ReaxFF Reactive Molecular Dynamics Simulation of the Oxidation of Silicon-Doped Amorphous Carbon Film in Heat-Assisted Magnetic Recording
LIU Qing-Kang SONG Wen-Ping HUANG Qi-Tao ZHANG Guang-Yu HOU Zhen-Xiu
ReaxFF Reactive Molecular Dynamics Simulation of the Oxidation of Silicon-Doped Amorphous Carbon Film in Heat-Assisted Magnetic Recording
LIU Qing-Kang*SONG Wen-Ping*HUANG Qi-Tao ZHANG Guang-Yu HOU Zhen-Xiu
()
Heat-assisted magnetic recording (HAMR) is one of the promising ways to extend the magnetic recording area density to 1 Tb·in−2in hard disk drives (HDDs). High temperature induced by laser heating can cause carbon overcoat (COC) oxidation. Reactive molecular dynamics (MD) simulations are performed to investigate the oxidation process of silicon-doped amorphous carbon (a-C:Si) films for HAMR application. The atomic details of the structure evolution and oxidation process are investigated, and, the oxidation mechanism of the a-C:Si film is clarified. The effect of the duration of laser irradiation on the oxidation of the a-C:Si film is investigated. The oxidation occurs during heating and the beginning of cooling process. Both volume expansion during heating process and cluster of carbon atoms during cooling process increase the rate of2carbon. Because of the decrease in the amount of unsaturated silicon atoms and low diffusion coefficient of atomic oxygen, the oxidation rate of the a-C:Si film decreases with laser irradiation cycles. The molecular oxygen is the oxidant due to surface defect of a-C:Si film. The atomic strains break the O―O bonds in Si―O―O―Si linkages and rearrange the surface oxide layers, and process the oxidation of the a-C:Si film.
Heat-assisted magnetic recording; Silicon-doped amorphous carbon; Oxidation; ReaxFF; Molecular dynamics simulations
1 Introduction
Heat-assisted magnetic recording (HAMR) is one type of techniques which is able to extend the magnetic recording area density to 1 Tb∙in−2in HDDs1,2. The pulsed laser is used in HAMR to temporarily heat the magnetic recording media above the Curie temperature (> 700 K) for a heating rate of around 1011–1012K∙s−13. The amorphous carbon (a-C) deposited on the magnetic recording media can be weakened or damaged due to laser heating, such as graphitization or oxidation4–8. The doping of silicon into a-C (a-C:Si) can improve the thermal stability9. This makes a-C:Si film potentially being used as a protective layer for HAMR applications. Hence, understanding the oxidation process of a-C:Si films induced by pulsed laser is crucial to improve the reliability in HDDs.
The a-C:Si film doped by 12.8% and 19.2% (atomic fraction) of silicon was thermal stability annealed at 400 °C in furnace, and an increase in the rate of silicon can increase the graphitization temperature of a-C:Si film9,10. This phenomenon also was found in Er’s work, and they found the thickness loss induced by oxidation decreased with increasing the rate of silicon11. The silicon oxide layer on the film surface can improve the thermal stability12. The oxidation process of a-C:Si was investigated experimentally by Kim.13The carbon escaped from the film in the form of CO or CO2, and the oxygen penetrated into the film. Nonetheless, the reasons of this phenomenon are not clear. The thickness of a-C:Si films in the works above is more than 100 nm which is much thicker than that in HDDs. In addition, all the experiments researched on the thermal stability and oxidation of a-C:Si are carried out at fixed temperature, this is not consistent with the HAMR condition. However, oxidation process of ultra-thin a-C:Si films with ultra-fast heating and cooling rate have not been investigated experimentally. There are a lot of difficulties in experimentally studying the oxidation process of a-C:Si films because of the superfast heating rates and the nanoscale thickness.
Molecular dynamics (MD) simulation is a promising way to study the oxidation process of a-C:Si film induced by laser with reactive force field. It has been proved by Newsome14that the oxidation processes of silicon carbide at various temperatures (in the range of 500 to 5000 K) in the O2and H2O environment can occur naturally with the ReaxFF reactive force field, which can calculate accurately the O2and H2O oxidation rateconstant15and activation barriers of polar faces of 3C-SiC16during the oxidation processes. The oxidation mechanism of SiC with thick silicon dioxide layer at ~1450 K was investigated using first-principles method17. The simulation conditions in works above also are conducted at fixed temperatures which are not consistent with superfast heating and cooling process in HAMR. The difference between the amorphous structure and crystal structure can affect the oxidation process. The works focus on the oxidation of ultrathin a-C:Si film under super-fast laser irradiation cycles are scarce. It is necessary to understand the oxidation process of a-C:Si film in atomic level.
In this work, the oxidation processes of a-C:Si film for the HAMR application are investigated by MD simulations using the ReaxFF reactive force field. The a-C:Si film is heated form 300 K to 800 K and cooled down to 300 K. The effect of irradiation duration is studied. The structure evolution and oxidation mechanism of a-C:Si film are analyzed in details.
2 Modeling and simulation process
The reactions with ReaxFF reactive force field18in MD simulations can occur naturally with time following the initial conditions, such as number of atoms, pressure and temperature. ReaxFF reactive force field18have been widely used to study the oxidation processes of systems, such as hydrocarbon19, silicon/silicon dioxide20, RP-321, epoxy resin22,-nitrophenol23and Octanitrocubane24. In this study, the ReaxFF reactive force field which parameters were obtained by fitting against experimental data and quantum mechanics (QM)14was used to assess the full oxidation process of a-C:Si film.
