基于咪唑基配体的Mnギ配合物的水热合成及结构、理论计算和三阶非线性光学性质
张载超 唐果东*,, 汤婷婷 Lance F.Culnane张 宇 宋瑛林 李荣清 夏 敏
(1淮阴师范学院化学化工学院,江苏省低维材料化学重点实验室,淮安 223300)
(2Department of Chemistry and Biochemistry,University of Colorado,Boulder,CO 80309,USA)
(3苏州大学物理科学与技术学院,苏州 215006)
0 Introduction
Recently,interest in the third-order nonlinear optical (NLO)materials has been rapidly growing because of their potential applications in various fields ranging from laser technology,optical communication,and optical data storage to optical signal processing[1-6].From a technological point of view,having a class of materials with inherently high nonlinearity,synthetic flexibility,high laser damage threshold and tunability afforded by functional substitutions make the organic materials highly attractive[7-10].However,most of the organic NLO crystals usually have poor mechanical and thermal properties and are susceptible to damage during processing even though they have high NLO efficiency.Additionally,it is difficult to grow largesize and optical-quality crystals of these materials for device applications.Purely inorganic NLO materials have excellent mechanical and thermal properties but possess relatively modest optical nonlinearity because of a lack of extended π-electron delocalization[11-16].Employed as inorganic-organic hybrid materials,metal-organic complexeshave attracted a lotof attention considering the possibility of tuning their NLO response by appropriate design at the molecular level,while remaining relatively stable.During the preparation of the inorganic-organic composite materials,it is quite important to conduct the careful selection of a suitable organic ligand and functional metal ion. Among the organic ligands,large conjugated ligands,such as imidazole derivatives,have been frequently used as building blocks in the synthesis of the functional molecular materials,due to their interesting electronic and photonic properties.
The ligand 1,10-phenanthroline has played a particularly important role in the structural designs of supramolecular networks because of its imbedded πelectron system[17].A series of 1,10-phenanthroline derivatives have been synthesized and characterized.Accordingly,investigations of their applications have been reported in areas of chemistry,physics,biology and material science[18-20].Up to now,1,10-phenanthroline and its derivatives have attracted particular attention because oftheirunique properties as chelating agents,and are commonly used as ligands in metal-organic coordination polymers[21-23].1,10-phenanthroline and substituted derivatives play a key role in the performance of biological systems,and act as antimalarial,antifungal,antitumoral,anti-allergic,antiinflammatory and antiviral drugs[24-26].The DNA-binding,DNA-photocleavage,luminescent material,and asymmetric catalysis of 1,10-phenanthroline derivatives and their metal complexes were also reported[27-28].
4-(1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)benzaldehyde (IPB)is one of the derivatives of 1,10-phenanthroline,and the structure of it has been studied for many years[29-35].However,detailed NLO properties of the its complexes have not been reported by far.To further develop this promising field and search for better NLO materials,we selected the ligand IPB as a candidate with potential NLO properties in that IPB has a large conjugated system and strong coordination with two nitrogen atoms and an oxygen atom,and can combine with a variety of metals.Herein,we have synthesized the complex with phenanthrolin and imidazoleskeletons,and have characterized the structure by X-ray crystallography,meanwhile,the IR,UV-Vis,fluorescence,NLO properties,and theoretical calculations are investigated.
1 Experimental and theoretical calculation
1.1 General
Phenanthroline and terephthalaldehyde were purchased from Aldrich Chemical Company and were used as received;ammonium acetate and glacial acetic acid were purchased from Nanjing Chemistry Reagent Limited Company;dimethyl sulfoxide (DMSO)was purchased from Shanghai Chemistry Reagent Limited Company.Solvents were purified and dried according to standard procedure.1,10 phenanthroline-5,6-dione was synthesized according to our previous work[36].
