because the PEG causes PEG-sealing(rather than PEG-fusion). To produce good recovery using PEG, the crushed segments of such nerves almost certainly need be ablated and an allograft inserted and PEG-fused.
As a second example, PEG-fusion in each animal has its own unique characteristics due to nerve anatomy, exact lesion site, length of ablation, skill of the surgeon on that day,etc., as does surgery on a sciatic nerve in each human patient. That is,results fall within a range of recoveries, rather than exactly the same recovery SFI at each tested day for each animal (see Figure 5 in Ghergherehchi et al., 2016; See Figure 3 in Riley et al.,2015). The excellent behavioral recoveries seen after PEG-fusion compared to the poor or no recovery seen in Negative Controls are for transected or ablated PNI’s in a proximal major nerve (mid-thigh sciatic lesions in rats). Short length (1–3 mm) crush lesions made by microforceps in mice and rats often recover very well in weeks (Figure 1D; Brushart, 2011).Such short-length crush lesions almost never occur in large mammals like humans (Green and Wolfe, 2011).
Third, PEG-fused allografts are not in a privileged environment but are not rejected despite not being tissue matched nor immune suppressed, even if the donor is not of the same stain (or species; unpublished data) as the host. PNI allografts are indeed very allogenic, as documented by their rapid rejection in Negative Control protocols in which all solutions are used except PEG or in which PEG is used but microsutures do not closely appose cut ends. Although the mechanism is not yet known,one working hypothesis is that rapid restoration of axonal transport through a fusion site may allow the host to introduce its MHC proteins into donor axons in the grafted segment,thus disguising the graft as “self”, thereby escaping immune surveillance and targeting. Regardless of mechanism, the lack of rejection of PEG-fused allografts is very different from allografttransplant repair of other tissues (Murphy and Weaver, 2016).That is, the 50% PEG solution used in a PEG-fusion protocol may have some neuroprotective effects but PEG-fusion is a very different functional use of PEG compared to its use in PEG-hydrogels as a neuroprotective agent (Kwon et al., 2009)
Fourth, PEG-fusion works by non-specifically joining open cut axonal ends (Riley et al., 2015; Bittner et al.,2016, 2017; Ghergherehchi et al., 2016). For allografts, this non-specificity includes different numbers of axons in donor and host nerve segments (Riley et al., 2015; Bittner et al., 2016). Such PEG-fused axons immediately restore action potential conduction across the lesion sites, as demonstrated by CAPs, CMAPs and muscle twitches (Figure 1A, B). Good recovery of behaviors usually occurs after several weeks(Figure 1D), but before any axons regenerating by outgrowth have reached muscle masses that remain innervated.These muscle masses do not atrophy because PEG-fused axons do not undergo Wallerian degeneration (Table 1).Outcomes from PEG-fusion repair of allografts are superior to PEG-fusion repair of single transections, almost certainly because the allograft is sized to be longer than the ablated segment after all cut ends are carefully trimmed, thereby eliminating any deleterious strain/tension of any host or donor axons (Riley et al., 2015). The behavioral recovery after PEG-fusion of a single transection or an ablation/allograft insertion presumably occurs by activating peripheral and CNS plasticities to a much greater extent than most neuroscientists currently believe to be possible (Riley et al., 2015;Bittner et al., 2016, 2017). As part of this conundrum/confusion, we note that PEG-fusion does not prevent regeneration by outgrowth, presumably from axons that were not successfully PEG-fused (Table 1; Bittner et al., 2016). However,such outgrowth adds little to the recovery already obtained by surviving PEG-fused axons in allografts after segment ablation of a major peripheral nerve (Figure 1D).
As one specific example of errors in interpretation of the PEG-fusion mechanism and/or results, a recent publications(Robinson and Madison, 2016) attempted “to assess motor neuron regeneration accuracy” after PEG-fusion. The authors concluded that PEG-fusion led to inaccuracy in regeneration by outgrowth and hence would probably fail as a clinical technique. However, these researchers did not perform a positive control (CAP conduction across the site of PEG-fusion)to demonstrate that they produced PEG-fusion at the time of their original surgery or a positive control behavioral test,such as the SFI, to demonstrate that they maintained successful PEG-fusion at any time postoperatively, especially when they attempted to assess the innervation accuracy of axons.
More importantly, even if one assumes that Robinson and Madison (2016) did produce successful PEG-fusion in the absence of evidence, the experimental protocol employed to assess the specificity of axons regenerating by outgrowth cannot distinguish between axons regenerating by outgrowth versus those that were PEG-fused immediately after injury to produce immediate reinnervation and have been maintained. Logically, one cannot label a nerve that contains both newly regenerated axons as well as maintained PEG-fused axons and then attribute all the results to axons regenerating by outgrowth, as the authors concluded (Bittner et al., 2017). They also failed to consider a set of relevant papers(Riley et al., 2015; Bittner et al., 2016) describing PEG-fusion recovery after allograft repair of ablated segments, at which time no regenerating axons have reached the denervated muscles, but instead reinnervation is solely due to by surviving PEG-fused axons. To date there is no study that has published data that validly addresses reinnervation by PEG-fusion versus regeneration by outgrowth from surviving proximal stumps.