By considering the computational expense without affecting the results, the a-C:Si film was simplified to a slab in this study. The a-C:Si slab with density around 2.96 g∙cm−3was modeled by liquid quenching method25. The content of silicon atoms was 20% (atomic fraction). The dimensions of a-C:Si slab along,anddirections were 2 nm, 2 nm and 2.1 nm, respectively. The length of simulation cell along the-axis direction was extended to 5.1 nm. The atmosphere was simplified to be pure oxygen environment. 200 O2molecules were randomly placed in the rest of simulation cell. Periodic boundary conditions (PBCs) were applied in all three directions. The cutoff distance of ReaxFF reactive force field was set to be 1 nm. The cutoff distances of O―O bonds, C―O bonds, C―C bonds and Si―O bonds were set as 1.8 nm, 1.7 nm, 1.73 nm and 2.1 nm, respectively14. The bonds can be elongated by thermal vibration, so the distances are slightly higher than standard bond lengths.
The system was firstly relaxed at 300 K in a canonical (NVT) ensemble controlled by Nose-Hoover thermostat at a time step of 0.25 fs for 1000 ps. This process minimizes energy of system and makes the oxygen molecules well-distributed on the surface of a-C:Si slab. The atomic configuration of system after the relaxation process at 300 K is shown in Fig.1(a). After the relaxation process, the model was heated from 300 K to 800 K, and then cooled down to 300 K. The heating rate and cooling rate were set as 1012K·s−1and 1010K·s−1, respectively. With cooling rate of 1010K·s−1, the system temperature can cool down naturally from 800 K to 300 K within 50 ns, this is consistent with laser off time in Ma’s work3, and the same cooling rate was used in our early work5. The evolution of potential energy and number of Si―O and C―O bonds were used to analyze the oxidation process of a-C:Si slab in HAMR application. The structure evolution of a-C:Si film was studied by radial distribution function (RDF). The visualization and analysis are conducted by OVITO26.
3 Results and discussion
3.1 The oxidation process of a-C:Si slab during the first laser irradiation cycle
In the relaxation process, the oxygen molecules bond with unsaturated silicon and carbon atoms in the form of Si―O―O―Si, C―O―O and Si―O―O linkages. The number of Si―O and C―O bonds fluctuates around 37 and 6 after the relaxation process, respectively. The atomic configurations of the a-C:Si slab in the first laser irradiation cycle at three different temperatures are shown in Fig.1. The atomic configuration after the relaxation process at 300 K is shown in Fig.1(a). When the a-C:Si slab is heated from 300 K to 800 K, the thicknesses of surface oxide layers and a-C:Si slab increase, as shown in Fig.1(b). The voids are formed and few oxygen atoms diffuse into the a-C:Si slab. The atomic configuration of a-C:Si slab does not change obviously when the system is cooled down to 300 K, as shown in Fig.1(c). More2carbons atoms are found near the surface oxide layer. Only one CO is emitted into the oxygen molecules environment. The emitting of CO into oxygen molecules environment and diffusion of atomic oxygen into a-C:Si slab are consistent with the experiment11. Because of low temperature, the CO is not oxidized to CO2. The central part of a-C:Si slab which does not contain the oxygen atoms is set as unoxidized a-C:Si slab with a thickness of 1.6 nm. The structure evolution of a-C:Si slab can be inferred from the unoxidized a-C:Si slab.
Fig.2 shows the evolution of system potential energy and number of Si―O and C―O bonds as a function of temperature in the first laser irradiation cycle. In the heating process, the potential energy decreases at around 450 K and 590 K, and decreases slowly when temperature is higher than 690 K. It keeps decreasing in the whole cooling process, even the temperature is lower than 600 K. The number of Si―O bonds and C―O bonds increases when temperature is above 450 K in the heating process. The number of Si―O and C―O bonds increases when system cools down from 800 K to 600 K. When the temperature is lower than 600 K, the number of Si―O and C―O bonds fluctuates around 90 and 20, respectively. The number of Si―O bonds is more than C―O bonds. According to the solid-state chemistry, the silicon atoms have high oxidation potential energy than carbon, oxygen atoms bond preferentially with silicon atoms. The silicon atoms are the main reducer in the oxidation process of a-C:Si slab. The oxidation state of a-C:Si slab can be inferred from the number of Si―O bonds and C―O bonds. The oxidation of a-C:Si slab mainly occurs in the heating process and at the beginning of cooling process.
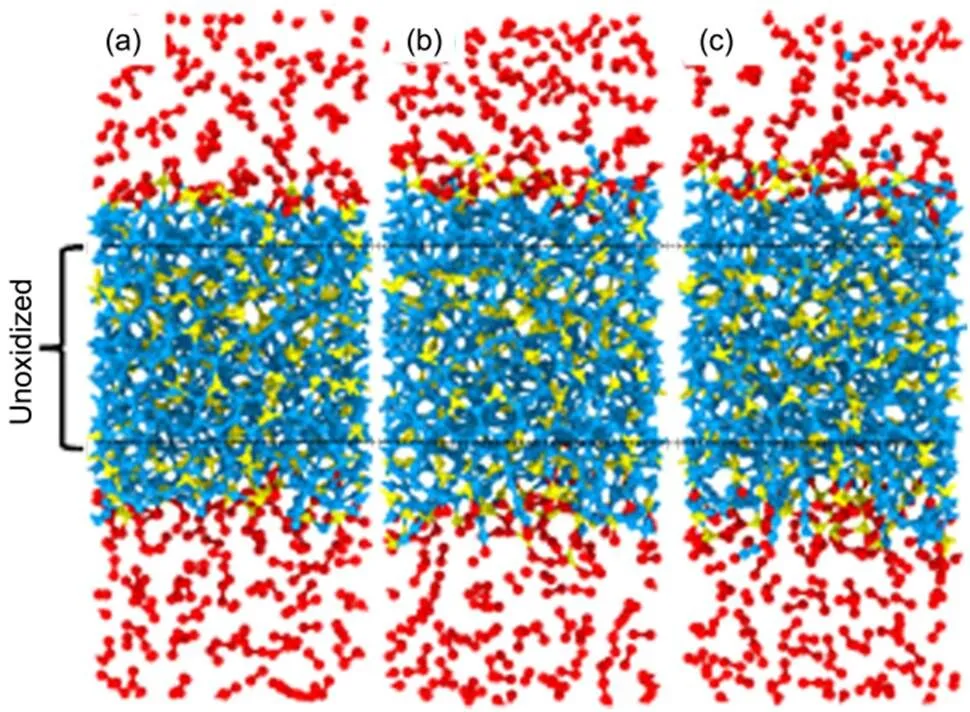
Fig.1 The atomic configuration of the system at three different temperatures in the first laser irradiation cycle.