1.2 Synthesis
1.2.1 Synthesis of 4-(1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)benzaldehyde
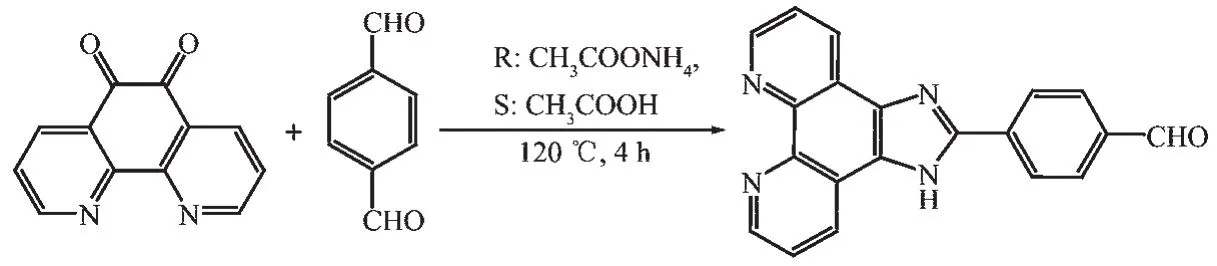
Scheme 1 Synthetic route for ligand IPB
The synthetic route is shown in Scheme 1.1,10 phenanthroline-5,6-dione (2.5 mmol,0.525 g),terephthalaldehyde (3.5 mmol,0.469 g)and ammonium acetate (50 mmol,3.80 g)were refluxed in glacial acetic acid (15 mL)for 2 h.The mixture was poured into 100 mL deionized ice water after cooling,and then the pH value was adjusted to about 6 with aqueous ammonia.Yellow solid was generated and recrystallized with ethanol.Yield:89%.Elemental Anal.Calcd.for C20N4OH12(%):C,74.06;H,3.73;N,17.27.Found(%):C,74.11;H,3.63;N,17.33.IR (KBr,cm-1):3 357 (s),2 852 (vs),2 768 (vs),1 695 (vs),1 607(vs).1H NMR (DMSO-d6):δ 13.05 (s,1H),12.95 (s,1H),7.53 (t,J=7.2 Hz 2H),7.49 (t,J=7.2 Hz 2H),7.42 (d,1H),7.35 (t,2H),7.27 (t,1H),6.98 (d,J=9.1 Hz 1H),6.94 (d,J=9.1 Hz 1H).UV-Vis (DMSO)λmax/nm:282(ε=3.33×104L·mol-1·cm-1).
1.2.2 Synthesis of complex Mn[(IPB)2Cl2]·4H2O
A mixture of the ligand IPB (0.2 mmol,0.064 8 g)and MnCl2·4H2O (0.5 mmol,0.099 8 g)were dissolved in deionized water (15 mL)and heated to 150 ℃ under autogenous pressure in a Teflon-lined steel bomb(25 mL)for 3 days,followed by slow cooling (10 ℃·h-1)to room temperature to afford yellow-colored block-shaped crystals.Elemental Anal.Calcd.for C40N8O5H32MnCl2(%):C,56.75;H,3.75;N,13.23.Found(%):C,56.88;H,3.31;N,13.56.IR (KBr,cm-1):ν(Mn-N)478.28 (vs).
1.3 Chemical and physical measurements
The FT-IR spectrum of the title complex was recorded as KBr discs using an AVATAR 360 spectrophotometer in the range of 400~4 000 cm-1at room temperature.1H NMR spectra was recorded in DMSO-d6solution on a Bruker Advance 400 MHz spectrometer.The electronic spectra was recorded on a UVVis 916 spectrophotometer in the region of 200~400 nm using DMSO as solvent.Elemental analysis was performed on a Perkin-Elmer 2400 LS elemental analyzer.The thermal gravimetric analysis was carried out with a NETZSCH STA 449F3 thermal analyzer with a heating rate of 10 K·min-1.
X-ray crystal diffraction measurements of the crystal was performed on a Bruker Smart Apex2 CCD diffractometer at 296 K.The intensity data were collected using graphite monochromatic Mo Kα radiation(λ=0.071 073 nm).No significant decay was observed during the data collection.The raw data were processed to give structure factors using the SAINT-plus program[37].Empirical absorption corrections were applied to the data sets using SADABS[38].The structure was solved by direction method and refined by full matrix least-squares against F2for all data using SHELXTL-97 software[39].All non-hydrogen atoms in the complex were anisotropically refined.Hydrogen atoms connected to C and N atoms were included in the calculated positions.The positions of H atoms in H2O were fixed according to the directions of corresponding H-bondings.All the H atoms were refined using a riding model with the isotropic thermal parameters of H atoms 1.2 times larger than those of the parent atoms.The crystal data,further details of the experimental conditions,and the structure refinement parameters for the complex are given in Table 1.
CCDC:1495344.
All optical measurements were conducted at room temperature with the complex dissolved in DMSO.TheNLO propertiesweremeasured as described in the literature[40],and were evaluated by the Z-scan technique with a pulse width of 4 ns at 1 064 nm and a 10 Hz repetition rate generated from a Q-switched frequency-doubled Nd∶YAG laser.The spatial profiles of the optical pulses were nearly Gaussian.The pulsed laser beam was focused onto the sample cell by use of a focusing mirror(focal length 30 cm).The spot radius of the laser beam was measured to be 55 μm.The energy of incident pulsesvaried bya NewportCom.attenuatorand was measured with a laser detector (Rjp-735 energy probe),which was linked to a computer by an IEEE interface,and simultaneously,the transmitted energy was gauged with another detector.The interval between the laser pulses was chosen to be 1s to avoid the long-term effects.The third-order NLO absorptive and refractive behaviors of title complex was determined by taking advantage of the Z-scan technique[41-42].The sample was mounted on a computer-controlled translation stage,which moved along the axis of the incident laser beam (Z-direction)with respect to the focal point.A 0.2 mm diameter aperture was installed in front of the transmission detector for determination of the sign and magnitude of the nonlinear refraction(closedaperture Z-scan)whereas for nonlinear absorption measurements,no aperture was applied (open-aperture Z-scan).Transmittance was recorded as a function of the sample position on the Z-axis.
