A journey to understand wheat Fusarium head blight resistance in the Chinese wheat landrace Wangshuibai
*
The Applied Plant Genomics Laboratory,Nanjing Agricultural University,Nanjing 210095,Jiangsu,China
1.Introduction
Fusarium head blight(FHB),caused by Fusarium graminearum,is arguably the most destructive disease in wheat.It greatly reduces yield and kernel quality in epidemic years and is almost not curable due to lack of immune germplasm[1–2].More than that,the fungal mycotoxin contaminated kernels render the grains unsuitable for food or feed[3].It has been a great challenge for wheat producers to avoid consequent economic damage.Among the measures adopted to curb this disease,deployment of scab-resistant varieties is the most preferred strategy for its economy and environmental friendliness.
Resistance to FHB in wheat is controlled by polygenes that usually have small effects and are vulnerable to environmental influences[4,5].Resistance is further complicated by its different manifestations,such as type I resistance against initial penetration,type II against fungal spread within spikes[6],type III for toxin decomposition[7],and less kernel infection and yield tolerance[8].Because of these complexities,understanding of the resistance mechanisms was very limited and progress in scab resistance breeding was slow and far from meeting our needs.
Since the end of last century,the application of molecular genetics to crops has greatly speeded up FHB resistance research in wheat.Buerstmayr et al.[9]summarized findings from 52 studies on FHB resistance QTL mapping using various germplasm accessions.Currently,more than 250 QTL distributed on all 21 chromosomes have been documented[10–28].As expected,most QTL have small effects and are yet to be verified.The effort is ongoing to cloning the FHB resistance QTL.Besides fine mapping of four major-effect QTL identified in common wheat,Fhb1[29,30],Fhb2[31],Fhb4[32]and Fhb5[33],a gene encoding a chimeric lectin on chromosome 3BS was reported to be responsible for FHB resistance conferred by Fhb1[34],which albeit is still under dispute.
In order to decipher the mechanisms underlying FHB resistance,our group has been conducting wheat FHB resistance research since 1998,mainly by forward genetics approach.Here we review the progress made in QTL mapping,cloning,functional gene discovery,and marker-assisted FHB resistance improvement.
2.Chromosome regions affecting FHB resistance
Many of the FHB resistance germplasms characterized and used in breeding programs worldwide can be traced back to the Chinese cultivar Sumai 3,developed by the Taihu Regional Institute of Agricultural Sciences,located in the Lower Yangtz River region where FHB occurs frequently and severely because of the warm and humid conditions during flowering.Cultivar Wangshuibai (WSB), indigenous to this region, displays excellent FHB resistance(Fig.1)that is at least as resistant as Sumai 3,but has not been successfully used in breeding programs.To determine the chromosomal regions conferring FHB resistance in WSB, we generated a recombinant inbred line(RIL)population from a cross of WSB with Nanda 2419[35]and constructed a molecular marker map of 4223.1 cM,comprising 887 loci[36].Nanda 2419 is a cultivar with moderate FHB resistance,compared to the most susceptible cultivars.Four field trials were conducted to evaluate the type I resistance of the RILs,which was measured as percentage of infected spikes(PIS)15 days post anthesis,after spray-inoculation at anthesis and scattering scabby wheat grains on the soil surface before anthesis[37].Seven QTL repeatedly detected in more than two trials were identified(Table 1).The WSB alleles of five of these QTL,including Qfhi.nau-4B and Qfhi.nau-5A the two with the largest effects,contributed to better resistance.
To detect type II resistance QTL,we evaluated the number of diseased spikelets(NDS)and the length of diseased rachis(LDR)of 10–20 spikes per line 20 days after single floret inoculation at anthesis in three trials.Three type II resistance QTL in WSB and one in Nanda 2419 were detected.Moreover,there was a minor type II resistance QTL from WSB repeatedly detected through composite interval mapping in the Xwmc419–Xbarc181 interval on chromosome 1B[35].Among the FHB resistance QTLfound in Nanda2419,the Qfhi.nau-2BandQfhi.nau-2Bintervalsoverlapped and were collectively referred to as Qfh.nau-2B thereafter.
The percentage of Fusarium-damaged kernels(FDK)directly reflects the damage level caused by FHB on wheat grains and its variation represents the so-called type IV scab resistance.To identify chromosomal regions governing type IV resistance and investigate its relationship with other scab resistance types,QTL associated with percent FDK were mapped[38].TypeI and typeII resistance QTL were found to be the major factors governing type IV resistance.In addition,the interval linked to Xcfd14 on chromosome 7D was associated with FDK and the number of spikelets per spike and compactness[38,39].The 2AS Xgwm328–Xgwm425interval was associated with FDK[38]and DON content[40].
The association of these identified intervals with FHB resistance was supported in other studies that used either different resistant lines or different mapping populations involving WSB[9].A few minor QTL for FHB resistance in WSB were detected using alternative susceptible mapping parents;for example,a QTL linked to Xgwm2 on chromosome 3AS[41],and a 7AL QTL linked to Xgwm276[42,43].Other major findings on chromosome locations of wheat FHB resistance QTLs are summarized in Table 2.As shown in the table,WSB carries the highest number of repeatedly identified FHB resistance QTL,in consistence with its performance against FHB.This implies the importance of QTL pyramiding for resistance enhancement.Next to WSB in terms of number of repeatedly identified QTL is Frontana with five QTL.
3.Fine mapping and cloning of FHB resistance genes
Because of limitations in population size and marker density of genetic maps in most primary mapping studies,and the dependence of QTL effects on environment,verification and marker saturation of detected QTL are always necessary for breeding and QTL cloning.To achieve this goal,we developed NILs with Qfh.nau-2B,Qfhs.nau-3B,Qfhi.nau-4B,Qfhi.nau-5A,and Qfhs.nau-6B through maker-assisted backcrossing using susceptible lines PH691(PH)and MY99-323(MY)as recurrent parents.Compared with the recurrent parents,the introduction of Qfhs.nau-3B and Qfhs.nau-6B substantially increased type II resistance,and introduction of Qfhi.nau-4B and Qfhi.nau-5A enhanced type I resistance[44](Fig.2).The improvements were statistically significant in field trials(Fig.3).NILs containing Qfh.nau-2B performed significantly better than the recurrent parents in both type I and type II responses.

Fig.1–Spikes of Wangshuibai in a field(left,photo taken on 24th of May 2016)compared with the spikes of a susceptible line in the same field(right,photo taken on the 20th of May 2016).Both cultivars have similar heading dates,but WSB still showed resistance even when allowing more time for disease development.
In order to reduce the genetic background effects in fine mapping,the NILs were used to develop secondary F2populations by crossing them with the respective recurrent parents.Recombinants covering the QTL intervals were identified through marker genotyping of populations each up to 20,000 plants.Evaluation of the derived homozygous recombinants in multiple trials showed that there were clear differences in FHB resistance between lines with the added QTLs and those without them[32,33].This allowed us to treat the QTL as single genes,a prerequisite for finemapping. After genotyping the QTL intervals in the recombinants with markers developed using comparative genomics,BACs for chromosome walking,and publicly available genomic and EST sequences,Qfhs.nau-3B(Fhb1),Qfhs.nau-6B(Fhb2),Qfhi.nau-4B(Fhb4),and Qfhi.nau-5A(Fhb5)were successfully fine mapped(Fig.4).
Qfhs.nau-3B was delimited to the Xwgrb597–Xmag9404 interval through genetic and BAC-physical mapping.Fhb1 in Sumai 3 was also placed in the same interval. There were three SNPs that distinguished WSB from Sumai 3 at the sequence level,but they appeared to have no genetic consequence.A candidate gene for Fhb1 was identified and when expressed in multiple transgenic lines it significantly enhanced FHB resistance(unpublished results).
