Theoretical and Experimental Studies on the Crystal Morphologyof Transition-Metal Carbohydrazide Perchlorate Complexes
YANG Li ZHANG Guo-Ying LIU Ying ZHANG Tong-Lai
Theoretical and Experimental Studies on the Crystal Morphologyof Transition-Metal Carbohydrazide Perchlorate Complexes
YANG Li*ZHANG Guo-Ying LIU Ying ZHANG Tong-Lai
()

Crystal morphology; Prediction; Attachment energy; Growth rate
1 Introduction
The shape or morphology of a crystal is extremely important to the energetic materials. It can have enormous impact on the physical and chemical properties, such as fluidity, apparent density, electrostatic accumulation, pressure resistance, stability, and so on1,2. These properties can directly affect initiating ability, sensitivity and other explosive performance. For example, it is well known that the acicular crystal of lead azide (LA) is poorer for fluidity and stability, but higher sensitivity than the columnar crystal3. The excellent crystal morphology can improve the safety and stability of the energetic materials, which is also helpful to industrial production and safe application4–6. Therefore, the study on the crystal morphology is vital to energetic materials.
Metal carbohydrazide complexes with strong oxidizing acid radical ions, used as initiating explosives, ignition composition, gas generator and burning rate modifier to propellants, have been extensively studied experimentally and theoretically7–15. Structures of metal carbohydrazide derivatives with sulfate, perchlorate, chloride and polymeric nitrogen were successively reported16–18using infrared spectrum analysis and X-ray single crystal diffraction analysis methods. It is found that metal carbohydrazide derivatives have excellent properties, such as appropriate sensitivity, good safety performance and strong initiating ability. Zhang.19–22carried out an in-depth research on the complexes. They found that cadmium carbohydrazide perchlorate and zinc carbohydrazide perchlorate had excellent properties and they were widely used as green initiating explosives, without using toxic and hazardous raw materials and eliminating waste in the manufacture and application processes.
In this work, we predicted the crystal morphology of manganese carbohydrazide perchlorate ([Mn(CHZ)3](ClO4)2), iron carbohydrazide perchlorate ([Fe(CHZ)3](ClO4)2), cobalt carbohydrazide perchlorate ([Co(CHZ)3](ClO4)2), nickel carbohydrazide perchlorate ([Ni(CHZ)3](ClO4)2) and cadmium carbohydrazide perchlorate ([Cd(CHZ)3](ClO4)2) by Bravais- Freidel-Donnay-Harker (BFDH) and growth morphology method. The crystal-morphologies of them are studied experimentally without crystal-control reagent.
2 Experimental and computational section
2.1 Computational method
The calculation was performed using the Universal force field (UFF)23,24, which was successfully applied to model a wide range of complexes metal complexes25–27, DNA28, and other organic systems. It is set based on the element, its hybridization and connectivity29.
The initial configurations of transition-metal carbohydrazide perchlorate complexes were obtained from the experimental data by X-ray single crystal diffraction method. Then the crystal structures were optimized by the density functional theory (DFT) using the CASTEP package30. We found that the GGA (PW91) proposed by Perdew and Wang31,32was more reliable to predict the structures. Therefore GGA (PW91) was used in all calculations. For the calculation, the cutoff energy was 300.0 eV on the plane wave. The-point grid is set as 2 × 2 × 1 in the Brillouin zone by using the Monkhost-Pack scheme. The convergence of total energies is less 0.01% under the selected kinetic energy and the-point grid. During the self-consistent field (SCF) calculations, the convergence tolerance of energy was set to 2.0 × 10−6eV, the maximum of residual force was 0.005 eV∙nm, the maximum of displacement of atoms was 0.02 nm and the maximum of residual bulk stress was 0.1 GPa. The optimized crystal structures were used as the starting point for the morphology calculations.
The morphology of the crystal structure of these complexes was studied using MORPHOLOGY code. BFDH and growth morphology method were used to predict the crystal growth in vacuum. BFDH method was based on the interplanar spacings of different crystal faces and took into account the crystal symmetry33. The growth morphology (AE model) was based on the intermolecular forces in crystallization by Hartman and Perdok34.
The attachment energy (att) is defined as the energy per molecule released when a new slice of depthdis attached to the crystal face35. It is the sum of the interaction energy per molecule (E()) between a slice of thicknessdand theth underlying slice.