PEG-fusion technologies could produce a paradigm shiftin current Neuroscience dogma that asserts that: 1) distal stumps of severed axons undergo obligate degeneration within days; 2) reinnervation can only occur by slowly growing regenerating processes from severed proximal stumps that need appropriately reinnervate denervated target tissues(that have often atrophied); and 3) allo-transplanted neuronal tissue is always rapidly rejected in unprotected immune environments.
In contrast, PEG-fusion results show that: 1) distal stumps of severed axons survive indefinitely, 2) reinnervation can occur within seconds to minutes by connecting axons in proximal and distal stumps to appropriately or inappropriately reinnervate denervated target tissues; 3) target tissues to not undergo atrophy; 4) remarkable behavioral restoration is obtained due to inappropriate connections that must be subject to substantial peripheral and/or CNS plasticities that support functional recovery; 5) PEG-fused transplanted allogenic neuronal tissue is most unexpectedly not rejected despite no tissue matching or immune suppression; and 6) PEG fusion works very similarly in humans in early case studies, as it does in rats.
All these results suggest that PEG-fusion technologies have the potential to produce a paradigm shift in clinical treatment of PNS nerve injuries.
Author contributions:All authors contributed to the writing of this review article.
Conflicts of interest:None declared.
Financial support: None.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Bittner GD, Sengelaub DR, Trevino RC, Ghergherehchi CL, Mikesh M(2017) Robinson and madison have published no data on whether polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J Neurosci Res 95:863-866.
Bittner GD, Sengelaub DR, Trevino RC, Peduzzi JD, Mikesh M, Ghergherehchi CL, Schallert T, Thayer WP (2016) The curious ability of polyethylene glycol fusion technologies to restore lost behaviors after nerve severance. J Neurosci Res 94:207-230.
Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, Zuzek A, Wilcott RW, Thayer WP, Winograd JM, Gonzalez-Lima F, Schallert T (2012) Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue, and polyethylene glycol after completely cutting rat sciatic nerves. J Neurosci Res 90:967-980.
Brushart TM (2011) Nerve Repair. New York: Oxford University Press.
Ghergherehchi CL, Bittner GD, Hastings RL, Mikesh M, Riley DC,Trevino RC, Schallert T, Thayer WP, Sunkesula SR, Ha TA, Munoz N, Pyarali M, Bansal A, Poon AD, Mazal AT, Smith TA, Wong NS,Dunne PJ (2016) Effects of extracellular calcium and surgical techniques on restoration of axonal continuity by polyethylene glycol fusion following complete cut or crush severance of rat sciatic nerves.J Neurosci Res 94:231-245.
Green DP, Wolfe SW (2011) Green’s Operative Hand Surgery. 6thed.Philadelphia: Elsevier/Churchill Livingstone.
Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ(2013) Principles of Neural Science. 5thed. New York: McGraw-Hill.
Kwon BK, Roy J, Lee JH, Okon E, Zhang H, Marx JC, Kindy MS (2009)Magnesium chloride in a polyethylene glycol formulation as a neuroprotective therapy for acute spinal cord injury: preclinical refinement and optimization. J Neurotrauma 26:1379-1393.
Murphy K, Weaver C (2016) Janeway’s Immunobiology. New York,NY: Garland Science/Taylor & Francis Group, LLC.
Riley DC, Bittner GD, Mikesh M, Cardwell NL, Pollins AC, Ghergherehchi CL, Bhupanapadu Sunkesula SR, Ha TN, Hall BT, Poon AD,Pyarali M, Boyer RB, Mazal AT, Munoz N, Trevino RC, Schallert T,Thayer WP (2015) Polyethylene glycol-fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J Neurosci Res 93:572-583.
Robinson GA, Madison RD (2016) Polyethylene glycol fusion repair prevents reinnervation accuracy in rat peripheral nerve. J Neurosci Res 94:636-644.
Spaeth CS, Robison T, Fan JD, Bittner GD (2012) Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J Neurosci Res 90:955-966.
- 中国神经再生研究(英文版)的其它文章
- Neural Regeneration Research: Information for Authors
- A growingfield: the regulation of axonal regeneration by Wnt signaling
- LETTER FROM THE EDITORS-IN-CHIEF
- Brain injury and neural stem cells
- Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease
- Tackling dipeptidyl peptidase IV in neurological disorders