Optical read-out and modulation of peripheral nerve activity
Arjun K. Fontaine, Hans E. Anderson John H. Caldwell, Richard F. Weir
1 Department of Bioengineering, University of Colorado — Anschutz Medical Campus, Aurora, CO, USA
2 Department of Cell and Developmental Biology, University of Colorado — Anschutz Medical Campus, Aurora, CO, USA
Introduction
A suitable neural interface is sought for numerous applications, including prosthesis/rehabilitation, medical treatment, and better understanding of physiological systems. Advanced prosthetic hands for limb replacement have the ability to mechanically substitute the degrees of motion of the biological hand; yet there exists no nervous system interface that allows for their full control/articulation and sensing. In the viscera,there is broad effort to understand and control neural pathways to address disorders affecting these organs.The range of disorders that could potentially be studied and treated by interfacing with visceral nervefibers is extensive. Vagus nerve stimulation (VNS) is known to elicit numerous clinically relevant modulatory effects;reduction of seizure frequency in intractable epilepsy(Gurbani et al., 2016) and attenuation of inflammation in rheumatoid arthritis (Koopman et al., 2016) are just two examples that have advanced to human treatment.The precise pathways that underlie function are poorly understood in many cases, however, and current therapeutic nerve stimulation is non-specific. These applications require the ability to decipher and modulate activity with a high degree of specificity (i.e., at the fascicle or individual axon level) in order to read and affect highly targeted activity. Furthermore, the neural mapping of structure and function in many systems needs to be established in order to implement precise therapeutic and restorative therapies.
Electrode-based nerve interfaces are quite limited in this respect; a primary limitation is their poor neural specificity. Cuff electrodes, such as the Flat Interface Nerve Electrode (FINE) Array (Schiefer et al., 2013;Tan et al., 2015), have demonstrated useful fascicle-specific stimulation, yet read-out and read-in with individual axons across the nerve is infeasible. Other electrode designs, such as the Utah Slant Array (Normann et al., 2005), impale the nerve. This often leads to tissue necrosis and scar tissue, resulting in poor longevity of the interface, while still incapable of single axon resolution. Given the needs of these applications and the limitations of current electrical interfaces, a novel approach is desired that can read-out (sense) and read-in (stimulate or silence) neural activity to specific axons within a nerve, for both the mapping of clinically relevant circuits and their functional manipulation.
Optics-Based Nerve Interfacing
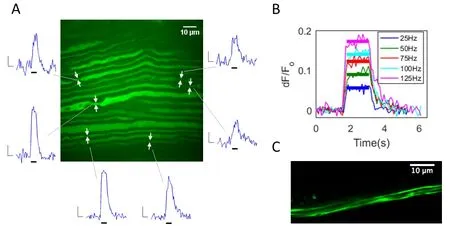
Figure 1 Imaging of neural activity with calcium sensitive fluorescent sensors.
An optical (optogenetic) approach is a promising methodology to meet the goals of a functional nerve interface. Single axon communication is feasible since sub-micron resolution is easily achievable with optical systems. Optogenetic reporters can facilitate activity read-out by sensing either membrane voltage or intracellular calcium, while optogenetic actuators such as ChannelRhodopsin2 (ChR2 or its variants)and HaloRhodopsin can stimulate or silence activity respectively in a light-driven fashion. Current voltage-sensitive fluorescent proteins are not suitable for in vivo application due to insufficient signal strength.However, calcium sensors such as the genetically encoded calcium indicator GCaMP6 have been shown to provide substantial signal in response to action potentials in vivo (Chen et al., 2013; Badura et al., 2014). Activity-dependent calcium-fluorescence transients have recently been demonstrated in peripheral nerve axons ex vivo, where signal dynamics were characterized with respect to action potential parameters using a synthetic calcium indicator molecule (Fontaine et al., 2017). In the peripheral nerve, myelinated axons produce a calcium signal at the node of Ranvier in response to activity(Figure 1A), while smaller diameterfibers exhibit relatively non-localized calcium transients along segments of axon. We have measured calcium signals with both synthetic (Calcium Green-1 dextran) and genetically encoded (GCaMP6f, GCaMP6s) sensors in individual axons at the single action potential level in the intact ex vivo peripheral nerve. The amplitude and duration of elicited calcium signals are well correlated to the underlying neural activity: signal amplitude is graded in proportion to the frequency and number of action potentials in a burst/train (Figure 1B), and signals persist for the duration of an action potential train (Fontaine et al.,2017). Both nodal signals from larger myelinated axon nodes, and non-localized signals in small-diameter axons have also been recorded in the vagus nerve using the genetically encoded calcium indicators GCaMP6f and GCaMP6s (our unpublished data).