(1)
The relationship between the lattice energy of the crystal (latt) and the energy of a growth slice of thicknessd(slice) is given by

Eatt = Elatt–Eslice(2)
The relative growth rate (R) of the crystal face is proportional of its attachment energy (att)36. The face with the lowest attachment energies are the slowest growing, and the most important to morphology.

Rij = Ri/Rj = Eatt,i/Eatt,j(3)
2.2 Experimental
[Mn(CHZ)3](ClO4)2, [Fe(CHZ)3](ClO4)2, [Co(CHZ)3](ClO4)2, [Ni(CHZ)3](ClO4)2and [Cd(CHZ)3](ClO4)2used in the experiment were synthesized, purified and dried according to the literature. The purities of products were more than 99.5%. In order to obtain the single crystal, the products of them were dissolved in deionized water (6.25 × 10−8S·cm−1), and kept the solution in the cups for 15 d. The crystal morphology of them was performed using BX51 microscope (Olympus Corp., Japan). The actual parameters of the equipment are as follow: Built-in kohler illuminator, voltage 12 V, and zoom magnification ×4 to ×100.
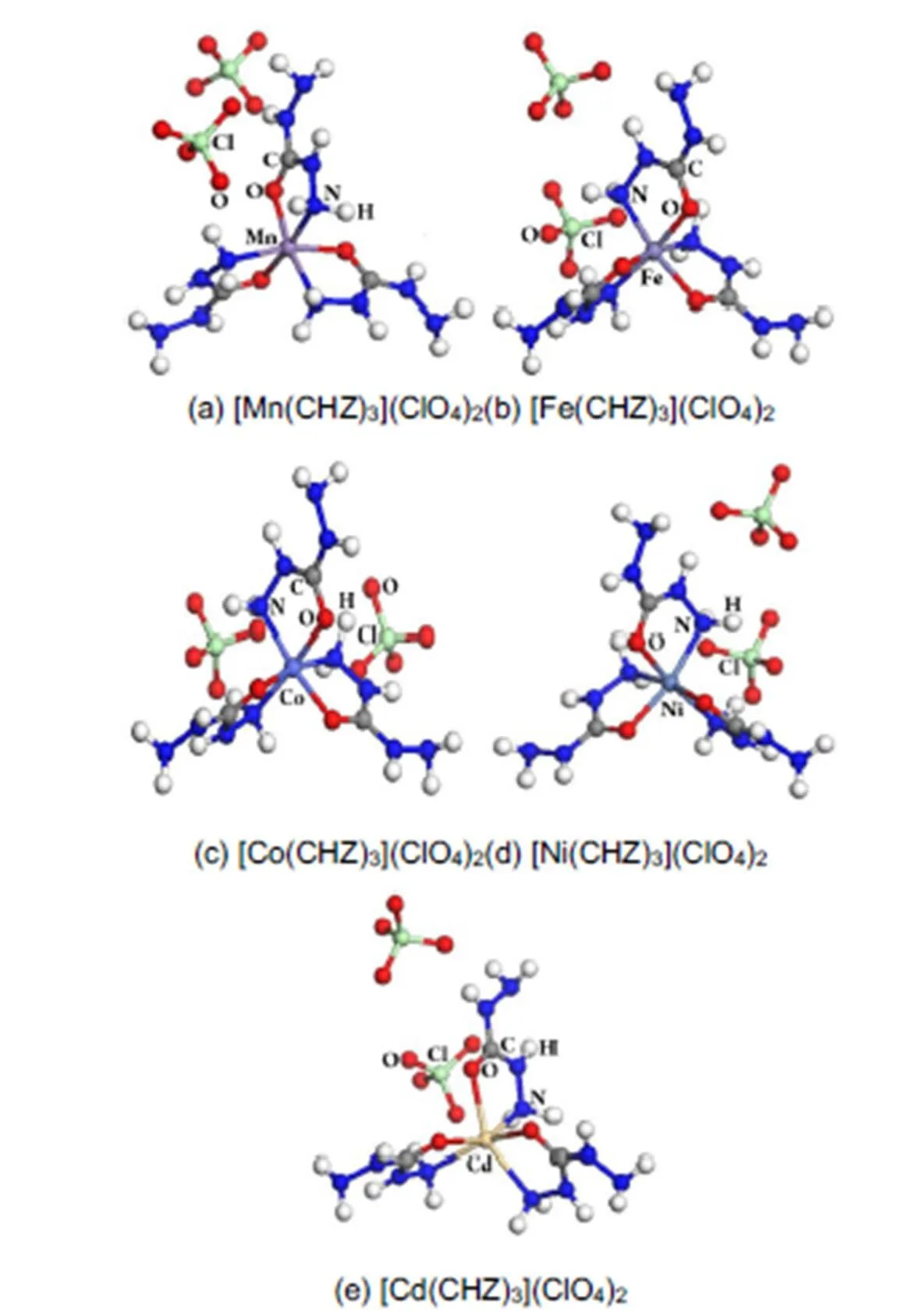
Fig.1 Molecular structure of transition carbohydrazide perchlorate complexes.