A gene encoding a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain(PFT)on chromosome 3BS was recently reported to be responsible for the Fhb1-conferring resistance[34].This conclusion was mainly based on association mapping studies with 40 cultivars,RNA interference experiments,mutant analysis and transgenic plant evaluations.However,we were unable to confirm that the resistance in WSB and Sumai 3was due to the existence of this gene.Firstly,in ourwork the PFT gene sequence of PH691 was identical to that of WSB and Sumai 3,but PH691 was highly susceptible to F.graminearum.Secondly,in the RIL population derived from Nanda 2419×WSB,a recombinant with the WSB PFT allele alone was susceptible in multiple trials(Fig.5-A).Recombinants with the WSB PFT allele alone obtained in secondary F2populations derived from the NILs showed similar results.Thirdly,among 151 cultivars used in an association analysis 44 had the WSB/Sumai 3 PFT allele,but only 12 of them were resistant(Fig.5-B).Fourthly,the expression levels of PFT in WSB,and particularly inNanda 2419,without F.graminearum infection were extremely low and became negligible after infection(Fig.5-C).We conclude that association of PFT with FHB resistance insome germplasms is due to its tight linkage to the resistance gene.

Table 1–QTL repeatedly detected in the WSB×Nanda 2419 population in different trials.(Excerpted from Lin et al.[35,37]and Ma et al.[38].)
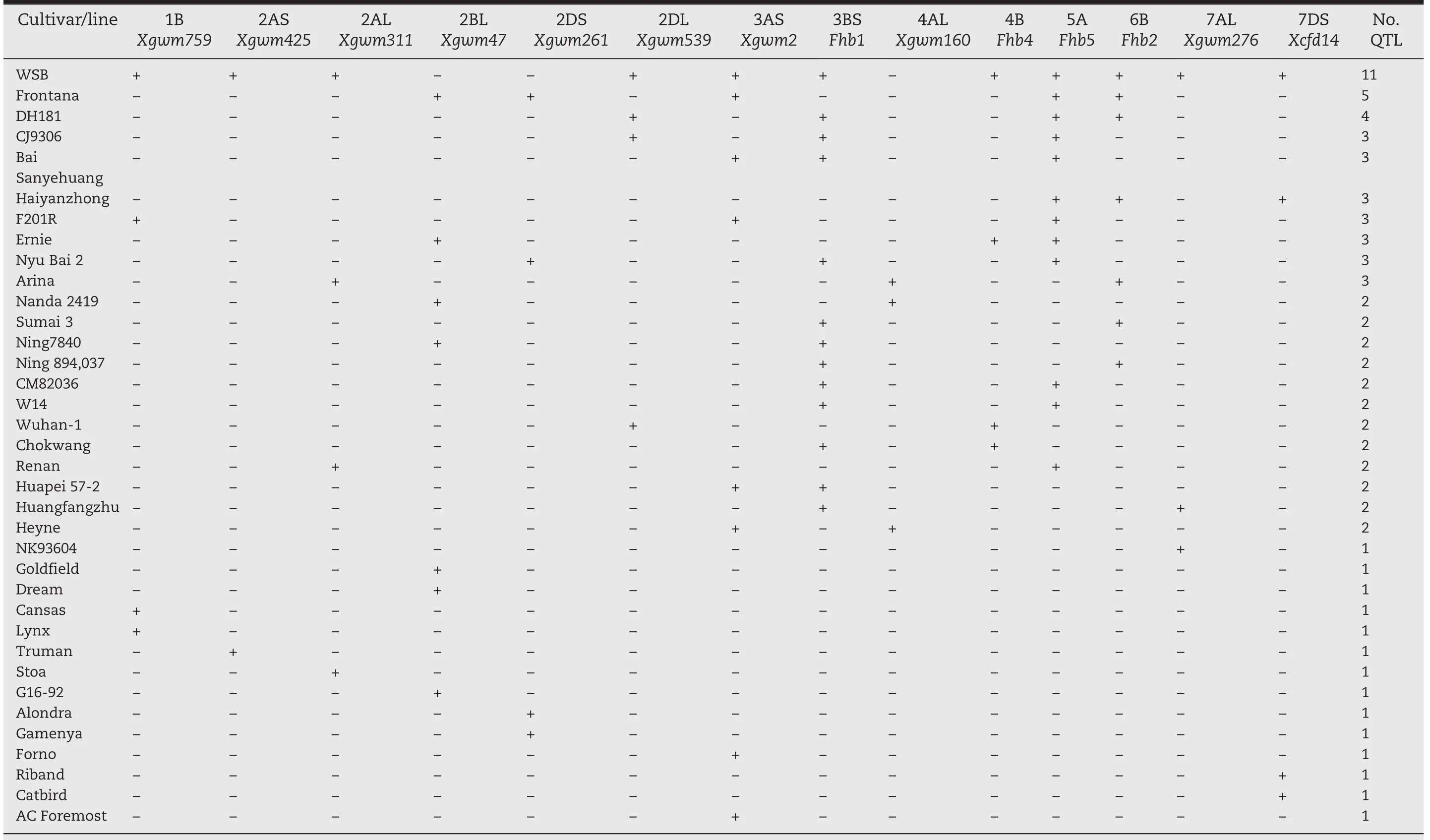
Table 2 – Distribution of repeatedly detected QTL in wheat germplasms a. +, QTL present; −, QTL not detected.
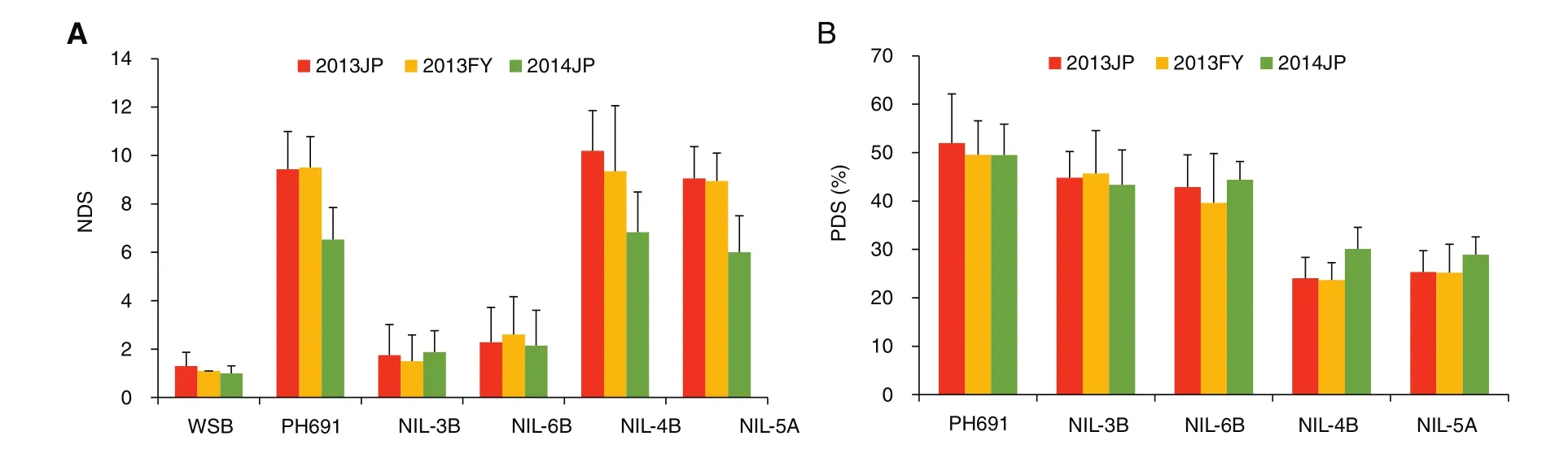
Fig.2–Numbers of diseased spikelets(NDS)(measures of type II resistance)20 days after single floret inoculation(A)and percent diseased spikelets(PDS)(measures of type I resistance)15 days after spray-inoculation of NILs with different FHB resistance QTL(B).The field trials were carried out in a randomized complete block design in two replications for each type of the inoculations at Jiangpu in Jiangsu province(2013JP,2014JP)and at Fengyang in Anhui province(2013FY).NIL-3B has Qfhs.nau-3B,NIL-6B has Qfhs.nau-6B,NIL-4B has Qfhi.nau-4B,and NIL-5A has Qfhi.nau-5A.
Qfhs.nau-6B was mapped to a 2.2 cM interval,in which Fhb2 was previously mapped[31].Qfhi.nau-4B was designated as Fhb4 and Qfhi.nau-5A as Fhb5[32,33](Fig.4).Fhb5 is located in an~50 Mb interval very close to the centromere of chromosome5A,a region of low recombination that makes map-based cloning a challenging task.However,the different mapping populations could help to solve some of the recombination suppression problems.For example,the recombination in the Fhb1 interval was severely suppressed in the combination ofFhb1 NIL×Mianyang 99-323,but more relaxed in the combination of Fhb1 NIL×PH691(unpublished results).