At (a) 300 K before laser irradiation; (b) 800 K and (c) 300 K after the laser irradiation cycle. The red, cyan and yellow atoms represent oxygen, carbon and silicon atoms, respectively.
The system potential energy in the ReaxFF reactive force field is the sum of oxygen, carbon and silicon potential energy. The potential energy of silicon fluctuates around −73920 kJ·mol−1in the first laser irradiation cycle which is not shown in this work. Fig.3 shows potential energy of oxygen and carbon as a function of temperature in the first laser irradiation cycle. As we can see, the potential energy of oxygen decreases rapidly in the heating process when temperature is above 450 K and in the cooling process when temperature decreases from 800 K to 600 K. The potential energy of oxygen is analogous with the number of Si―O bonds shown in Fig.2, but they are reverse. Once the oxygen atoms bond with the silicon or carbon atoms, the potential energy of oxygen atoms decreases. This is due to the parameter setting of ReaxFF force field14. It is unexpected that the potential energy of carbon fluctuates in the heating process and keeps decreasing in the cooling process. The reason for this will be explained in detail below.
The volume expansion of a-C:Si film can be inferred from the density of unoxidized a-C:Si slab. Fig.4 shows the density of unoxidized a-C:Si slab as a function of temperature in the first laser irradiation cycle. We can observe that the density of a-C:Si film drops from 2.96 g·cm−3to 2.927 g·cm−3when the temperature increases from 300 K to 500 K. The density drops sharply when temperature is above 500 K in the heating process. During the cooling process, the density drops slightly from 2.76 g·cm−3to 2.73 g·cm−3. We can conclude from the density that the volume expansion occurs when the temperature is higher than 500 K in the heating process. The volume expansion process is accompanied with the release of compression stress in the a-C:Si film.
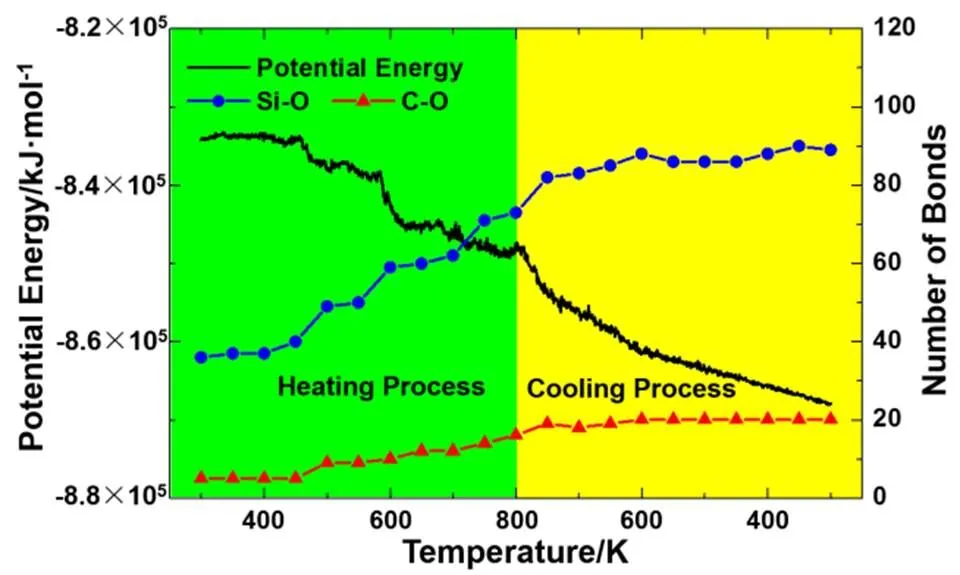
Fig.2 The system potential energy and number of Si―O and C―O bonds as a function of temperature in the first laser irradiation cycle.
The black line, blue circle and red triangle represent the potential energy, number of Si―O and C―O bonds, respectively.
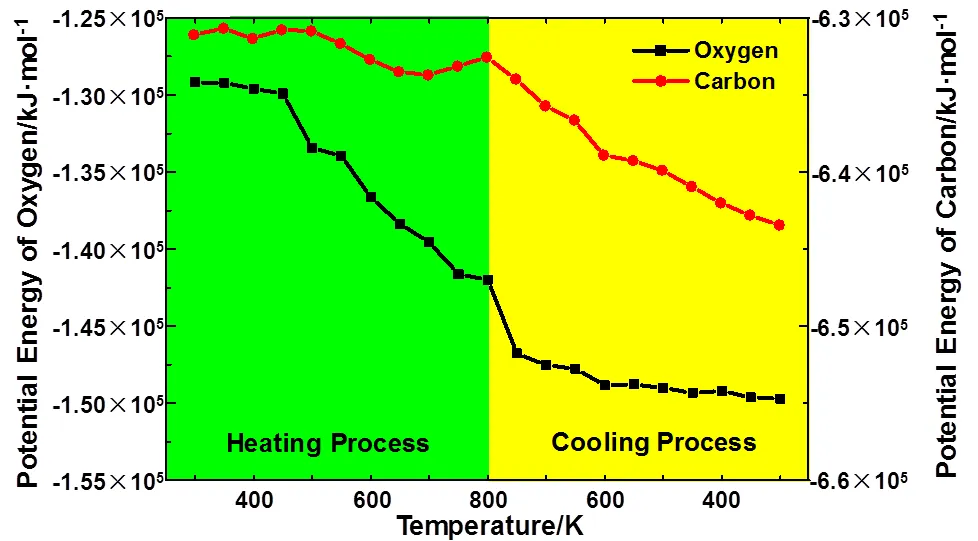
Fig.3 The potential energy of oxygen and carbon as a function of temperature in the first laser irradiation cycle.