Fig.2 Morphology of [Mn(CHZ)3](ClO4)2 in vacuum by BFDH model (a) and AE model (b).
3 Results and discussion
3.1 Prediction of the crystal morphology
The molecular structures of [Mn(CHZ)3](ClO4)2, [Fe(CHZ)3](ClO4)2, [Co(CHZ)3](ClO4)2, [Ni(CHZ)3](ClO4)2and [Cd(CHZ)3](ClO4)2are shown in Fig.1 The crystal structures of them in the solid state are in space group21/with= 4 in the unit cell. Crystal cell dimensions and cell angles are listed in Table 1.
According to the Arrhenius and Gibbs Thomson equations (equation (4)), the crystal nucleus formation is obtained37.

(4)
,,,,: lattice parameters,: the volume of the cell.
It can be seen that the big cell volume can decrease the nucleation rate from the equation (4). That means the order of nucleation rate for the complexes is in the following sequence: [Cd(CHZ)3](ClO4)2<[Mn(CHZ)3](ClO4)2<[Co(CHZ)3](ClO4)2<[Ni(CHZ)3](ClO4)2<[Fe(CHZ)3](ClO4)2when,,, andare certain.
The morphology of transition-metal carbohydrazide perchlorate complexes predicted using the BFDH and AE models in vacuum was shown in Fig.2–Fig.6. It can be seen that the morphology of them are close to oblong block shapes. The similar shapes may be attributed to the same the ligand and the outer ion of ClO4−. While the contribution of metal cation contribute to morphology is very little. The regular crystal shapes and the smooth surfaces of them are beneficial to improve the free-running property and safety.



(5)
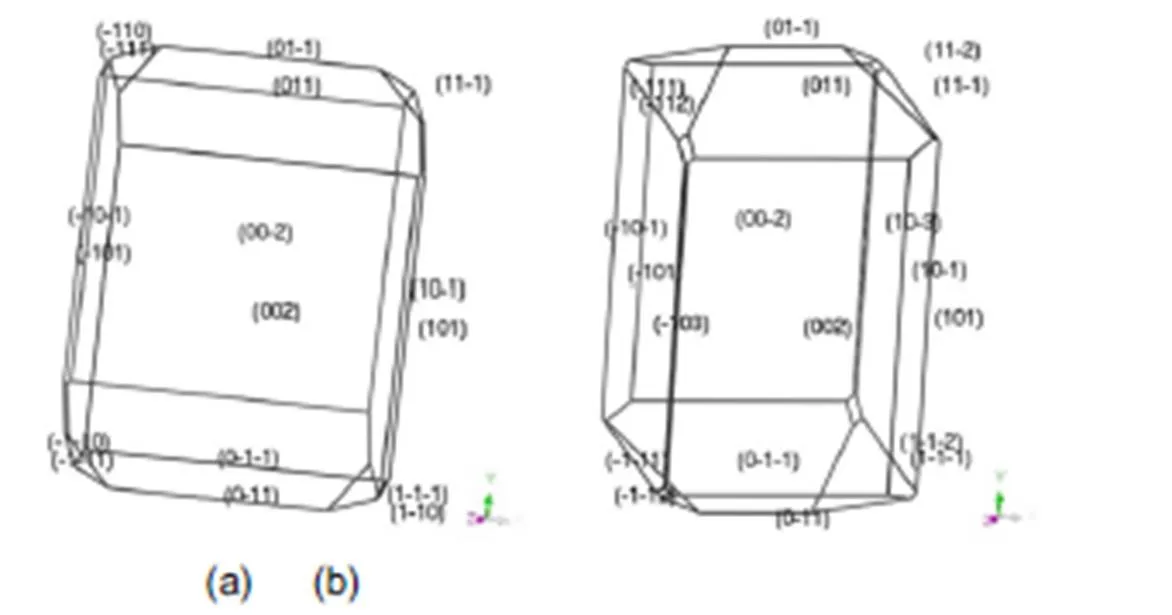
Fig.4 Morphology of [Co(CHZ)3](ClO4)2 in vacuum by BFDH model (a) and AE model (b).