4.Discovery of genes related to FHB response by reverse genetics
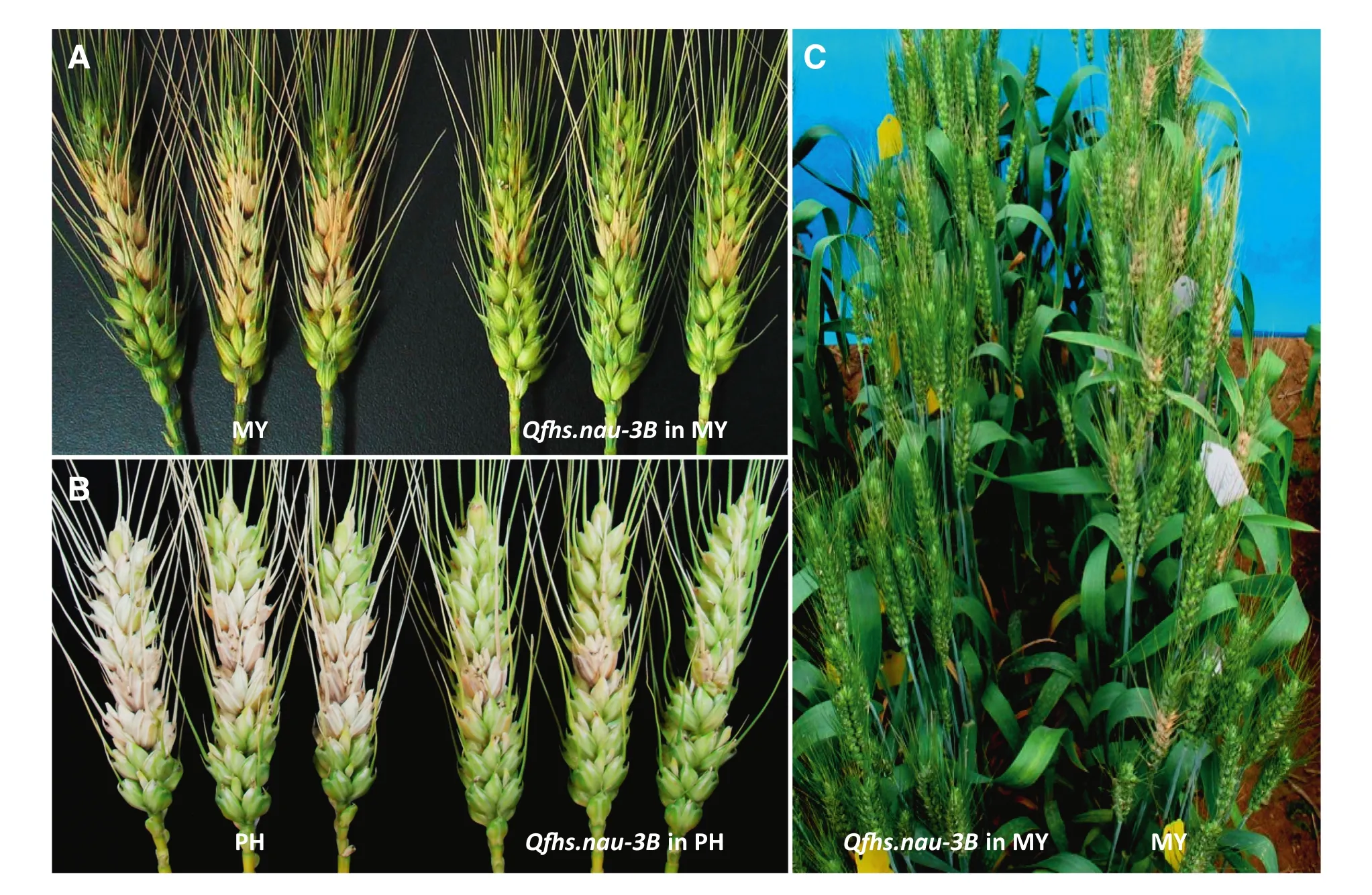
Fig.3–Effect of Qfhs-nau-3B when transferred to PH(PH691)and MY(MY99-323)on FHB resistance 20 days after single floret inoculation.A and B,close-up views;C.field view of the MY NIL with Qfhs-nau-3B compared to MY.
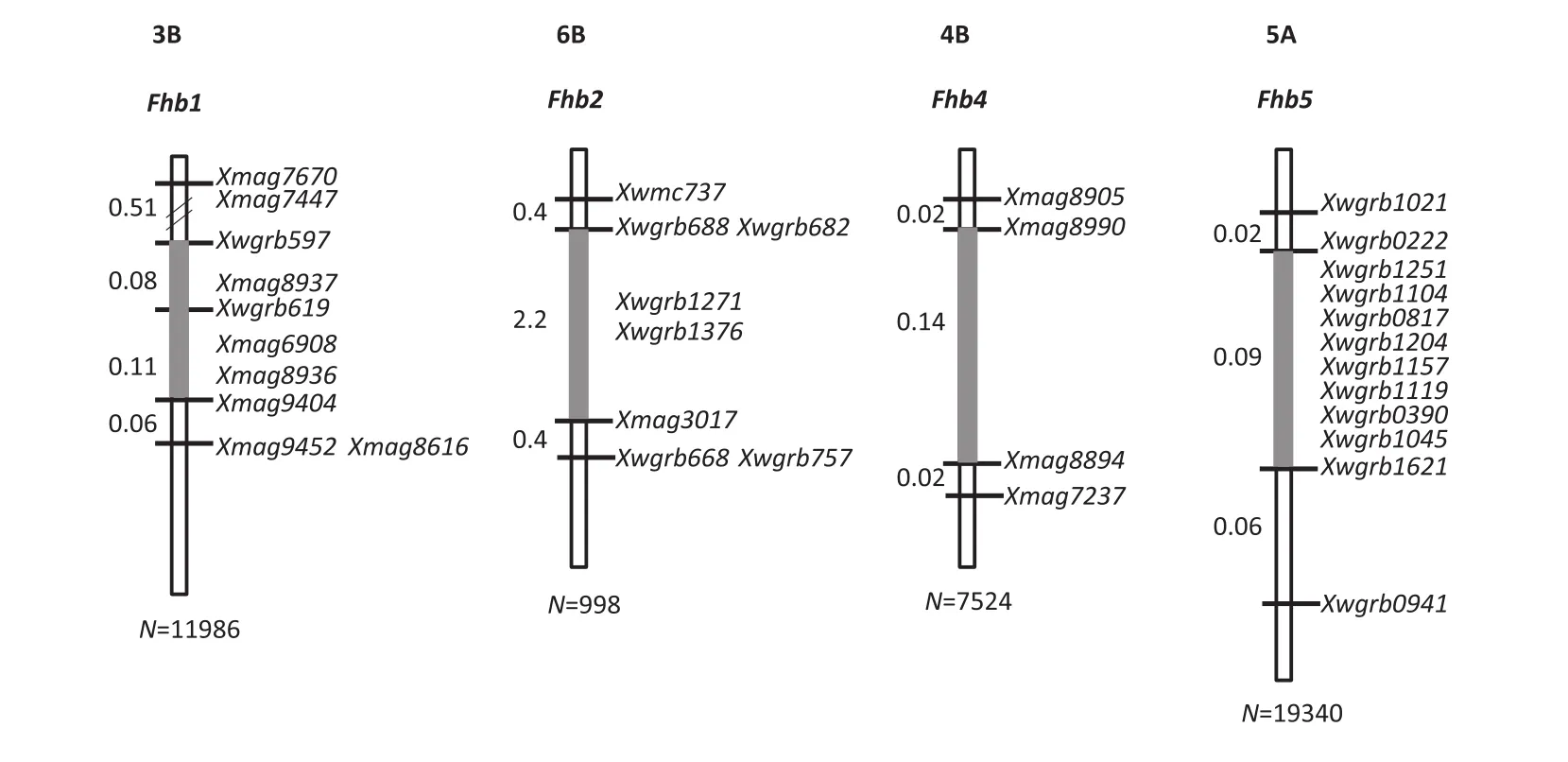
Fig.4–Marker maps surrounding individual FHB resistance QTL.The numbers to the left of each map indicate the genetic distances between adjoining markers.N,population size used in recombinant screening for each QTL.The grey bars indicate the QTL regions.