The black quadrate and red circle represent the potential energy of oxygen and carbon, respectively. The range of vertical axles is identical.
The atomic structure of a-C:Si slab changes during the volume expansion. The structural evolution of the a-C:Si system also can be inferred from unoxidized a-C:Si slab. There are three different types of bonds co-existed in a-C:Si slab: the C―C bonds, C―Si bonds and Si―Si bonds. In view of the decrease of carbon potential energy in the cooling process of the first laser irradiation cycle, only the pair radius distribution functions (RDFs) of C―C bonds in unoxidized a-C:Si slab at three different temperatures are shown in Fig.5. There are three peaks before laser irradiation which located at 1.2 Å , 1.5 Å and 2.5 Å, respectively. When the a-C:Si slab is heated to 800 K, the location of first peak does not change, but the peak height increases from 0.59 to 1.13. The height increase indicates that the content of1carbon increases. The location of second peak shifts from 1.5 Å to 1.47 Å, and the peak height decreases from 5.27 to 4.75. The volume expansion decrease the number of carbon atoms in the unoxidized a-C:Si slab. So the height of the second peak of C―C bonds decreases. The bonds are prolonged around the voids which are formed in the heating process. The high tensile stresses around the voids can transform carbon atoms into low volume density states, the2bonded carbon27. This is the reason for the left shift of second peak. The low volume densities make the2carbon atoms rearranging easily under deformation. The stresses are released easily by2bonds which have high rotation flexibilities28.
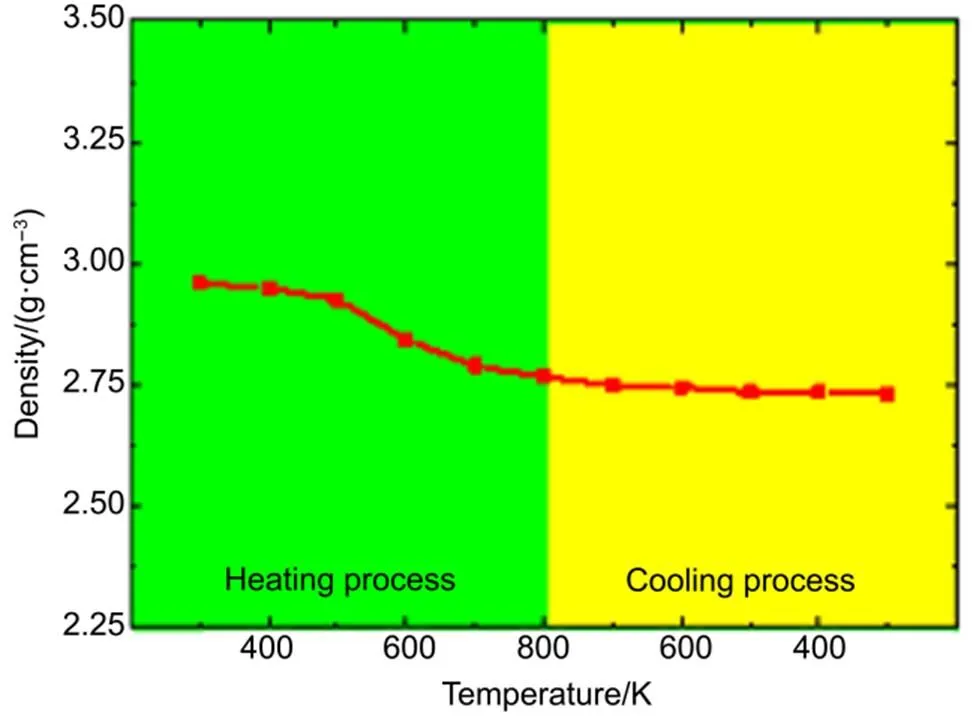
Fig.4 The density of unoxidized a-C:Si slab as a function of temperature in the first laser irradiation cycle.

Fig.5 The pair radius distribution functions (RDFs) of C―C bonds in unoxidized a-C:Si slab at different temperatures in the first laser irradiation cycle.
The vertical dotted lines indicate the C―C bond length of acetylene, graphite and diamond, respectively.
When the temperature of a-C:Si slab is cooled down to 300 K, the locations of the first and second peaks do not change. However, the peak heights of them increase from 1.13 and 4.75 to 1.72 and 5.85, respectively. This means the content of1and2carbon increases in the cooling process.