Fig.5 Morphology of [Ni(CHZ)3](ClO4)2 in vacuum by BFDH model (a) and AE model (b).
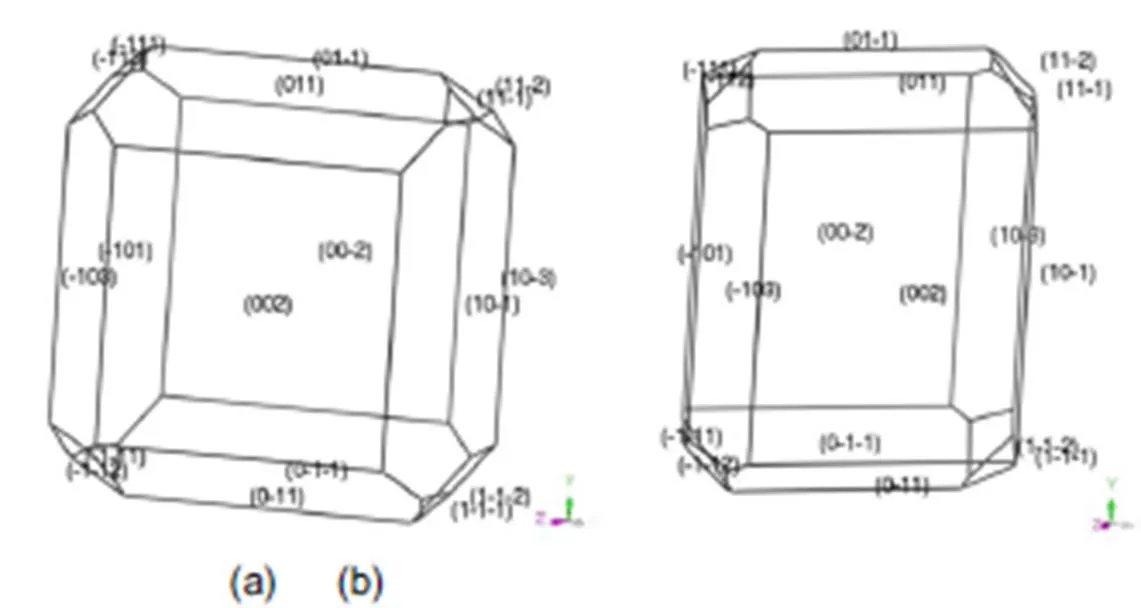
Fig.6 Morphology of [Cd(CHZ)3](ClO4)2 in vacuum by BFDH model (a) and AE model (b).

Table 2 Predicted Morphologies of the BDFH and AE models for transition carbohydrazide perchlorate complexes.

ComplexFaceBDFH/%AE/%Total facet areaEatt/(kcal∙mol−1)Rij [Ni(CHZ)3](ClO4)236.03130.3757247.670−27.8281.00 29.96019.6574690.440−33.2881.20 (011)30.71527.5706578.443−44.0851.58 0.5285.6891357.419−38.6741.39 (101)–12.0832883.167−33.2961.20 2.6354.2871022.946−48.2301.73 0.1300.11627.720−51.7361.86 (110)–0.22353.194−50.6641.82 Sum10010023860.999−327.801 [Cd(CHZ)3](ClO4)225.07033.974786.511−7.9481.00 24.05523.097534.700−10.6251.34 (011)34.22126.635616.606−13.1991.66 11.6849.879228.703−12.1241.53 4.2232.65961.565−16.0502.02 0.7463.75086.824−15.4831.95 –0.0050.116−16.6722.10 Sum1001002315.025−92.101
1 kcal∙mol−1= 4.187 kJ∙mol−1.
From the equation (5), the bigger crystal surface area can improve the crystal growth rate. In Table 2, the order of sum facet area is [Fe(CHZ)3](ClO4)2> [Co(CHZ)3](ClO4)2> [Ni(CHZ)3](ClO4)2> [Mn(CHZ)3](ClO4)2> [Cd(CHZ)3](ClO4)2. Therefore the order of crystal growth rate keeps the same sequence with sum facet area when,andare same.