Plant resistance to pathogen attack is a finely regulated process associated with a number of cellular events,such as oxidative burst induction[45],hypersensitive response[46],accumulation of toxic compounds[47],and fortification of cell walls[48].It is conceivable that a large gene network regulates resistance responses.Accordingly,a common approach in identification of FHB resistance-related genes is through reverse genetics.Following early studies that demonstrated the induction of defense-related genes by F.graminearum infection[49–51],numerous studies have tried to identify FHB resistance-related genes through high-throughput omics[52–67].
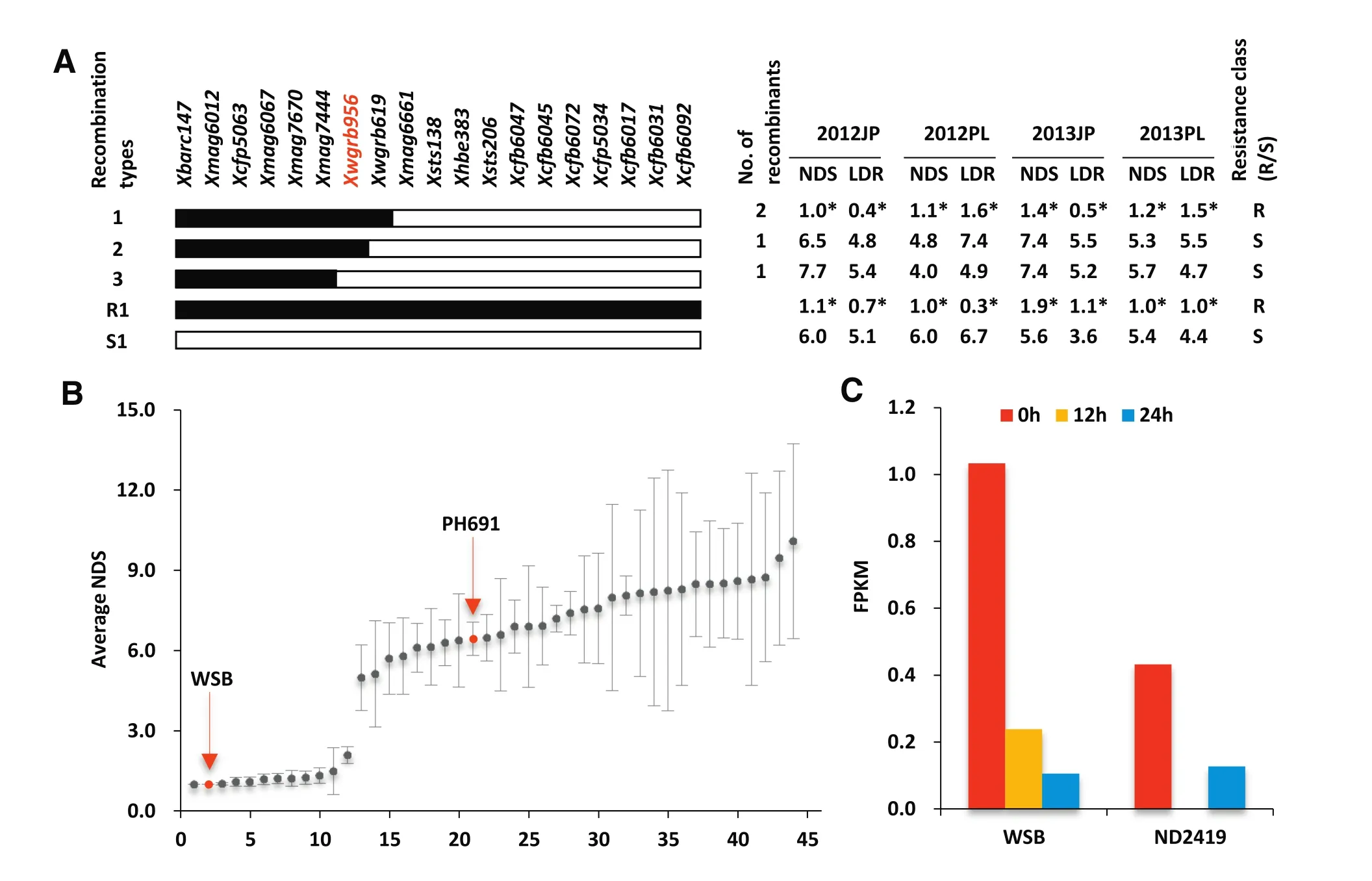
Fig.5–Relevancy of the WSB/S3 PFT allele with FHB resistance.(A)Genotypes and phenotypes of key recombinants identified in 530 RILs derived from Nanda 2419×WSB.WGRB956 is a PFT-specific marker.Black rectangles,WSB chromatin;open rectangles,Nanda 2419 chromatin.R1 and S1,resistant control and susceptible controls,respectively.*Significantly different from the susceptible control at P=0.05.(B)NDS(averaged over three separate trials in different years)distribution in 44 cultivars with the WSB/Sumai 3 PFTallele.(C)Expression levelsmeasured in FPKM(fragments per kilobasemillion) of the PFT gene after inoculation with F.graminearum in WSB and Nanda 2419.Spikelets were collected for RNA extraction and RNA-seq data generation at 0,12,and 24 h after inoculation with F.graminearum.
As an initial attempt,a proteomics-based search of proteins differentially expressed in resistantWSB 6–24 h afterF.graminearum infection was conducted in 2001 in our laboratory.Proteins associated with defense reactions were activated or translated shortly after inoculation[68].Later,by comparing the protein profiles of WSB and the susceptible WSB mutant Meh0106 created by EMS treatment,in spikes at 12 h after inoculation,we identified four jasmonic acid(JA)/ethylene(ET)biosynthesis proteins,one antimicrobial compound synthesis protein,one cell-wall formation-related protein,and three defense-related proteins,which were all differentially up-regulated in WSB[59].These results suggested the association of these proteins with FHB responses.To complement the proteomics approach,a comparative transcriptomic analysis of genes expressed in infected spikes and in spikes without pathogen infection was conducted.One hundred and sixty-three up-regulated defense-related unigenes were identified and classified into ten functional categories based on their putative functions(Table 3).Most of the up-regulated genes were involved in basal resistance or defense[52,56].Moreover,of the identified genes,nearly 50%encoded proteins for synthesis of antimicrobial compounds,modulation of the oxidative state,and resistance gene analogs(RGAs)or kinase proteins.The involvement of these genesinFHB resistance or defense was confirmed through expression profiling of WSB and the susceptible mutant,and in other studies[60–62].It is intriguing that some RGAs were found only in spikes under pathogen attack.This may imply unique roles in defense against F.graminearum. RGAs and kinases are important in regulation of crosstalk between signaling pathways mediated by phytohormones in response to pathogen infection[69].
Lignin is a major component of plant cell walls and is involved in defense against pathogen attack.Many genes,related to lignin biosynthesis,were up-regulated by F.graminearum infection in resistant lines[59,60];differences in lignin composition and structure were also observed inresistant and susceptible lines[70].Cell wall fortification could make the wall more resistant to cell wall degrading enzymes produced by pathogens and prevent the diffusion of pathogen-produced toxins[71].

Table 3–Functional classification of genes differentially expressed after F.graminearum infection.(Excerpted from Ding et al.[60].)
Jacalin-related lectins(JRLs)are a group of plant lectins with at least one domain homologous to jacalin protein first isolated from Artocarpus integrifolia[72].Most of the functionally characterized JRL genes in plants are associated with disease resistance,abiotic stress tolerance,wounding or insect damage,or even responses to multiple stresses[73,74].Interestingly,in mining JRL genes present in the wheat genome,at least 12 were inducible by F.graminearum infection[73].Over-expression of mannose-specific JRL-like gene TaJRL1 in Arabidopsis thaliana led to increased resistance to F.graminearum and Botrytis cinerea and to elevation of JA and SA levels,whereas attenuation of TaJRLL1 expression through virus-induced gene silencing increased susceptibility to F.graminearum and Blumeria graminis[75].Over-expression of TaJRL2.1in wheat up-regulated lignin synthesis-related genes and led to better FHB resistance(unpublished data).