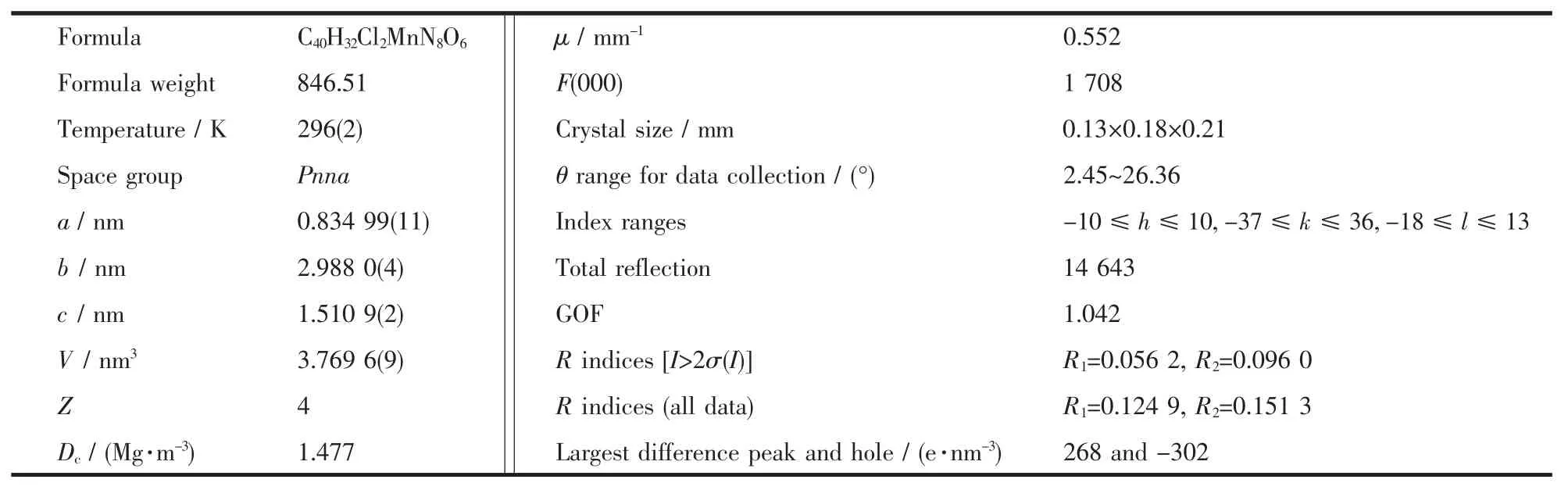
Table 1 Crystal data and structure refinement for complex
1.4 Methods of calculation
Geometry optimization is one ofthe most important steps in the theoretical calculations.This procedure proceeded in two steps.First,the initial geometry was constructed by MM+molecular modeling with the HyperChem 6.0 package[43].Then,the geometry was optimized by density functional theory (DFT)with LANL2DZ (Los Alamos ECP plus double-zeta)[44-45]basis sets.The most stable structure was found to be a minimum since there was no imaginary frequency in the frequency calculation.Lastly,the molecular orbital(MO)calculations were completed.All calculations were performed using the Gaussian 09W program package[46].