The atomic shear strain (Mises)29,30of atomwas introduced initially to quantify local inelastic deformation at atomic level. The atomic shear strain (Mises)29of atomis computed as:

where the ηimeans local Lagrangian strain matrix. In this work, the atomic shear strains are calculated by OVITO26 based on two atomic configurations, which have been used to investigative the graphitization5 and tensile behaviors27 of a-C films. Fig.6 shows the distribution of atomic shear strains at three different temperatures in the first laser irradiation cycle. As can be seen in Fig.6(a), there are high atomic shear strains on the surface oxide layers and low atomic shear strains inside the a-C:Si slab when the system is relaxed at 300 K. When the system is heated to 800 K, there are higher atomic shear strains which distribute broader on the surface oxide layers, as shown in Fig.6(b). When the temperature of system is cooled down to 300 K, as shown in Fig.6(c), high atomic shear strains also locate on surface oxide layers. But there are higher atomic shear strains inside the a-C:Si slab than that before laser irradiation, especially in the region near the surface oxide layers.
At (a) 300 K before laser irradiation, (b) 800 K and (c) 300 K after laser irradiation cycle, respectively.

Table 1 The number of cluster size of carbon atoms at three different temperatures in the first laser irradiation cycle.
The atoms are excited to higher vibrational states at higher temperature. This is due to the fact that the atomic shear strains increase with temperature. The oxidation of a-C:Si slab at the beginning of cooling process is the result of high atomic shear strain at high temperature. It was found that the oxidation of silicon on the surface can cause the strain accumulation31. The strain accumulation accelerates the oxidation by emitting the silicon or carbon atoms from substrate to the oxidizing layers. This makes CO emitted into oxygen molecules environment. The small atomic elastic modulus of2carbon atoms can cause large atomic strains under deformation28,32. Due to the increase of2carbon atoms after laser irradiation, the atomic shear strains inside the a-C:Si slab becomes higher.
After viewing and analyzing the atoms configuration in the cooling process, we infer that the decrease of potential energy of carbon is caused by cluster of carbon atoms. The cluster sizes of carbon atoms are counted at three different temperatures in the first laser irradiation cycle, as shown in Table 1. The cluster size is determined by the number of carbon atoms in the cluster. There is only one large cluster which consists of 178 carbon atoms before laser irradiation, and a lot of small carbon clusters which only contain one or a few atoms. When the system is heated to 800 K, the largest carbons cluster contains 271 carbon atoms and the number of carbon clusters which contain one or two atoms decreases. When the a-C:Si slab is cooled down to 300 K, the carbon cluster which contains 625 carbon atoms is formed.
The C―C bond is the most stable bond in this system due to its high bond energy and low cohesive energy. The cluster of carbon atoms forms more stable C―C bonds which can lower potential energy. The cluster of carbon atoms was also found in the irradiation swelling process of silicon carbide33. The high atomic strains in the a-C:Si slab assist the carbon atoms to overcome the energy barriers and cluster even at a low temperature5,27. This is the reason for potential energy of carbon keeps decreasing in the cooling process. In addition, the clustering of carbon in the a-C:Si slab which has large free volume increases the rate of2carbon.
3.2 The effect of laser irradiation cycles on the oxidation of a-C:Si slab
The a-C:Si film is irradiated for thousands of cycles in the HAMR application. It is necessary to understand the performance of a-C:Si film under repetitive laser irradiation. By considering the calculation time and evolution of the a-C:Si slab, the a-C:Si slab is heated for 10 cycles in this study. The effect of laser irradiation cycles on the number of Si―O and C―O bonds and system potential energy is shown in Fig.7. The data was counted at 300 K after the each laser irradiation cycles. As we can see, violently oxidation occurs in the first laser irradiation. The number of Si―O and C―O bonds increases sharply in the first laser irradiation and increases linearly after the third laser irradiation cycle. The potential energy decreases sharply in the first laser irradiation cycle. The decrement of potential energy decreases with laser irradiation cycles which indicates that system stabilizes slowly.

Fig.7 The effect of laser irradiation cycles on the number of Si―O and C―O bonds and system potential energy.
The red circle, blue triangle and black quadrate represent number of Si―O and C―O bonds and potential energy of system, respectively. The 0 means data measured before the laser irradiation, the 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 mean dates measured after the corresponding laser irradiation cycles.

Fig.8 The effect of laser irradiation cycles on the largest carbon cluster size and density of unoxidized a-C:Si slab.
The black quadrate and red triangle represent the largest carbon cluster size and density of unoxidized a-C:Si slab respectively. The 0 means data measured before the laser irradiation, the 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 means dates measured after the corresponding laser irradiation cycles.
The effect of laser irradiation cycles on the largest carbon cluster size and density of unoxidized a-C:Si slab is shown in Fig.8. The size of the largest carbon cluster increases sharply in the first laser irradiation cycle. The incremental quantity of largest carbon cluster size increases slowly with laser irradiation cycles after the first laser irradiation cycle. We can conclude that the contribution of carbon cluster on the decrease of potential energy after the first laser irradiation cycle is slight. The decrease of system potential energy is mainly contributed by the oxidation of a-C:Si slab. The decrement of density is large in the first laser irradiation cycle. After the sixth laser irradiation cycle, the density fluctuates around 2.66 g∙cm−3. The volume expansion of the a-C:Si slab is finished after six laser irradiation cycles.
After viewing the atomic configuration, we find the roughness and thickness of surface oxide layers increase with number of laser irradiation cycles. The rearrangement of surface oxide layers which is caused by the surface shear strains can make the unsaturated silicon and carbon atoms exposing to be oxidized. The decrease of unsaturated silicon and carbon atoms on the surface slow the oxidation of a-C:Si slab. The diffusion of oxygen atoms into the a-C:Si slab is only found in the first laser irradiation cycle. This can be explained by “hot atomic mechanism”34which will be discussed in next section. The energy barrier for atomic oxygen to diffuse into the a-C:Si is small than that of molecular oxygen17. But the energy grain of oxygen atoms is not enough to overcome the diffusion energy barrier even at 800 K. The compressive stress in the a-C:Si can slow the oxidation reaction by restricting the diffusion of atomic oxygen. This phenomenon is analogous with the self-limiting oxidation35,36of silicon nanowires37. The bond length of C―C and C―Si is shorter than that of Si―Si, the self-limiting oxidation is more notable. The oxidation of a-C:Si film is restricted by almost saturated oxidation surface and low diffusion coefficient of atomic oxygen after the third laser irradiation cycle.