Thus far, a great number of genes inducible by F. graminearum infection have been identified through reverse genetics in different laboratories.However,it is worth noting that substantial differences in identified genes and their expression profiles occur between studies,probably due to the differences in wheat genotypes,experimental procedures and technologies.The big challenge facing us now is to functionally verify the association of these genes with FHB response.A few wheat genes that were either differentially expressed after F. graminearum inoculation or mycotoxin treatment,or cloned based on the association of homologs in other plant species with defense responses,have been used in wheat transformation for functional analysis and practical application[76–80].Their effects on FHB responses were mostly observed in greenhouse conditions.Large-scale field evaluations are still needed to justify their use in breeding programs[76,81].
5.The role of hormones in FHB response
The mechanisms underlying resistance to necrotrophic and hemi-biotrophic pathogens,such as F.graminearum,are complicated.Many studies demonstrated that the JA and ET signaling pathways play important roles[82–86].However,there are conflicting findings for the association of the SA signal-mediated defense pathway,the central player in resistance to biotrophic pathogens [87], with resistance to necrotrophic and hemi-biotrophic pathogens[88–92].A transcriptome analysis showed that defense pathways mediated by calcium ions,SA,and PA,as well as JA and SA,are all required for resistance responses to infection by F.graminearum[59],in accordance with many other studies[53,55,58,62,63,67,92].A time-course expression profile comparison between WSB and its susceptible mutant,of a selected set of genes related to these pathways,revealed a biphasic regulation of defense signaling.This,together with observation of a sequential increase in endogenous SA and JA contents occurring in spikes after infection,points to a hypothesized model of the early cellular events,in which the early defense reactions to F.graminearum infection start with the initiation of Ca2+and SA signaling before JA signaling[59].Plants can synthesize SA via PAL or ICS1[93,94],but only PAL was inducible by F. graminearum infection. Similarly,Arabidopsis plants synthesize SA via the PAL pathway in response to the necrotrophic pathogen Botrytis cinerea[95].Based on the altered expression profiles in the mutant,it was postulated that timely and orderly activation of the SA and JA defense pathways would be critical for them to coordinately regulate resistance[60].
6.Marker-assisted improvement of FHB resistance
In theNIL evaluations, we showed the effectiveness of individual QTL from WSB in conferring FHB resistance.To accelerate their employment in wheat production, we designed a backcross-based QTL pyramiding strategy.Starting by crossing WSB with susceptible elite lines,plants in each BCF1that carried all target QTL were selected for further backcrossing.In each generation of backcrossing selection was firstly performed for the target QTL with markers linked to them(foreground selection),and then against the donor backgrounds using markers dispersed across the whole genome(background selection).After three generations of backcross,plants homozygous for the QTL and with over 95%reconstitution of the recurrent parental genotype were selected for phenotype evaluation.QTL pyramiding was also performed by crossing different NILs.Atotal of 19 lines with different QTL combinations in the PH691 and MY backgrounds were obtained.These lines were evaluated atFengyang inAnhui province,and atJiangpu in Jiangsu in randomized complete block design trials in 2013 and 2014.The type I and type II resistance levels in PH691 were increased by over 80%by introduction of Fhb4+Fhb5 and Fhb1+Fhb2,respectively(Fig.6-A,B).Additive effects were particularly significant for Fhb4+Fhb5.Differences in disease symptoms of recurrent parents and lines with combined QTL were very clear after F. graminearum inoculation(Fig.6-C).Similar results were obtained for the MY-derived lines regardless of locations and years.The experiments have now been expanded to more elite lines from Henan,Shandong,Sichuan and Jiangsu.Preliminary results obtained so far are very promising.Hopefully,the developed lines will be promoted to production trials and used as breeding parents.
7.Perspectives
FHB has been documented in China for almost 90 years.We are still in the process of finding effective strategies to improve resistance due to the complexity of this disease.However,after about 20 years of intensive effort worldwide,basic understanding of FHB resistance genetics and dozens of resistance QTL have been achieved.The mechanisms underlying FHB resistance are starting to emerge through use of transcriptomics and proteomics.More importantly,practices in our laboratory and others have demonstrated the feasibility of breeding resistant cultivars.The use of marker-assisted selection will make the breeding process more effective and cost-efficient.Breeders can now achieve breeding goals much easier than previously.

Fig.6–Phenotypes of 13 developed lines with different combinations of QTL,compared with the susceptible control.(A)PDS 15 days after spray-inoculation for assessment of type I resistance.(B)NDS 20 days after single floret inoculation,for assessment type II resistance(2013,Jiangpu).Multiple comparisons were made by Duncan's test(P=0.01).(C)Comparison of the susceptible response of PH691 and a derived line with Fhb1,Fhb2,Fhb4 and Fhb5,20 days after single floret inoculation.
Many questions still remain.Most of the identified QTL are yet to be fully validated,the number of ready-to use QTL is limited,the resistance mechanisms are far from clear,and reasons for significant differences in genetic background effects are still unknown.Moreover,comprehensive evaluation of newly bred candidate resistant lines is still required for application in production.Since FHB resistance,agronomical and quality traits are all controlled by polygenes,selection for all traits simultaneously is still difficult.Currently,we suggest prioritizing selection of the major resistance QTL in early generations by MAS before selection ofothertraits.Genome-wide selection could be a promising approach for multiple trait improvement in the future.
Acknowledgments
We are very grateful to staff and many previous students who are not listed as co-authors but have made significant contribution to FHB research.We are also grateful to many colleagues,nationally and internationally,whose support made our work possible andmeaningful.We apologize for not citing all excellent researches on wheat FHB resistance due to limited scope of this paper.The project was mainly supported by National Key Research and Development Program (2016YFD0101802,2016YFD0101004),National Basic Research Program of China(2004CB117205,2012CB125902),National Key Technology R&D Program of China(2002AA224161),National Science and Technology Major Project(2009ZX08009-049B,2012ZX08009003),Jiangsu Collaborative Innovation Initiative for Modern Crop Production,‘111'project B08025,Natural Science Foundation of Jiangsu Province(BK20131316),Innovation Team Program for Jiangsu Universities(2014),and particularly,the long-term funding from the National Science Foundation ofChina(30025030,30430440,30721140555,31030054,30671295).
R E F E R E N C E S
[1]D.W.Parry,P.Jenkinson,L.Mcleod,Fusarium ear blight(scab)in small grain cereals:a review,Plant Pathol.44(1995)207–238.
[2]R.S.Goswami,H.C.Kistler,Heading for disaster:Fusarium graminearum on cereal crops,Mol.Plant Pathol.5(2004)515–525.
[3]J.Gilbert,A.Tekauz,Review:recent developments in research on Fusarium head blight of wheat in Canada,Can.J.Plant Pathol.22(2000)1–8.
[4]C.H.A.Snijders,J.Perkowski,Effect of head blight caused by Fusarium culmorum on toxin content and weight of wheat kernels,Phytopatholgy 80(1990)566–570.
[5]T.Ban,K.Suenaga,Genetic analysis of resistance to Fusarium head blight caused by Fusarium graminearum in Chinese wheat cultivar Sumai 3 and the Japanese cultivar Saikai 165,Euphytica 113(2000)87–99.
[6]H.W.Schroeder,J.J.Christensen,Factors affecting resistance of wheat to scab caused by Gibberella zeae,Phytopathology 53(1963)831–838.
[7]J.D.Miller,J.C.Young,D.R.Sampson,Deoxynivalenol and Fusarium head blight resistance in spring cereals,J.Phytopathol.113(1985)359–367.
[8]A.Mesterhazy,Types and components of resistance to Fusarium head blight of wheat,Plant Breed.114(1995)377–386.
[9]H.Buerstmayr,T.Ban,J.A.Anderson,QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat:a review,Plant Breed.128(2009)1–26.
[10]S.Liu,M.D.Hall,C.A.Griffey,A.L.McKendry,Meta-analysis of QTL associated with Fusarium head blight resistance in wheat,Crop Sci.49(2009)1955–1968.