2 Results and discussion
2.1 Structure description
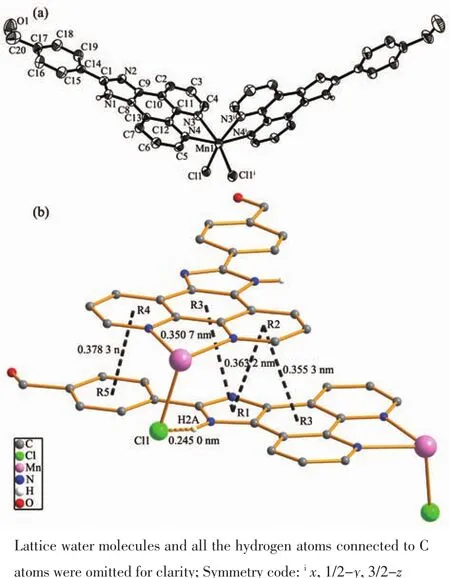
Fig.1 (a)Asymmetric unit of complex Mn[(IPB)2Cl2]·4H2O with 30%ellipsoid probability level;(b)Intermolecular weak interaction in the crystal of Mn[(IPB)2Cl2]·4H2O
The complex structure was determined by singlecrystal X-ray diffraction.The molecular configuration of complex is displayed in Fig.1.The crystallographic data and structure refinement of the complex is listed in Table 1.The crystal system of the complex is orthorhombic,and the space group is Pnna.The coordination sphere around the Mn1 center (Fig.1a)is a distorted octahedral structure,and four nitrogen atoms(N3,N4,N3i,N4i)from two IPB ligands and two chlorine atoms (Cl1,Cl1i)coordinate to the Mn1 center.The Mn-Cl distances of title complex (0.251 5(17)nm)is slightly longer than the corresponding reported bond length (0.249 6 nm).The Mn-N distances of the complex range from 0.226 3(4)to 0.230 3(5)nm,which are slightly shorter than the corresponding reported bond lengths (0.232 0~0.235 5 nm)[47].The binding angle of coordinating nitrogen in the IPB ligand,NMn-N and N-Mn-Cl (N3-Mn1-N4,N3-Mn1-Cl1,N3-Mn1-Cl1i,N4-Mn1-N4i,N4-Mn1-Cl1 and N4-Mn1-Cl1i),are 72.66(16)°,87.94(12)°,164.40(11)°,157.6(2)°,91.89(12)° and 103.44(12)°,respectively.Selected bond lengths and bond angles are listed in Table 2.
It is worth mentioning that the IPB ligands of two adjacent molecules in complex are essentially parallel and display an intermolecular weak interaction(Fig.1b).The intermolecular weak interaction between the R1-R2 ring (R1:N1-C1-N2-C9-C8,R2:N4-C5-C6-C7-C13-C12)has an interplanar separation of 0.363 2 nm,and the dihedral angle is 1.256°.For the R1-R3 ring (R3:C8-C9-C10-C11-C12-C13),the interplanar separation is approximately 0.350 7 nm,and the dihedral angle is 1.413°.For the R2-R3 ring and the R4-R5 ring (R4:N3-C4-C3-C2-C10-C11,R5:C14-C15-C16-C17-C18-C19),the interplanar separations are approximately 0.355 3 and 0.378 3 nm,respectively,and the dihedral angles are 1.892°and 3.783°,respectively.There is a hydrogen bond between the N atom of the imidazole ring and the adjacent Cl atom N1-H1A…Cl1 (0.238 0 nm,161.00°).The other hydrogen bond data are listed in Table 3.
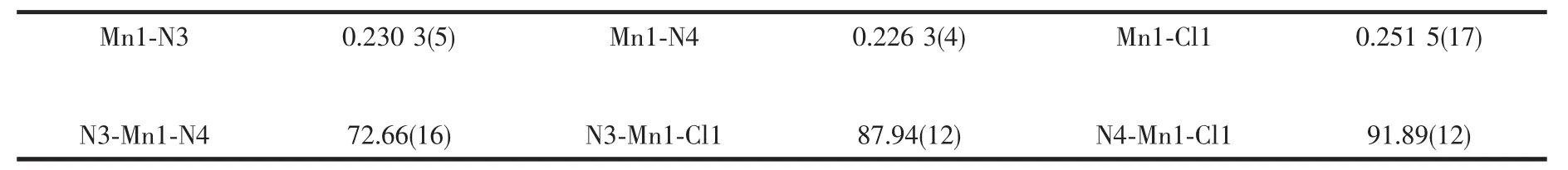
Table 2 Selected bond distances(nm)and bond angles(°)for the complex
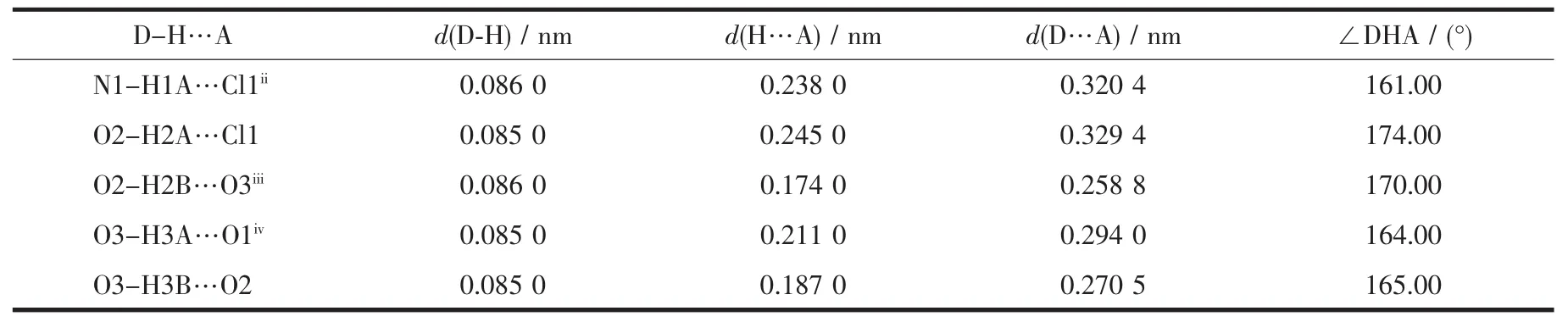
Table 3 Hydrogen bond parameters for the complex
2.2 Spectral analysis
The electronic absorption spectrum of the complex measured in DMSO solution at room temperature is displayed in Fig.2.It shows that the spectra range from 400 to 500 nm and have almost negligible linear absorption in the visible region.In the complex,there are three absorption peaks at 268 nm,282 nm,and 361 nm.The absorption peak at 268 nm (ε=7.90×104L·mol-1·cm-1)is a shoulder peak,the maximum absorption wavelength at 282 nm (ε=9.15×104L·mol-1·cm-1)can be assigned to the benzene absorption,and the absorption peak at 361 nm (ε=8.74×104L·mol-1·cm-1)can be assigned to the L→L charge transfer(LLCT,n-π*and π-π*transition)for the ligand.