3.3 The oxidation mechanism of a-C:Si slab in the HAMR
As we have stated above, the oxygen molecules can bond with unsaturated silicon or carbon atoms on the surfaces in the form of Si―O―O―Si, Si―O―O and C―O―O linkages. The oxidation of a-C:Si slab is mainly contributed by Si―O―O―Si and Si―O―O linkages. The Si―O―O linkage bonds with unsaturated silicon atoms and forms the Si―O―O―Si linkage. The oxidation mechanism of a-C:Si slab is shown in Fig.9. As shown in Fig.9(a), there are two different types of O―O bond as marked by the green and blue ellipse, respectively. The O―O bond in the green ellipse is a part of Si―O―O―Si linkage and the other one in the blue ellipse is free oxygen molecule. The O―O bond in the Si―O―O―Si linkage is broken and then two Si―O―Si linkages are formed, as shown in the green circles in Fig.9(b). Meanwhile one C―Si bond is broken, the silicon atom bonded with new formed atomic oxygen becomes unsaturated. The unsaturated silicon atom bonds the free oxygen molecule in blue ellipse, and the new Si―O―O―Si linkage is formed, as shown in Fig.9(c). With evolution of oxidation reaction, as shown in Fig.9(d), the oxygen atom in green circle diffuses into the a-C:Si slab and bonds with silicon atom in the next layer. The O―O bond in new formed Si―O―O―Si linkage is broken, the Si―O and Si―O―C linkage are formed, as shown in the blue circle in Fig.9(e). The formation process of atomic oxygen is consistent with the oxidation process of Si-face of SiC which covered by 1 nm amorphous SiO2layer17.
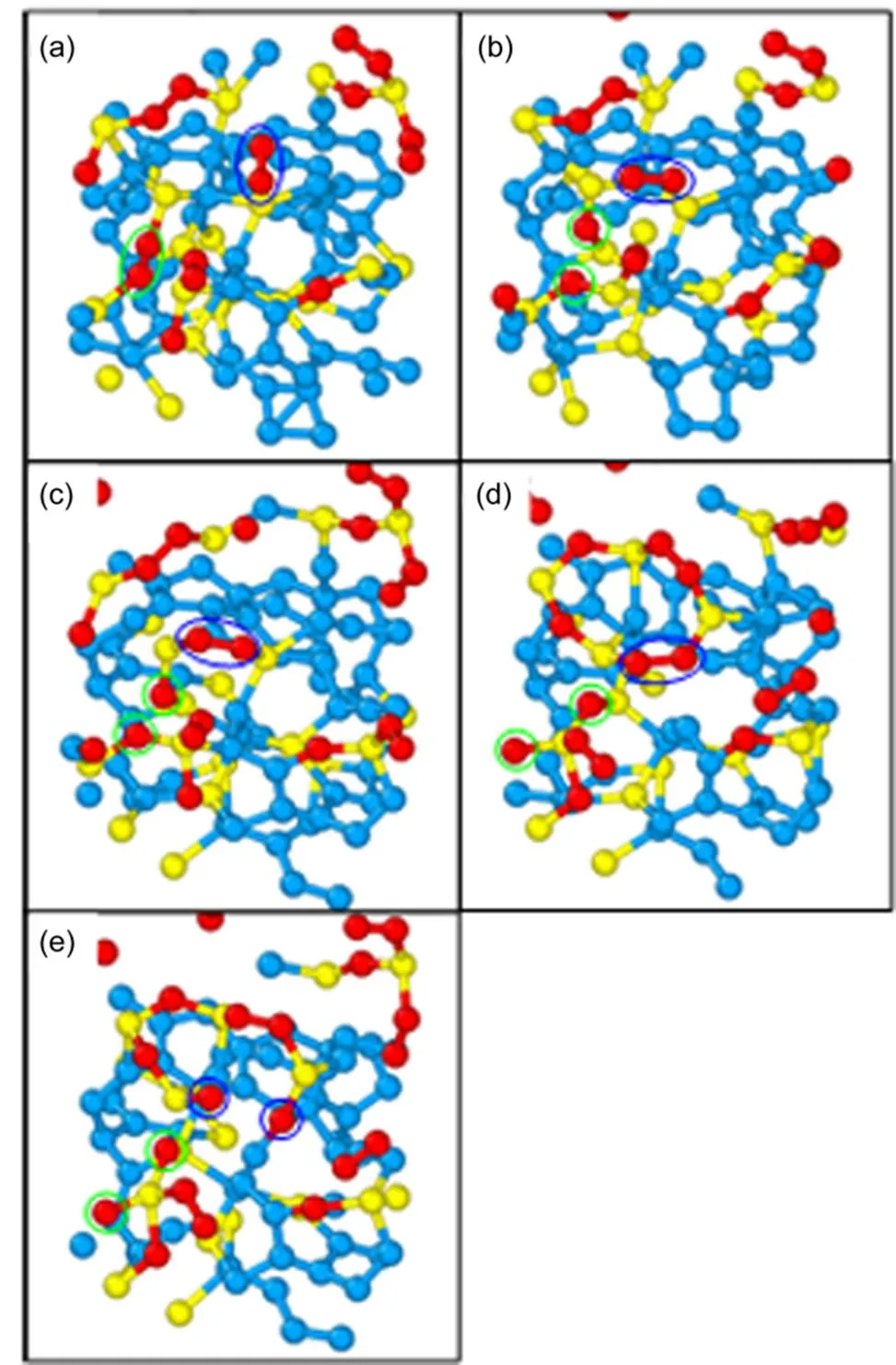
Fig.9 The oxidation mechanism of a-C:Si slab.