[11]S.Y.Liu,C.A.Griffey,M.D.Hall,A.L.McKendry,J.L.Chen,W.S.Brooks,G.Brown-Guedira,D.Van Sanford,D.G.Schmale,Molecular characterization of field resistance to Fusarium head blight in two US soft red winter wheat cultivars,Theor.Appl.Genet.126(2013)2485–2498.
[12]J.Haberle,J.Holzapfel,G.Schweizer,L.Hartl,A major QTL for resistance against Fusarium head blight in European winter wheat,Theor.Appl.Genet.119(2009)325–332.
[13]X.L.Zhang,X.R.Shen,Y.F.Hao,J.J.Cai,H.W.Ohm,L.R.Kong,A genetic map of Lophopyrum ponticum chromosome 7E,harboring resistance genes to Fusarium head blight and leaf rust,Theor.Appl.Genet.122(2011)263–270.
[14]C.G.Chu,Z.X.Niu,S.B.Zhong,S.A.Chao,T.L.Friesen,S.Halley,E.M.Elias,Y.H.Dong,J.D.Faris,S.S.Xu,Identification and molecular mapping of two QTL with major effects for resistance to Fusarium head blight in wheat,Theor.Appl.Genet.123(2011)1107–1119.
[15]T.Li,G.H.Bai,S.Y.Wu,S.L.Gu,Quantitative trait loci for resistance to Fusarium head blight in a Chinese wheat landrace Haiyanzhong,Theor.Appl.Genet.122(2011)1497–1502.
[16]D.Jayatilake,G.Bai,Y.Dong,A novel quantitative trait locus for Fusarium head blight resistance in chromosome 7A of wheat,Theor.Appl.Genet.122(2011)1189–1198.
[17]T.Miedaner,T.Wurschum,H.P.Maurer,V.Korzun,E.Ebmeyer,J.C.Reif,Association mapping for Fusarium head blight resistance in European soft winter wheat,Mol.Breed.28(2011)647–655.
[18]M.Buerstmayr,M.Lemmens,B.Steiner,H.Buerstmayr,Advanced backcross QTL mapping of resistance to Fusarium head blight and plant morphological traits in a Triticum macha×T.aestivum population,Theor.Appl.Genet.123(2011)293–306.
[19]M.Buerstmayr,K.Huber,J.Heckmann,B.Steiner,J.C.Nelson,H.Buerstmayr,Mapping of QTL for Fusarium head blight resistance and morphological and developmental traits in three backcross populations derived from Triticum dicoccum×Triticum durum,Theor.Appl.Genet.125(2012)1751–1765.
[20]M.Buerstmayr,A.Alimari,B.Steiner,H.Buerstmayr,Genetic mapping of QTL for resistance to Fusarium head blight spread(type 2 resistance)in a Triticum dicoccoides×Triticum durum backcross-derived population,Theor.Appl.Genet.126(2013)2825–2834.
[21]X.H.Zhang,H.Y.Pan,G.H.Bai,Quantitative trait loci responsible for Fusarium head blight resistance in Chinese landrace Baishanyuehuang,Theor.Appl.Genet.125(2012)495–502.
[22]Q.J.Zhang,J.E.Axtman,J.D.Faris,S.M.Chao,Z.C.Zhang,T.L.Friesen,S.B.Zhong,X.W.Cai,E.M.Elias,S.S.Xu,Identification and molecular mapping of quantitative trait loci for Fusarium head blight resistance in emmer and durum wheat using a single nucleotide polymorphism-based linkage map,Mol.Breed.34(2014)1677–1687.
[23]C.Burt,A.Steed,N.Gosman,M.Lemmens,N.Bird,R.Ramirez-Gonzalez,S.Holdgate,P.Nicholson,Mapping a Type 1 FHB resistance on chromosome 4AS of Triticum macha and deployment in combination with two Type 2 resistances,Theor.Appl.Genet.128(2015)1725–1738.
[24]A.Giancaspro,S.L.Giove,D.Zito,A.Blanco,A.Gadaleta,Mapping QTL for Fusarium head blight resistance in an interspecific wheat population,Front.Plant Sci.7(2016)1381.
[25]S.Petersen,J.H.Lyerly,P.V.Maloney,G.Brown-Guedira,C.Cowger,J.M.Costa,Y.H.Dong,J.P.Murphy,Mapping of Fusarium head blight resistance quantitative trait loci in winter wheat cultivar NC-Neuse,Crop Sci.56(2016)1473–1483.
[26]N.Prat,C.Guilbert,U.Prah,E.Wachter,B.Steiner,T.Langin,O.Robert,H.Buerstmayr,QTL mapping of Fusarium head blight resistance in three related durum wheat populations,Theor.Appl.Genet.130(2017)13–27.
[27]P.Arruda Marcio,P.Brown,G.Brown-Guedira,A.M.Krill,C.Thurber,K.R.Merrill,B.J.Foresman,F.L.Kolb,Genome-wide association mapping of Fusarium head blight resistance in wheat using genotyping-by-sequencing,Plant Genome 9(2016)10.3835.
[28]X.Li,Z.P.Xiang,W.Q.Chen,Q.L.Huang,T.G.Liu,Q.Li,S.F.Zhong,M.Zhang,J.W.Guo,L.Lei,P.G.Luo,Reevaluation of two quantitative trait loci for type II resistance to Fusarium head blight in wheat germplasm PI 672538,Phytopatholgy 107(2017)92–99.
[29]P.A.Cuthbert,D.J.Somers,J.Thomas,S.Cloutier,A.Brulé-Babel,Fine mapping Fhb1,a major gene controlling Fusarium head blight resistance in bread wheat(Triticum aestivum L.),Theor.Appl.Genet.112(2006)1465–1472.
[30]S.Liu,X.Zhang,M.O.Pumphrey,R.W.Stack,B.S.Gill,J.A.Anderson,Complex microcolinearity among wheat,rice,and barley revealed by fine mapping of the genomic region harboring a major QTL for resistance to Fusarium head blight in wheat,Funct.Integr.Genomics 6(2006)83–89.
[31]P.A.Cuthbert,D.J.Somers,A.Brule-Babel,Mapping of Fhb2 on chromosome 6BS:a gene controlling Fusarium head blight field resistance in bread wheat(Triticum aestivum L.),Theor.Appl.Genet.114(2007)429–437.
[32]S.L.Xue,G.Q.Li,H.Y.Jia,F.Xu,F.Lin,M.Z.Tang,Y.Wang,X.An,H.B.Xu,L.X.Zhang,Z.X.Kong,Z.Q.Ma,Fine mapping of Fhb4,a major QTL conditioning resistance to Fusarium infection in bread wheat(Triticum aestivum L.),Theor.Appl.Genet.121(2010)147–156.
[33]S.L.Xue,F.Xu,M.Z.Tang,Y.Zhou,G.Q.Li,X.An,F.Lin,H.B.Xu,H.Y.Jia,L.X.Zhang,Z.X.Kong,Z.Q.Ma,Precise mapping Fhb5,a major QTL conditioning resistance to Fusarium infection in bread wheat(Triticum aestivum L.),Theor.Appl.Genet.123(2011)1055–1063.
[34]N.Rawat,M.O.Pumphrey,S.Liu,X.Zhang,V.K.Tiwari,K.Ando,H.N.Trick,W.W.Bockus,E.Akhunov,J.A.Anderson,B.S.Gill,Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight,Nat.Genet.48(2016)1576–1580.
[35]F.Lin,Z.X.Kong,H.L.Zhu,S.L.Xue,J.Z.Wu,D.G.Tian,J.B.Wei,C.Q.Zhang,Z.Q.Ma,Mapping QTL associated with resistance to Fusarium head blight in the Nanda 2419×Wangshuibai population.I.Type II resistance,Theor.Appl.Genet.109(2004)1504–1511.
[36]S.L.Xue,Z.Z.Zhang,F.Lin,Z.X.Kong,Y.Cao,C.J.Li,H.Y.Yi,M.F.Mei,H.L.Zhu,J.Z.Wu,H.B.Xu,D.M.Zhao,D.G.Tian,C.Q.Zhang,Z.Q.Ma,A high-density intervarietal map of the wheat genome enriched with markers derived from expressed sequence tags,Theor.Appl.Genet.117(2008)181–189.