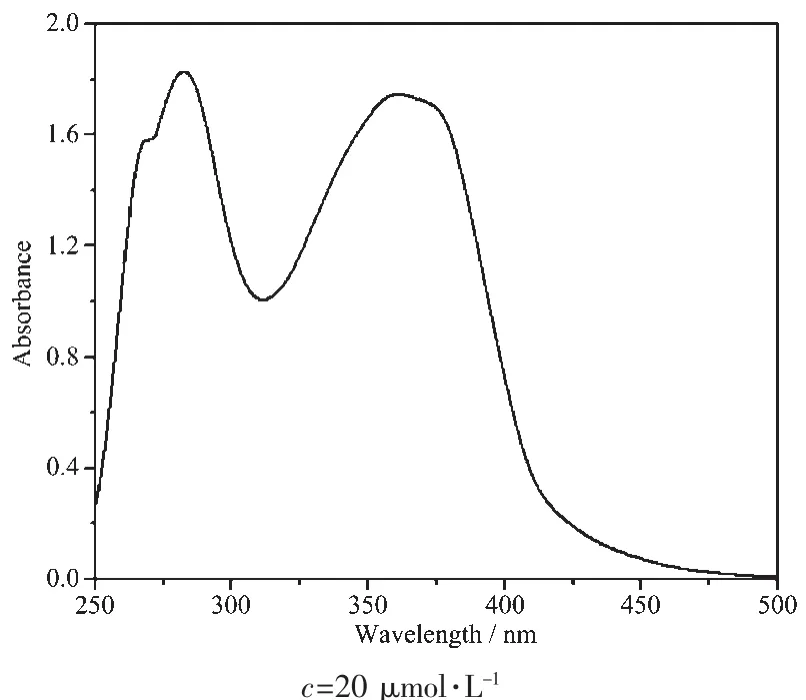
Fig.2 UV-Vis spectrum of the complex in DMSO solution
Photoluminescence is very important in photochemistry and photophysics.Interestingly,the photoluminescence of the complex and the free ligand,IPB,were measured in the 2 μmol·L-1DMSO solution,as shown in Fig.S1.The fluorescent emission peaks are at 408 and 429 nm for the ligand IPB (λex=287 nm).The complex has no fluorescence properties,because the metal quenches the fluorescence[48-49].
2.3 Nonlinear optical property
The third-order NLO property of the DMSO solution of title complex was investigated with 1064 nm laser pulses of 4 ns duration by Z-scan experimentation.The nonlinear absorption components of the complex was evaluated by the Z-scan method under an open-aperture configuration and the experimental NLO absorptive data for title complex was obtained(Fig.3).They clearly illustrate that the absorption increases as the incident light irradiance rises.The normalized transmittance isabout93% fortitle complex at 45 μJ per pulse at the focal point,and the nonlinear absorption coefficient β is-4.5 × 10-11m·W-1for the complex.It is obvious that the complex exhibits strong NLO absorption.The third-order NLO absorption data can be well-represented by Eq. (1)and (2)[41,50],which describe a third-order NLO absorptive process:
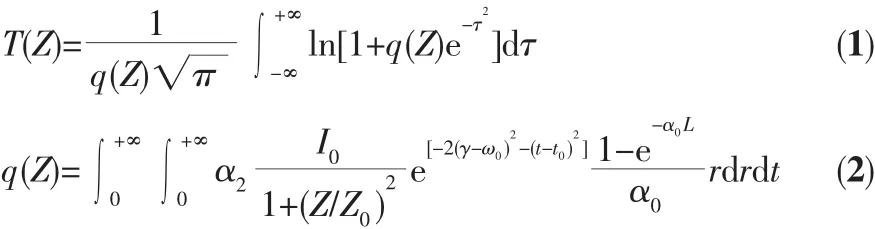
where α0and α2are linear and effective third-order NLO absorptive coefficients;light transmittance T is a function of the sample′s Z-position (with respect to focal point Z=0);Z is the distance of the complex sample from the focalpoint;L isthe sample thickness;I0is the peak irradiation intensity at focus;Z0=πω02/λ,where ω0is the spot radius of the laser pulse at focus and λ is the laser wavelength;r is the radial coordinates;t is the time,and t0is the pulse width.