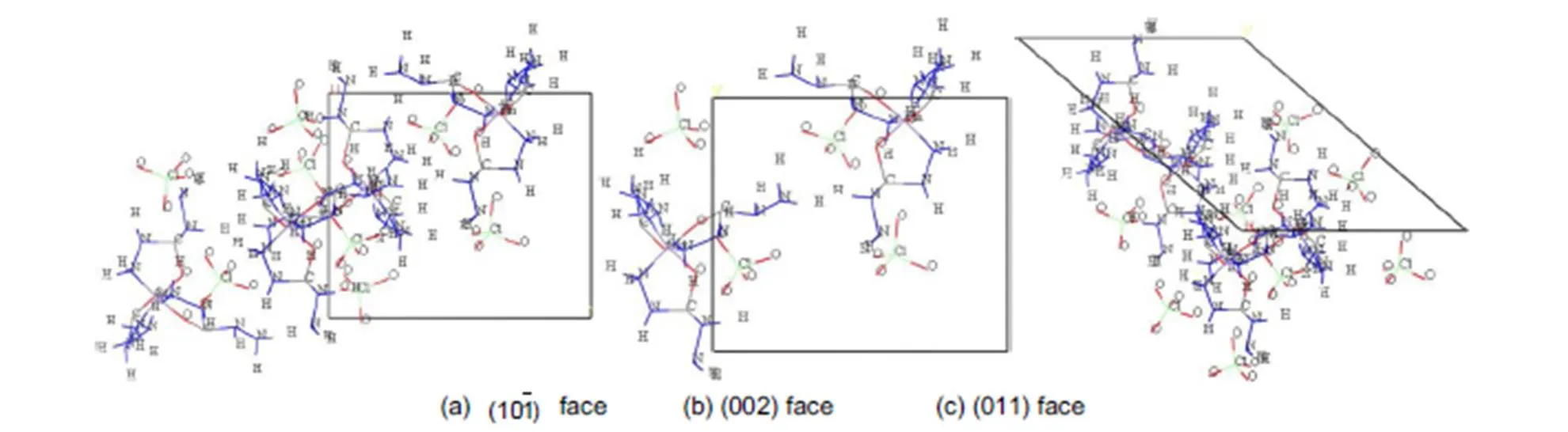
Fig.7 Cleaved main crystal faces of [Mn(CHZ)3](ClO4)2.