[37]F.Lin,S.L.Xue,Z.Z.Zhang,C.Q.Zhang,Z.X.Kong,G.Q.Yao,D.Q.Tian,H.L.Zhu,C.J.Li,Y.Cao,J.B.Wei,Q.Y.Luo,Z.Q.Ma,Mapping QTL associated with resistance to Fusarium head blight in the Nanda 2419×Wangshuibai population.II:Type I resistance,Theor.Appl.Genet.112(2006)528–535.
[38]Z.Q.Ma,S.L.Xue,F.Lin,S.H.Yang,G.Q.Li,M.Z.Tang,Z.X.Kong,Y.Cao,D.M.Zhao,H.Y.Jia,Z.Z.Zhang,L.X.Zhang,Mapping and validation of scab resistance QTL in the Nanda 2419×Wangshuibai population,Cereal Res.Commun.36(2008)245–251.
[39]C.Li,H.Zhu,C.Zhang,F.Lin,S.Xue,Y.Cao,Z.Zhang,L.Zhang,Z.Ma,Mapping QTL associated with Fusariumdamaged kernels in the Nanda 2419×Wangshuibai population,Euphytica 163(2008)185–191.
[40]Z.Q.Ma,D.M.Zhao,C.Q.Zhang,Z.Z.Zhang,S.L.Xue,F.Lin,Z.X.Kong,D.G.Tian,Q.Y.Luo,Genetic analysis of five spikerelated traits in wheat using RIL and immortalized F2populations,Mol.Gen.Genomics.277(2007)31–42.
[41]H.X.Ma,K.M.Zhang,L.Gao,G.H.Bai,H.G.Chen,Z.X.Cai,W.Z.Lu,Quantitative trait loci for resistance to Fusarium head blight and deoxynivalenol accumulation in Wangshuibai wheat under field conditions,Plant Pathol.55(2006)739–745.
[42]J.B.Yu,G.H.Bai,S.B.Cai,Y.H.Dong,T.Ban,New Fusarium head blight-resistant sources from Asian wheat germplasm,Crop Sci.48(2008)1090–1097.
[43]W.C.Zhou,F.L.Kolb,J.B.Yu,G.H.Bai,L.K.Boze,L.L.Domier,Molecular characterization of Fusarium head blight resistance in Wangshuibai with simple sequence repeat and amplified fragment length polymorphism markers,Genome 47(2004)1137–1143.
[44]G.Jia,P.D.Chen,G.J.Qin,G.H.Bai,X.Wang,S.L.Wang,B.Zhou,S.H.Zhang,D.J.Liu,QTL for Fusarium head blight response in a wheat DH population of Wangshuibai/Alondra‘s',Euphytica 146(2005)183–191.
[45]S.Xue,G.Li,H.Jia,F.Lin,Y.Cao,F.Xu,M.Tang,Y.Wang,X.Wu,Z.Zhang,L.Zhang,Z.Kong,Z.Ma,Marker-assisted development and evaluation of near-isogenic lines for scab resistance QTL of wheat,Mol.Breed.25(2010)397–405.
[46]C.Lamb,R.A.Dixon,The oxidative burst in plant disease resistance,Annu.Rev.Plant Physiol.Plant Mol.Biol.48(1997)251–275.
[47]N.Watanabe,E.Lam,Arabidopsis Bax inhibitor-1 functions as an attenuator of biotic and abiotic types of cell death,Plant J.45(2006)884–894.
[48]B.P.Thomma,I.Nelissen,K.Eggermont,W.F.Broekaert,Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola,Plant J.19(1999)163–171.
[49]N.H.Bhuiyan,G.Selvaraj,Y.Wei,J.King,Role of lignification in plant defense,Plant Signal.Behav.4(2009)158–159.
[50]C.Pritsch,G.J.Muehlbauer,W.R.Bushnell,D.A.Somers,C.P.Vance,Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum,Mol.Plant-Microbe Interact.13(2000)159–169.
[51]W.L.Li,J.D.Faris,S.Muthukrishnan,D.J.Liu,P.D.Chen,B.S.Gill,Isolation and characterization of novel cDNA clones of acidic chitinases and-1,3-glucanases from wheat spikes infected by Fusarium graminearum,Theor.Appl.Genet.102(2001)353–362.
[52]W.M.Kruger,C.Pritsch,S.Chao,G.J.Muehlbauer,Functional and comparative bioinformatic analysis of expressed genes from wheat spikes infected with Fusarium graminearum,Mol.Plant-Microbe Interact.15(2002)445–455.
[53]W.Zhou,F.L.Kolb,D.E.Riechers,Identification of proteins induced or upregulated by Fusarium head blight infection in the spikes of hexaploid wheat(Triticum aestivum),Genome 48(2005)770–780.
[54]A.Bernardo,G.Bai,P.Guo,K.Xiao,A.C.Guenzi,P.Canaan,Fusarium graminearum-induced changes in gene expression between Fusarium head blight resistant and susceptible wheat cultivars,Funct.Integr.Genomics 7(2007)69–77.
[55]L.Kong,H.W.Ohm,J.M.Anderson,Expression analysis of wheat defense related genes in wheat in response to infection by Fusarium graminearum,Genome 50(2007)1038–1048.
[56]G.Li,Y.Yen,Jasmonate and ethylene signaling pathway may mediate Fusarium Head Blight resistance in wheat,Crop Sci.48(2008)1888–1896.
[57]J.Geddes,F.Eudes,A.Laroche,L.B.Selinger,Differential expression of proteins in response to the interaction between the pathogen Fusarium graminearum and its host,Hordeum vulgare,Proteomics 8(2008)545–554.
[58]H.Jia,S.Cho,G.J.Muehlbauer,Transcriptome analysis of a wheat near isogenic line pair carrying Fusarium head blightresistant and-susceptible alleles,Mol.Plant-Microbe Interact.22(2009)1366–1378.
[59]B.Steiner,H.Kurz,M.Lemmens,H.Buerstmayr,Differential gene expression of related wheat lines with contrasting levels of head blight resistance after Fusarium graminearum inoculation,Theor.Appl.Genet.118(2009)753–764.
[60]L.Ding,H.Xu,H.Yi,L.Yang,Z.Kong,L.Zhang,S.Xue,H.Jia,Z.Ma,Resistance to hemi-biotrophic F.graminearum infection is associated with coordinated and ordered expression of diverse defense signaling pathways,PLoS One 6(2011)e19008.
[61]R.Gunnaiah,A.C.Kushalappa,R.Duggavathi,S.Fox,D.J.Somers,Integrated metabolo-proteomic approach to decipher the mechanisms by which wheat QTL(Fhb1)contributes to resistance against Fusarium graminearum,PLoS One 7(2012),e40695.
[62]X.Zhang,J.Fu,Y.Hiromasa,H.Pan,G.Bai,Differentially expressed proteins associated with Fusarium head blight resistance in wheat,PLoS One 8(2013),e82079.
[63]J.Xiao,X.Jin,H.Wang,A.Cao,W.Zhao,H.Pei,Z.Xue,L.He,Q.Chen,X.Wang,Transcriptome-based discovery of pathways and genes related to resistance against Fusarium head blight in wheat landrace Wangshuibai,BMC Genomics 14(2013)197.
[64]K.G.Kugler,G.Siegwart,T.Nussbaumer,C.Ametz,M.Spannagl,B.Steiner,M.Lemmens,K.F.X.Mayer,H.Buerstmayr,W.G.Schweiger,Quantitative trait loci-dependent analysis of a gene co-expression network associated with Fusarium head blight resistance in bread wheat(Triticum aestivum L.),BMC Genomics 14(2013)728.
[65]K.Al-Taweel,W.G.Fernando,A.L.Brûlé-Babel,Transcriptome profiling of wheat differentially expressed genes exposed to different chemotypes of Fusarium graminearum,Theor.Appl.Genet.127(2014)1703–1718.
[66]M.Erayman,M.Turktas,G.Akdogan,T.Gurkok,B.Inal,E.Ishakoglu,E.Ilhan,T.Unver,Transcriptome analysis of wheat inoculated with Fusarium graminearum,Front.Plant Sci.6(2015)867.