As shown in Fig.3,a reasonably good fit between the experimentaldataand the theoreticalcurve suggests that the experimentally-obtained NLO effects are third-order in nature.The figures clearly illustrate that the absorption increases as the incident light irradiance rises,since light transmittance (T)is a function of the sample′s Z position.
From Fig.3a,the nonlinear refraction is found to have a negative sign for the complex indicating that the complex exhibits self-focusing property.The change of transmittance is very large in the open aperture case (Fig.3a),which signifies that the complex has strong excited-state absorption,and reverse saturable absorption (RSA)at532 nm.The theoretical explanation of these phenomena has been reported[51].
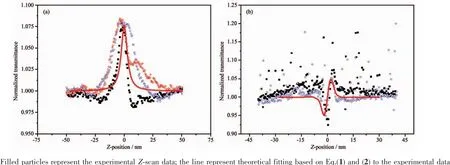
Fig.3 Z-Scan measurement of complex in 1.1 mmol·L-1DMSO solution:(a)data collected under the close-aperture configuration;(b)data obtained by dividing the normalized Z-scan data obtained under the open-aperture configuration by the normalized Z-scan data
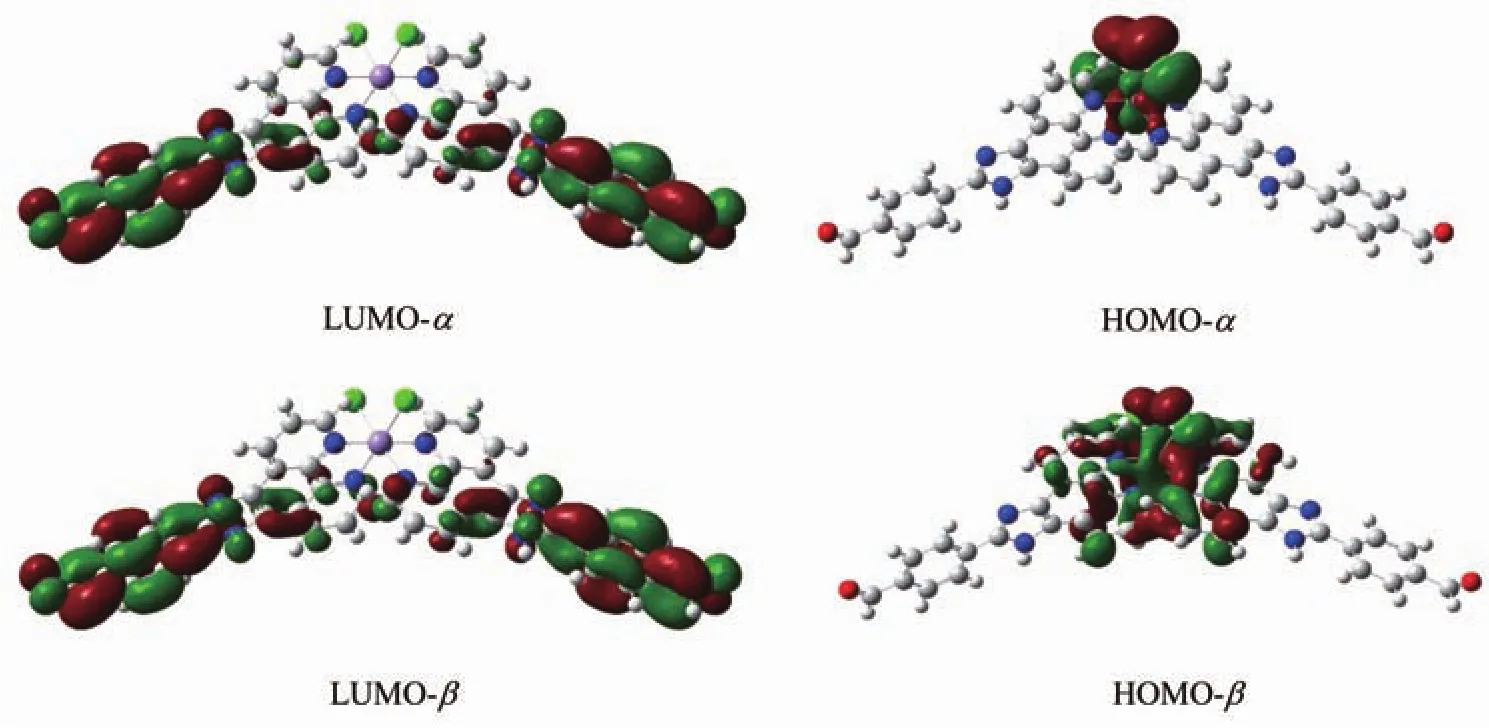
Fig.4 Examples of HOMOs and LUMOs of the complex
In DMSO solution,the complex shows strong NLO absorptive properties as depicted in Fig.3b.The obtained normalized transmittance with the peak followed by the valley manifests that the sign of the nonlinear refractive index is positive,i.e.there may be self-focusing in DMSO solutions of the complex,and the nonlinear refractive index,γ,of the complex is 1.0 ×10-19m2·W-1.