The green and blue ellipses highlight the O-O bonds, and green and blue circles highlight the atomic oxygen.
The break of O―O bond in the Si―O―O―Si linkage can be explained by the bond energy. The energies of Si―O bond and C―O bond are ~4.674 eV (451 kJ·mol−1) and ~3.71 eV (358 kJ·mol−1), respectively. But the energy of single O-O bond is ~1.51 eV (146 kJ·mol−1). With assistance of atomic shear strains, the O―O bond breaks easily than other two. The dissociation of O―O bond on a-C:Si slab surface activate the new formed oxygen atoms and provide more kinetic energy for them to incorporate into the a-C:Si slab. The energy gain makes the oxygen atoms as the “hot atoms”. This kind of oxygen reaction behavior was explained by “hot atom mechanism” which is independent of oxidation temperature34. The “hot” oxygen atoms rearrange the surface bonding states and assist the oxidation process. Since the oxygen molecules in the environment is abundant, the oxidation kinetics of a-C:Si slab in the HAMR application come from the molecular mechanism. The surface of a-C:Si slab is defective, such as dangling bonds, this makes the molecular oxygen to be the effective oxidant. The result is identical with the oxidation mechanism of C-face of SiC17.
4 Conclusions
The oxidation process of a-C:Si film induced by a pulsed laser in HAMR application is investigated by MD simulations. Using the ReaxFF reactive force field, we captured the atomic details of structure evolution and oxidation process, and clarified the oxidation mechanism of a-C:Si film. The oxidation of a-C:Si film occurs in the heating process and beginning of cooling process because of the high atomic shear strains. The volume expansion of a-C:Si film occurs in the heating process accompanying the release of inner stress. The cluster of carbon atoms which occurs in the cooling process stabilizes and lowers the potential energy of a-C:Si film. The atomic stains assist the cluster of carbon atoms and increase the rate of2carbon in the first laser irradiation cycle. Because of the decrease of unsaturated silicon and carbon atoms on the surfaces, the oxidation rate of a-C:Si film decreases with the laser irradiation cycles. Due to the low temperature and high compressive stress of a-C:Si film, the diffusion coefficient of atomic oxygen into a-C:Si film is low. The molecular oxygen works as an oxidant due to the surface defect. The oxygen molecules bond with unsaturated silicon atoms and form Si―O―O―Si linkages. The atomic strains break O―O bonds, rearrange the surface oxide layer and promote the oxidation of a-C:Si film.
(1) Kryder, M. H.; Gage, E. C.; Mcdaniel, T. W.; Challener, W. A.; Rottmayer, R. E.; Ju, G. P.; Hsia, Y. T.; Erden, M. F.2008,(11), 1810. doi: 10.1109/JPROC.2008.2004315
(2) Weller, D.; Moser, A.1999,(6), 4423. doi: 10.1109/20.809134
(3) Ma, Y. S.; Chen, X. Y.; Liu, B.2012,(3), 337. doi: 10.1007/s11249-012-0032-7
(4) Pathem, B. K.; Guo, X. C.; Rose, F.; Marchon, B.2013,(7), 3721. doi: 10.1109/TMAG.2012.2236645
(5) Liu, Q. K.; Li, L. Q.; Zhang, H. T.; Huang, Q. T.; Zhang, G. Y.; Hou, Z. X.2017,(3), 3301007. doi: 10.1109/TMAG.2016.2626344
(6) Jones, P. M.; Ahner, J.; Platt, C. L.; Tang, H.; Hohlfeld, J.2014,(3), 144. doi: 10.1109/TMAG.2013.2285599
(7) Wang, N.; Komvopoulos, K.2011,(9), 2277. doi: 10.1109/TMAG.2011.2139221
(8) Ma, Y. S.; Ji, R.; Man, Y. J.; Shakerzadeh, M.; Zheng, R. Y.; Seet, H. L.; Hu, J. F.2016,(2), 3300606. doi: 10.1109/TMAG.2015.2494857
(9) Wu, W. J.; Hon, M. H.1999,(2–3), 134. doi: 10.1016/S0257-8972(98)00719-1
(10) Khriachtchev, L.; Vainonen-Ahlgren, E.; Sajavaara, T.; Ahlgren, T.; Keinonen, J.2000,(4), 2118. doi: 10.1063/1.1305831
(11) Er, K. H.; So, M. G.2013,(1), 134.