[67]D.Dhokane,S.Karre,A.C.Kushalappa,C.McCartney,Integrated metabolo-transcriptomics reveals Fusarium head blight candidate resistance genes in wheat QTL-Fhb2,PLoS One 11(2016),e0155851.
[68]S.Karre,A.Kumar,D.Dhokane,A.C.Kushalappa,Metabolo-transcriptome profiling of barley reveals induction of chitin elicitor receptor kinase gene(HvCERK1)conferring resistance against Fusarium graminearum,Plant Mol.Biol.93(2017)247–267.
[69]Y.Wang,L.M.Yang,H.B.Xu,Q.F.Li,Z.Q.Ma,C.G.Chu,Differential proteomic analysis of proteins in wheat spikes induced by Fusarium graminearum,Proteomics 5(2005)4496–4503.
[70]O.Martinez de Ilarduya,Q.Xie,I.Kaloshian,Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions,Mol.Plant-Microbe Interact.16(2003)699–708.
[71]V.Lionetti,A.Giancaspro,E.Fabri,S.L.Giove,N.Reem,O.A.Zabotina,A.Blanco,A.Gadaleta,D.Bellincampi,Cell wall traits as potential resources to improve resistance of durum wheat against Fusarium graminearum,BMC Plant Biol.15(2015)6.
[72]S.E.Sattler,D.L.Funnell-Harris,Modifying lignin to improve bioenergy feedstocks:strengthening the barrier against pathogens?Front.Plant Sci.4(2013)70.
[73]M.M.Bunn-Moreno,A.Campos-Neto,Lectin(s)extracted from seeds of Artocarpus integrifolia(jackfruit):potent and selective stimulator(s)of distinct human T and B cell function,J.Immunol.127(1981)427–429.
[74]M.Song,W.Xu,Y.Xiang,H.Jia,L.Zhang,Z.Ma,Association of jacalin-related lectins with wheat responses to stresses revealed by transcriptional profiling,Plant Mol.Biol.84(2014)95–110.
[75]D.Weidenbach,L.Esch,C.Moller,G.Hensel,J.Kumlehn,C.Hofle,R.Huckelhoven,U.Schaffrath,Polarized defense against fungal pathogens is mediated by the jacalin-related lectin domain of modular Poaceae-specific proteins,Mol.Plant 9(2016)514–527.
[76]Y.Xiang,M.Song,Z.Wei,J.Tong,L.Zhang,L.Xiao,Z.Ma,Y.Wang,A jacalin-related lectin-like gene in wheat is a component of the plant defense system,J.Exp.Bot.62(2011)5471–5483.
[77]C.A.Mackintosh,J.Lewis,L.E.Radmer,S.Shin,S.J.Heinen,L.A.Smith,M.N.Wyckoff,R.Dill-Macky,C.K.Evans,S.Kravchenko,G.D.Baldridge,R.J.Zeyen,G.J.Muehlbauer,Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight,Plant Cell Rep.26(2007)479–488.
[78]L.Ma,Y.Shang,A.Cao,Z.Qi,L.Xing,P.Chen,D.Liu,X.Wang,Molecular cloning and characterization of an up-regulated UDP-glucosyltransferase gene induced by DON from Triticum aestivum L.cv.Wangshuibai,Mol.Biol.Rep.37(2010)785–795.
[79]I.Bahrini,M.Sugisawa,R.Kikuchi,T.Ogawa,H.Kawahigashi,T.Ban,H.Handa,Characterization of a wheat transcription factor,TaWRKY45,and its effect on Fusarium head blight resistance in transgenic wheat plants,Breed.Sci.61(2011)121–129.
[80]X.Zhu,Z.Li,H.Xu,M.Zhou,L.Du,Z.Zhang,Overexpression of wheat lipid transfer protein gene TaLTP5 increases resistances to Cochliobolus sativus and Fusarium graminearum in transgenic wheat,Funct.Integr.Genomics 12(2012)481–488.
[81]A.Perochon,J.Jia,A.Kahla,C.Arunachalam,S.R.Scofield,S.Bowden,E.Wallington,F.M.Doohan,TaFROG encodes a Pooideae orphan protein that interacts with SnRK1 and enhances resistance to the mycotoxigenic fungus Fusarium graminearum,Plant Physiol.169(2015)2895–2906.
[82]C.S.Gao,X.J.Kou,H.P.Li,J.B.Zhang,A.S.I.Saad,Y.C.Liao,Inverse effects of Arabidopsis NPR1 gene on Fusarium seedling blight and Fusarium head blight in transgenic wheat,Plant Pathol.62(2013)383–392.
[83]B.P.H.J.Thomma,K.Eggermont,I.A.M.A.Penninckx,B.Mauch-Mani,R.Vogelsang,B.P.A.Cammue,W.F.Broekaert,Separate jasmonate-dependent and salicylate-dependent defense response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens,Proc.Natl.Acad.Sci.U.S.A.95(1998)15107–15111.
[84]B.P.Geraats,P.A.Bakker,L.C.van Loon,Ethylene insensitivity impairs resistance to soilborne pathogens in tobacco and Arabidopsis thaliana,Mol.Plant-Microbe Interact.15(2002)1078–1085.
[85]O.Lorenzo,R.Piqueras,J.J.Sánchez-Serrano,R.Solano,ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense,Plant Cell 15(2003)165–178.
[86]M.Pré,M.Atallah,A.Champion,M.De Vos,C.M.Pieterse,J.Memelink,The AP2/ERF domain transcription factor ORA59 integrates jasmonic acid and ethylene signals in plant defense,Plant Physiol.147(2008)1347–1357.
[87]P.F.Qi,M.Balcerzak,H.Rocheleau,W.Leung,Y.M.Wei,Y.L.Zheng,Jasmonic acid and abscisic acid play important roles in host-pathogen interaction between Fusarium graminearum and wheat during the early infection stages of Fusarium head blight,Physiol.Mol.Plant Pathol.93(2016)39–48.
[88]E.Luna,T.J.A.Bruce,M.R.Roberts,V.Flors,J.Ton,Nextgeneration systemic acquired resistance,Plant Physiol.158(2012)844–853.
[89]M.Berrocal-Lobo,A.Molina,Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum,Mol.Plant-Microbe Interact.17(2004)763–770.
[90]A.Johansson,J.Staal,C.Dixelius,Early responses in the Arabidopsis-Verticillium longisporum pathosystem are dependent on NDR1,JA-and ET associated ET associated signals via cytosolic NPR1 and RFO1,Mol.Plant-Microbe Interact.19(2006)958–969.
[91]B.A.Adie,J.Pérez-Pérez,M.M.Pérez-Pérez,M.Godoy,J.J.Sánchez-Serrano,E.A.Schmelz,R.Solano,ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis,Plant Cell 19(2007)1665–1681.
[92]S.H.Spoel,J.S.Johnson,X.Dong,Regulation of tradeoffs between plant defenses against pathogens with different lifestyles,Proc.Natl.Acad.Sci.U.S.A.104(2007)18842–18847.
[93]R.Makandar,V.J.Nalam,H.Lee,H.N.Trick,Y.Dong,J.Shah,Salicylic acid regulates basal resistance to Fusarium head blight in wheat,Mol.Plant-Microbe Interact.25(2012)431–439.
[94]H.I.Lee,J.León,I.Raskin,Biosynthesis and metabolism of salicylic acid,Proc.Natl.Acad.Sci.U.S.A.92(1995)4076–4079.
[95]M.C.Wildermuth,J.Dewdney,G.Wu,F.M.Ausubel,Isochorismate synthase is required to synthesize salicylic acid for plant defense,Nature 414(2001)562–565.
- The Crop Journal的其它文章
- The landscape of molecular mechanisms for salt tolerance in wheat
- Genetic improvement of heat tolerance in wheat:Recent progress in understanding the underlying molecular mechanisms
- Wheat genome editing expedited by efficient transformation techniques: Progress and perspectives
- Corrigendum to “A new method for evaluating the drought tolerance of upland rice cultivars”[Crop J. 5 (2017) 488–489]
- Mapping stripe rust resistance genes by BSR-Seq:YrMM58 and YrHY1 on chromosome 2AS in Chinese wheat lines Mengmai 58 and Huaiyang 1 are Yr17
- Wheat breeding in the hometown of Chinese Spring