In order to explore and understand the relationships between the molecular composition and NLO properties of the complex,the frontier molecules orbitals of the complex was calculated (Fig.4).
According to the frontiermolecularorbital theory,the orbitals of the highest occupied molecular orbital (HOMO)and the lowest unoccupied molecular orbital (LUMO)are important to the electronic propertiesofthe complexes.TheHOMO orbitalis primarily composed of contributions from d orbitals on the Mn atom and the lone-pair electrons on N atoms of the phenanthroline ring.While the LUMO orbital is mainly composed of the phenyl ring on the 4-aldehyde group,imidazole ring,and has a small contribution from the other benzene ring in the ligand.
Generallyspeaking,theNLO propertiesare highly affected by the energy gap between HOMO and LUMO,and the smaller the energy gap,the better the NLO performance[52].The theoretical calculations show that the title complex tends to have the smallest energy gap (1.32 eV),which result in the fact that it may possess good NLO properties.This is consistent with the experimental NLO results.
3 Conclusions
The complex,Mn[(IPB)2Cl2]·4H2O (IPB=4-(1H-imidazo[4,5-f][1,10]phenanthrolin-2-yl)benzaldehyde),was synthesized and characterized.The central Mn ion within the title complex molecule shows a distorted octahedralenvironment.The UV-Vis and NLO properties are also reported.The results can be summarized as follows: (1)In DMSO solutions,the complex display strong NLO absorptive properties.(2)The normalized transmittance is about 93%with a 45 μJ pulse at the focal point,and the nonlinear absorption coefficient,β,is-4.5×10-11m·W-1,the third-order nonlinear refraction index,γ,is 1.0×10-19m2·W-1for the complex,exhibits self-focusing property.(3)In the UV-Vis spectrum,the maximum absorption wavelength is at 282 nm (ε=9.15×104L·mol-1·cm-1).The results seem to suggestthatmetal-organic complexes indeed exhibit a promising combination of tunability and stability,and might be appropriate candidates for the development of NLO materials.
Supporting information is available at http://www.wjhxxb.cn
[1]Alam M Z,Leon I D,Boyd R W.Science,2016,352:795-797
[2]Chelucci G,Thummel R.Chem.Rev.,2002,102:3129-3170
[3]Nakano M,Champagne B.J.Phys.Chem.Lett.,2015,6:3236-3256
[4]Jayabharathi J,Thanikachalam V,Perumal M V.Spectrochim.Acta Part A,2012,95:614-621
[5]SONG Ying-Lin(宋瑛林),LI Zhong-Guo(李中国).Infrared Laser Engineering(红外与激光工程),2017,46(5):1-5
[6]OU Yong-Cong(区泳聪),ZHONG Jun-Xing(钟均星),SONG Ying-Yi(宋萦怡),et al.Chinese J.Inorg.Chem.(无机化学学报),2016,32(4):738-744
[7]Shanthi D,Selvarajan P,HemaDurga K K,et al.Spectrochim.Acta Part A,2013,110:1-6
[8]Manivannan S,Dhanuskodi S.J.Cryst.Growth,2014,262:473-478
[9]Demir S,Tinmaz F,Dege N,et al.J.Mol.Struct.,2016,1108:637-648
[10]Jagadeesha M R,Kumarb H M S,Kumari R A.Optik,2015,126:4014-4018