This work has demonstrated the potential of using activity-dependent calcium transients as a read-out of neural activity in individual axons. Optical read-in has been demonstrated in the in vivo rodent peripheral nerve in prior studies which incorporated blue light activation of genetically targeted axonal ChR2 for activation of motor units (Llewellyn et al., 2010; Towne et al., 2013).
Requisite Methods and Challenges
Fiber-coupled optical device
Imaging of activity within the in vivo nerve will require miniaturizedfiber-coupled microscopes (FCMs)capable of delivering/detecting light between a laser and neural targets. The incorporation of a high-density optical fiber bundle enables lateral resolution for imaging at the distal end of the optical fiber, and an electro-wetting lens (Terrab et al., 2015) can facilitate rapid axial scanning with no moving parts, to achieve three-dimensional imaging. Such devices are in development, including a system by Ozbay et al. (2015),which has demonstrated three-dimensional two-photon imaging in the in vivo mouse brain (manuscript under review). Imaging activity across numerous neuronal processes in this manner is not trivial due to the challenge of exciting and detecting optical signals with meaningful brightness and resolution through the device. To be functionally useful, the system needs to collect enough signal, while scanning over a sufficient volume of tissue/axons, at an appropriate speed. It is likely that sensors such as GCaMP will continue to be improved in the future with enhancements in sensitivity and dynamic range, rendering optical signals of activity even more robust. The continued development of red-shifted sensors and actuators (Klapoetke et al.,2014; Dana et al., 2016) may also enable expanded multi-wavelength systems.
The optical read-in to single axons is also technically challenging, but read-in to a population or subset of axons is relatively less challenging. A single optical fiber can be used to deliver light for broad illumination of the nerve and achieve specificity determined by the genetic targeting of the actuator, and by targeting spectrally separate opsins to different axonal populations. (The cross-sectional portion of nerve that can be reached with sufficient power is dependent on the size of the nerve and the optical penetration). In both cases,a nerve ‘cuff’ could position the distal end of the FCM or opticalfiber to abut the neural tissue.
Adeno-associated viral vectors
The genetically encoded protein that serves as the sensor or effector must be delivered to the cell type of interest. Adeno-associated viruses (AAVs) have become a widely used vector for gene delivery, with numerous AAV based gene therapies currently in clinical trials,and one approved by the European Medicines Agency(Naso et al., 2017). AAV particles, lacking viral DNA and loaded with genes of interest, can provide a safe and effective method for gene delivery, with relatively limited immunogenic and mutagenic concerns. AAVs are poorly immunogenic compared with other viruses,yet potential responses to its viral components and the delivered transgene/protein are challenges that need to be addressed. AAVs can be tailored for specific applications through serotype and promoter selection, as well as consideration of serotype specific transduction/transport properties. Many serotypes undergo axonal and trans-synaptic transport, thus enabling a myriad of potential transduction strategies. AAVs have been employed in numerous studies to transduce neurons and neuronal processes of the central and peripheral nervous systems with optogenetic proteins (Andrasfalvy et al., 2010; Kravitz et al., 2010; Towne et al., 2013;Christensen et al., 2016; Williams et al., 2016). Using intramuscular injection (anterior tibialis muscle) and retrograde transport, our group delivered GCaMP6f to axons of the common peroneal nerve (Figure 1C)and detected robust action potential-elicited calcium transients in these axons (Anderson et al., 2017). Other studies have demonstrated the functional delivery of ChR2 to peripheral axons (Towne et al., 2013; Williams et al., 2016). Although transgene delivery would impose a significant regulatory hurdle for clinical use, the AAV-mediated targeting of optically-sensitive reporters and actuators to functionally pertinent axons may be clinically achievable.
Discussion
An optical approach to nerve interfacing has the potential to open up new avenues of study and therapy.Established studies demonstrate the feasibility to both read activity in axons of the PNS with calcium-sensitive reporters and actuate them with light activated opsins. Bioelectronic medicines, which strive to modulate therapeutically relevant processes by intervening with their neural pathways (Birmingham et al., 2014), may benefit greatly from optogenetic techniques. The methods described in this paper for axon-specific communication could be used to both identify axon subtypes that are pertinent to given functions, and subsequently monitor and/or modulate them. A closed loop system could activate or silence genetically identifiable axons of an organ in response to a real-time reading of molecular markers in the blood (glucose, for example) or neural activity itself. Given the frequency-modulated nature of activity-dependent calcium signals, neural frequency or ‘strength’ in a given pathway can be inferred. Once such a fluorescence signal is detected from an axon, it can be used in a variety of ways, including as a command signal to a prosthetic actuator.Motor axon interrogation in the limb may be useful for the control of artificial hands/limbs (Fontaine et al.,2018), provided that the interface can monitor a suffi-cient number of units at an appropriate speed. Sensory feedback may also be possible with the induction of activity in proprioceptive or mechanoreceptive afferents.With the current and developing palette of reporters and actuators, and the capability of specific genetic targeting, there are numerous ways in which an optogenetic system could be employed in the peripheral nervous system for the study of neural pathways, as well as interventional therapies such as physiological system modulation and device control.