(12) Hatada, R.; Baba, K.; Flege, S.; Ensinger, W.2016,, 93. doi: 10.1016/j.surfcoat.2016.08.011
(13) Kim, J. W.; Hong, B. X.; Lim, D. C.; Lee, D. B.2005,(1), 288. doi: 10.1016/j.surfcoat.2004.08.168
(14) Newsome, D. A.; Sengupta, D.; Foroutan, H.; Russo, M. F.; van Duin, A. C. T.2012,(30), 16111. doi: 10.1021/jp306391p
(15) Newsome, D. A.; Sengupta, D.; van Duin, A. C.2013,(10), 5014. doi: 10.1021/jp307680t
(16) Sun, Y.; Liu, Y. J.; Xu, F.2015,(9), 096203. doi: 10.1088/1674-1056/24/9/096203
(17) Shen, X.; Tuttle, B. R.; Pantelides, S. T.2013,, 033522. doi: 10.1063/1.4815962
(18) van Duin, A. C. T.; Dasgupta, S.; Lorant, F.; Goddard, W. A.2001,(41), 9396. doi: 10.1021/jp004368u
(19) Chenoweth, K.; van Duin, A. C. T.; Goddard, W. A.2008,(5), 1040. doi: 10.1021/jp709896w
(20) van Duin, A. C. T.; Strachan, A.; Stewman, S.; Zhang, Q. S.; Xu, X.; Goddard, W. A.2003,(19), 3803. doi: 10.1021/jp0276303
(21) Liu, X. L.; Li, X. X.; Han, S.; Qiao, X. J.; Zhong, B. J.; Guo, L.2016,(6), 1424. [刘晓龙, 李晓霞, 韩 嵩, 乔显杰, 钟北京, 郭 力. 物理化学学报, 2016,(6), 1424.] doi: 10.3866/PKU.WHXB201603233
(22) Diao, Z. J.; Zhao, Y. M.; Chen, B.; Duan, C. L.2012,, 2037. [刁智俊, 赵跃民, 陈 博, 段晨龙. 化学学报, 2012,, 2037.] doi: 10.6023/A12070451
(23) Wang, Z. M.; Zheng, M.; Xie, Y. B.; Li, X. X.; Zeng, M.; Cao, H. B.; Guo, L.2017,(7), 1399. [王子民, 郑 默, 谢勇冰, 李晓霞, 曾 鸣, 曹宏斌, 郭 力.物理化学学报, 2017,(7), 1399.] doi: 10.3866/PKU.WHXB201704132
(24) Yang, Z.; He, Y.2016,(4), 921. [杨镇,何远航. 物理化学学报, 2016,(4), 921.] doi: 10.3866/PKU.WHXB201512251
(25) Li, L. Q.; Xu, M.; Song, W. P.; Ovcharenko, A.; Zhang, G. Y.; Jia, D.2013,, 287. doi: 10.1016/j.apsusc.2013.09.073
(26) Stukowski, A.2010,, 1. doi: 10.1088/0965-0393/18/1/015012
(27) Bai, L. C.; Srikanth, N.; Wu, H.; Liu, Y.; Liu, B.; Zhou, K.2016,, 8. doi: 10.1016/j.jnoncrysol.2016.03.025
(28) Namilae, S.; Radhakrishnan, B.; Sarma, G.2007,, 1302. doi: 10.1016/j.compscitech.2006.10.002
(29) Shimizu, F.; Ogata, S.; Li, J.2007,(11), 2923. doi: 10.2320/matertrans.MJ200769
(30) Tang, C.; Wong, C. H.2016,, 61. doi: 10.1016/j.intermet.2015.12.010
(31) Kageshima, H.; Shiraishi, K.1998,(26), 5936. doi: 10.1103/PhysRevLett.81.5936
(32) Kelires, P. C.2000,(23), 15686. doi: 10.1103/PhysRevB.62.15686
(33) Li, Y. Y.; Xiao, W.; Li, H. L.2016,, 75. doi: 10.1016/j.jnucmat.2016.08.004
(34) Pamungkas, M. A.; Joe, M.; Kim, B. H.; Lee, K. R.2011,, 053513. doi: 10.1063/1.3632968
(35) Kao, D. B.; Mcvittie, J. P.; Nix, W. D.; Saraswat, C. K.1988,(1), 25. doi: 10.1109/16.2412
(36) Liu, H. I.; Biegelsen, D. K.; Johnson, N. M.; Ponce, F. A.; Pease, R. F. W.1993,(6), 2532. doi: 10.1116/1.586661
(37) Khalilov, U.; Pourtois, G.; Bogaerts, A.; van Duin, A. C. T.; Neytsa, E. C.2013,, 719. doi: 10.1039/C2NR32387G
热辅助存储磁盘硅掺杂非晶碳薄膜氧化的ReaxFF反应力场分子动力学模拟
刘青康*宋文平*黄其涛 张广玉 侯珍秀
(哈尔滨工业大学机电工程学院,哈尔滨 150001)
热辅助磁存储技术是一种提高磁盘存储面密度到1 Tb·in−2的方法,在数据写入过程中的激光局部加热会使磁盘非晶碳薄膜氧化。本文采用ReaxFF反应力场分子动力学方法,建立硅掺杂非晶碳(a-C:Si)薄膜在激光诱导氧化的模型,从原子尺度分析a-C:Si薄膜的结构变化、氧化过程,确定了氧化机理以及激光加热次数对氧化的影响规律。a-C:Si薄膜的氧化发生在加热阶段和初始降温阶段,在加热过程中a-C:Si薄膜的体积扩张和降温过程中原子应变引起碳原子团簇,均使薄膜中2碳含量增加。随着加热次数的增加,表面未饱和原子数量减少和氧原子低扩散率使薄膜氧化速率逐渐降低。此外,非晶薄膜的表面缺陷使分子氧成为氧化剂,表面原子剪切应变使Si―O―O―Si链中O―O键断裂,重构氧化表面,进而促进a-C:Si薄膜的氧化。
热辅助磁存储;硅掺杂非晶碳薄膜;氧化;ReaxFF;分子动力学模拟
O643
10.3866/PKU.WHXB201706222
May 3, 2017;
June 9, 2017;
June 22, 2017.
Corresponding authors. LIU Qing-Kang, Email: qingkangliu86@gmail.com; Tel: +86-451-86413111. SONG Wen-Ping, Email: songwenping@hit.edu.cn; Tel: +86-451-86413111.
The project was supported by the National Natural Science Foundation of China (51405103), China Post-doctoral Science Foundation (2014M551230, 2015T80335).
国家自然科学基金(51405103)与中国博士后科学基金(2014M551230, 2015T80335)资助项目