[11]Suresh P,Janarthanan S,Samuel R S,et al.Spectrochim.Acta Part A,2015,135:732-735
[12]Govindasamy P,Gunasekaran S.Spectrochim.Acta Part A,2015,136:1543-1556
[13]Wang L,Ni L,Yao J.Solid State Sci.,2012,14:1361-1366
[14]Wang X L,Chen Y Q,Liu G C,et al.Inorg.Chem.Acta,2010,363:773-778
[15]Chen T F,Liu Y A,Zheng W J,et al.Inorg.Chem.,2010,49:6366-6368
[16]Li Z D,Wu K C,Su G B,et al.Opt.Mater.,2002,20:295-299
[17]Biswas P,Dutta S,Ghosh M.Polyhedron,2008,27:2105-2112
[18]Jiang C W,Chao H,Li R H,et al.Polyhedron,2001,20:2187-2193
[19]Gao J,Wang Z P,Yuan C L,et al.Spectrochim.Acta Part A,2011,79:1815-1822
[20]WANG Run-Xue(王润雪),WANG Shu-Wen(王姝文),QI Yan-Juan(齐艳娟).Chinese J.Inorg.Chem.(无机化学学报),2012,28(3):536-540
[21]Bian Z Q,Wang K Z,Jin L P.Polyhedron,2002,21:313-319[22]Zhang Q L,Liu J G,Liu J,et al.J.Inorg.Biochem.,2001,85:291-296
[23]Shavaleev N M,Adams H,Weinstein J A.Inorg.Chem.Acta,2007,360:700-704
[24]Xu Z D,Liu H,Xiao S L,et al.J.Inorg.Biochem.,2002,90:79-84
[25]Sun D D,Wang W Z,Mao J W,et al.Bioorg.Med.Chem.Lett.,2012,22:102-105
[26]Khan I M,Ahmad A,Aatif M.J.Photochem.Photobiol.B,2011,105:6-13
[27]Tan L F,Chao H,Zhou Y F,et al.Polyhedron,2007,26:3029-3036
[28]Chelucci G,Thummel R P.Chem.Rev.,2002,102:3129-3170
[29]Koike M,Murakami K,Fujinami T,et al.Inorg.Chim.Acta,2013,399:185-192
[30]Arnold U,Walter O,Dring M.Inorg.Chim.Acta,2006,359:327-333
[31]Jagenbrein M,Monakhov K,Braunstein P.J.Organomet.Chem.,2015,796:11-16
[32]Ghosh A,Rao K P,Sanguramath R A,et al.J.Mol.Struct.,2009,927:37-42
[33]Mei H X,Huang H Q,Zhang T,et al.J.Mol.Struct.,2016,1107:266-277
[34]Salah A M B,Nali H,Arczyński M,et al.J.Organomet.Chem.,2016,805:42-48
[35]Keum C,Kim M C,Lee S Y.J.Mol.Catal.A Chem.,2015,408:69-74
[36]ZHENG Ren-Hua(郑 人 华 ),JIANG Hua-Jiang(蒋 华 江 ).Journal of Taizhou University(台州学院学报),2004,26(6):68-70
[37]APEX2 and SAINT,Bruker AXS Inc.Madison Wisconsin,USA,2008.
[38]Sheldrick G M.Acta Crystallogr.Sect.A:Found.Crystallogr.,2008,A64:112-122
[39]Sheldrick G M.Acta Crystallogr.Sect.C:Cryst.Struct.Commun.,2015,C71:3-8
[40]Zhang C,Song Y L,Xu Y,et al.Inorg.Chim.Acta,2000,311:25-32
[41]Bahae M S,Said A A,Wei T H,et al.IEEE J.Quantum Electron.,1990,26:760-769
[42]Hou H W,Xin X Q,Liu J,et al.J.Chem.Soc.Dalton Trans.,1994:3211-3214
[43]Hyper Chem Pro.Release 6.03,Hypercube Inc.,USA,2000.
[44]Zhanpeisov N U,Fukumura H.J.Phys.Chem.C,2007,111:16941-16945
[45]Nicklass A,Dolg M,Stoll H,et al.J.Chem.Phys.,1995,102:8942-8952
[46]Frisch M J,Trucks G W,Schlegel H B.Gaussian 09,Revision B.03,Gaussian Inc.,Pittsburgh PA,2009.
[47]Zhu P,Li H M.J.Mol.Struct.,2011,992:106-110
[48]Alexiev A,Rubio S,Deyanove M.Anal.Chim.Acta,1994,295:211-219
[49]Ci Y X,Li Y Z,Chang W B.Anal.Chim.Acta,1991,248:589-594
[50]Said A A,Sherk-Bahae M,Hagan D J,et al.J.Opt.Soc.Am.B:Opt.Phys.,1992,9:405-414
[51]Li K,Tang G D,Kou S S,et al.Spectrochim.Acta Part A,2015,139:54-62
[52]Tang G D ,Cao Y,Zhang J F,et al.Synth.Met.,2008,158:264-272