Fig.8 Cleaved main crystal faces of [Fe(CHZ)3](ClO4)2.
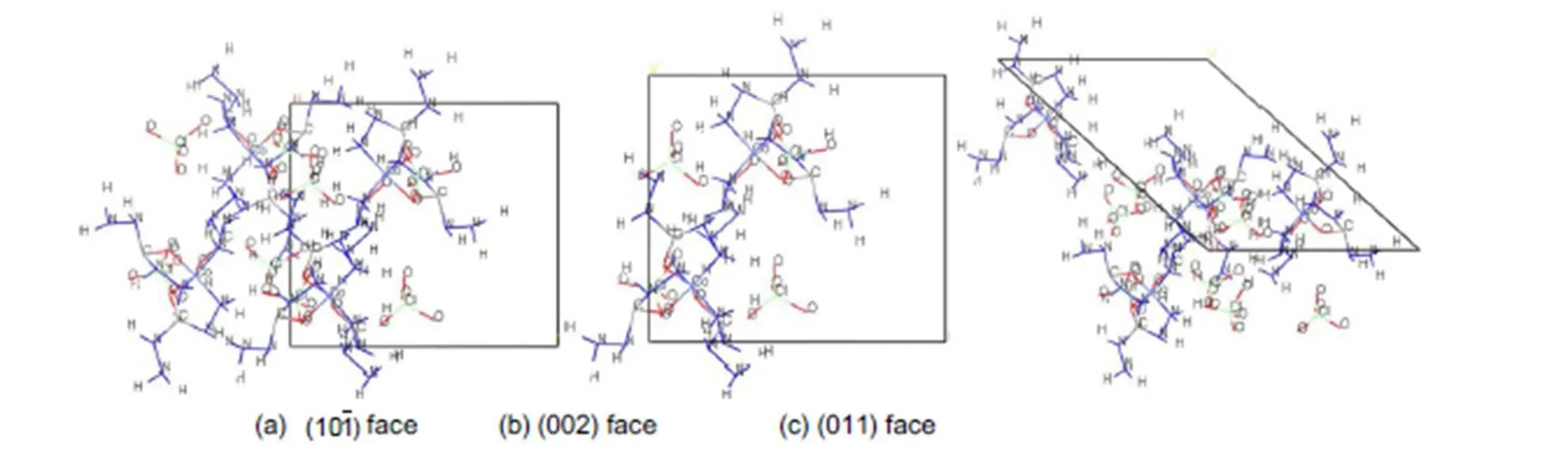
Fig.9 Cleaved main crystal faces of [Co(CHZ)3](ClO4)2.

Fig.10 Cleaved main crystal faces of [Ni(CHZ)3](ClO4)2.

Fig.11 Cleaved main crystal faces of [Cd(CHZ)3](ClO4)2.