Author contributions:AKF wrote the manuscript, and performed all experiments with the exception of the AAV-mediated GCaMP transduction experiments. HEA performed the virally transduced GCaMP work. RFW and JHC have directed this research, with JHC overseeing laboratory experimentation.
Conflicts of interest:None declared.
Financial support:None.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under identical terms.
Anderson HE, Fontaine AK, Caldwell JH, Weir RF (2017) Imaging of electrical activity in small diameterfibers of the murine peripheral nerve with GCaMP6f. Sci Rep (accepted for publication).
Andrasfalvy BK, Zemelman BV, Tang J, Vaziri A (2010) Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc Natl Acad Sci U S A 107:11981-11986.
Badura A, Sun XR, Giovannucci A, Lynch LA, Wang SS (2014) Fast calcium sensor proteins for monitoring neural activity. Neurophotonics 1:025008.
Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, Mc-Laughlin B, Pasricha P, Weber D, Ludwig K, Famm K (2014) Bioelectronic medicines: a research roadmap. Nat Rev Drug Discov 13:399-400.
Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL,Svoboda K, Kim DS (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499:295-300.
Christensen AJ, Iyer SM, Francois A, Vyas S, Ramakrishnan C, Vesuna S, Deisseroth K, Scherrer G, Delp SL (2016) In vivo interrogation of spinal mechanosensory circuits. Cell Rep 17:1699-1710.
Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K,Kim DS (2016) Sensitive red protein calcium indicators for imaging neural activity. Elife 5:e12727.
Fontaine AK, Gibson EA, Caldwell JH, Weir RF (2017) Optical readout of neural activity in mammalian peripheral axons: calcium signaling at nodes of ranvier. Sci Rep 7:4744.
Fontaine AK, Segil JL, Caldwell JH, Weir RF (2018) Real-time prosthetic digit actuation by optical read-out of activity-dependent calcium signals in the peripheral nerve. In: 2018 IEEE International Conference on Robotics and Automation (ICRA). Brisbane,Australia: IEEE Xplore (paper under review).
Gurbani S, Chayasirisobhon S, Cahan L, Choi S, Enos B, Hwang J,Lin M, Schweitzer J (2016) Neuromodulation therapy with vagus nerve stimulation for intractable epilepsy: a 2-year efficacy analysis study in patients under 12 years of age. Epilepsy Res Treat 2016:9709056.
Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V,Constantine-Paton M, Wong GK, et al. (2014) Independent optical excitation of distinct neural populations. Nat Methods 11:338-346.
Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ,Tak PP (2016) Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A 113:8284-8289.
Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K,Kreitzer AC (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466:622-626.
Llewellyn ME, Thompson KR, Deisseroth K, Delp SL (2010) Orderly recruitment of motor units under optical control in vivo. Nat Med 16:1161-1165.
Naso MF, Tomkowicz B, Perry WL 3rd, Strohl WR (2017) Adeno-associated virus (AAV) as a vector for gene therapy. Biodrugs 31:317-334.
Normann RA, McDonnall D, Clark GA, Stein RB, Branner A (2005)Physiological activation of the hind limb muscles of the anesthetized cat using the utah slanted electrode array. Proc Int Jt Conf Neural Networks 5:3103-3108.
Ozbay BN, Losacco JT, Cormack R, Weir R, Bright VM, Gopinath JT, Restrepo D, Gibson EA (2015) Miniaturized fiber-coupled confocal fluorescence microscope with an electrowetting variable focus lens using no moving parts. Opt Lett 40:2553-2556.
Schiefer MA, Freeberg M, Pinault GJ, Anderson J, Hoyen H, Tyler DJ, Triolo RJ (2013) Selective activation of the human tibial and common peroneal nerves with a flat interface nerve electrode. J Neural Eng 10:056006.
Tan DW, Schiefer MA, Keith MW, Anderson JR, Tyler DJ (2015)Stability and selectivity of a chronic, multi-contact cuff electrode for sensory stimulation in human amputees. J Neural Eng 12:026002.
Terrab S, Watson AM, Roath C, Gopinath JT, Bright VM (2015)Adaptive electrowetting lens-prism element. Opt Express 23:25838-25845.
Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL (2013)Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One 8:e72691.
Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, Liberles SD (2016) Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166:209-221.
- 中国神经再生研究(英文版)的其它文章
- Neural Regeneration Research: Information for Authors
- A growingfield: the regulation of axonal regeneration by Wnt signaling
- LETTER FROM THE EDITORS-IN-CHIEF
- Brain injury and neural stem cells
- Modulation of mitochondrial bioenergetics as a therapeutic strategy in Alzheimer’s disease
- Tackling dipeptidyl peptidase IV in neurological disorders