3.2 Experimental morphology
The crystal-morphology of [Mn(CHZ)3](ClO4)2, [Fe(CHZ)3](ClO4)2, [Co(CHZ)3](ClO4)2and [Ni(CHZ)3](ClO4)2without crystal-control reagent was synthesized and observed by BX51 microscope (Olympus Corp., Japan) in Fig.15.
It can be seen that the crystal morphology of [Mn(CHZ)3](ClO4)2, [Fe(CHZ)3](ClO4)2, [Ni(CHZ)3](ClO4)2and [Cd(CHZ)3](ClO4)2are obviously short columnar polyhedrons on the crystal morphology. In literature40, [Co(CHZ)3](ClO4)2also appear columnar polyhedrons shapes. Through the comparison of BDFH and AE model, it can be concluded that AE model are nearer to experimental morphology, and more better to predict crystal growth morphology. Therefore, we ascertain that the predicted crystal morphologies for carbohydrazide perchlorates by AE model are reliable.
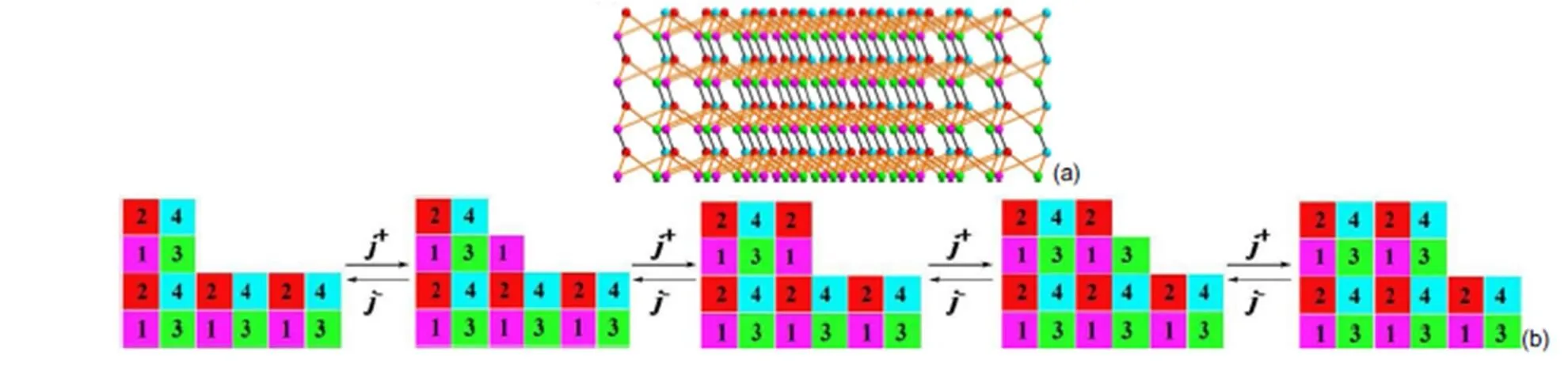
Fig.12 The bonding network of the (002) face; (a) top view of the face, (b) schematic image of incorporation of growth units.
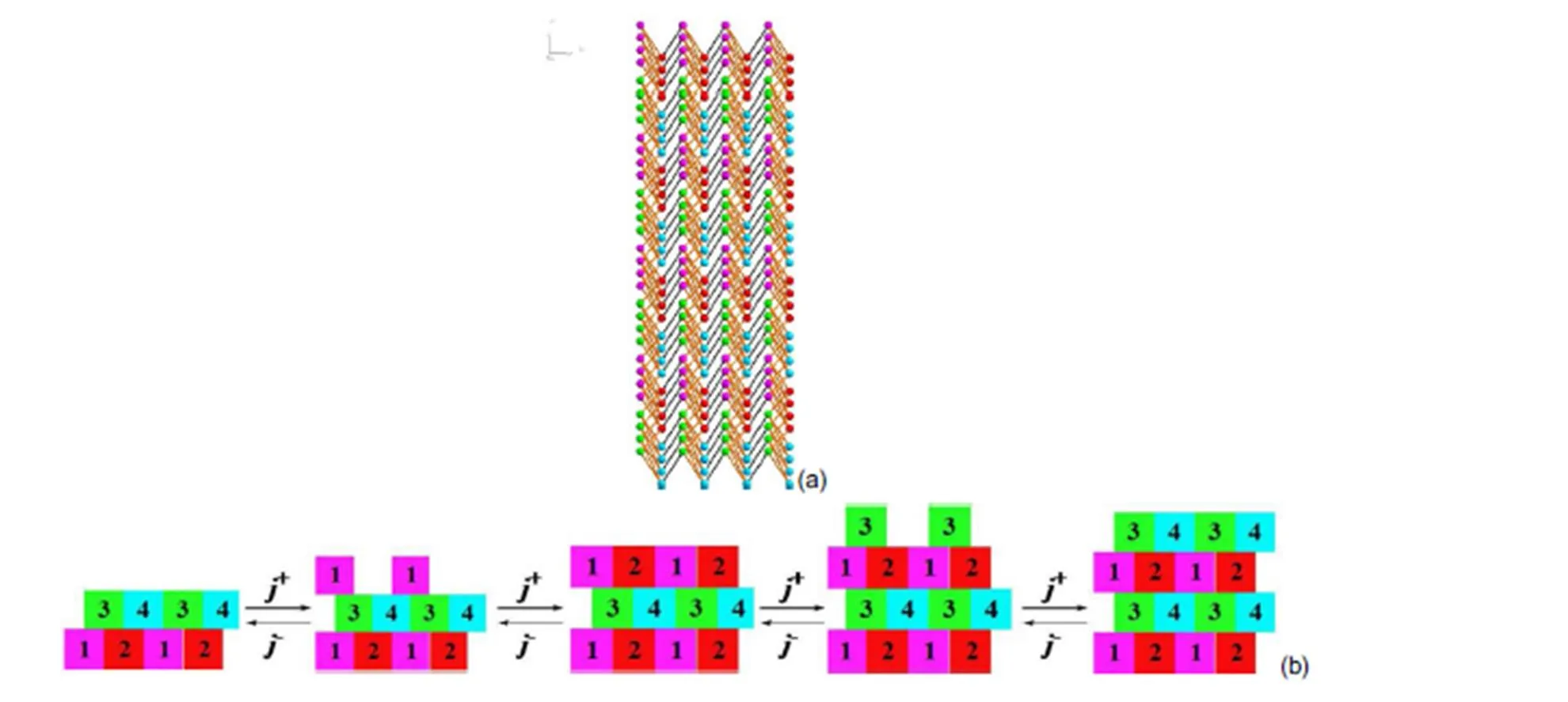
Fig.13 The bonding network of theface; (a) top view of the face, (b) schematic image of incorporation of growth units.
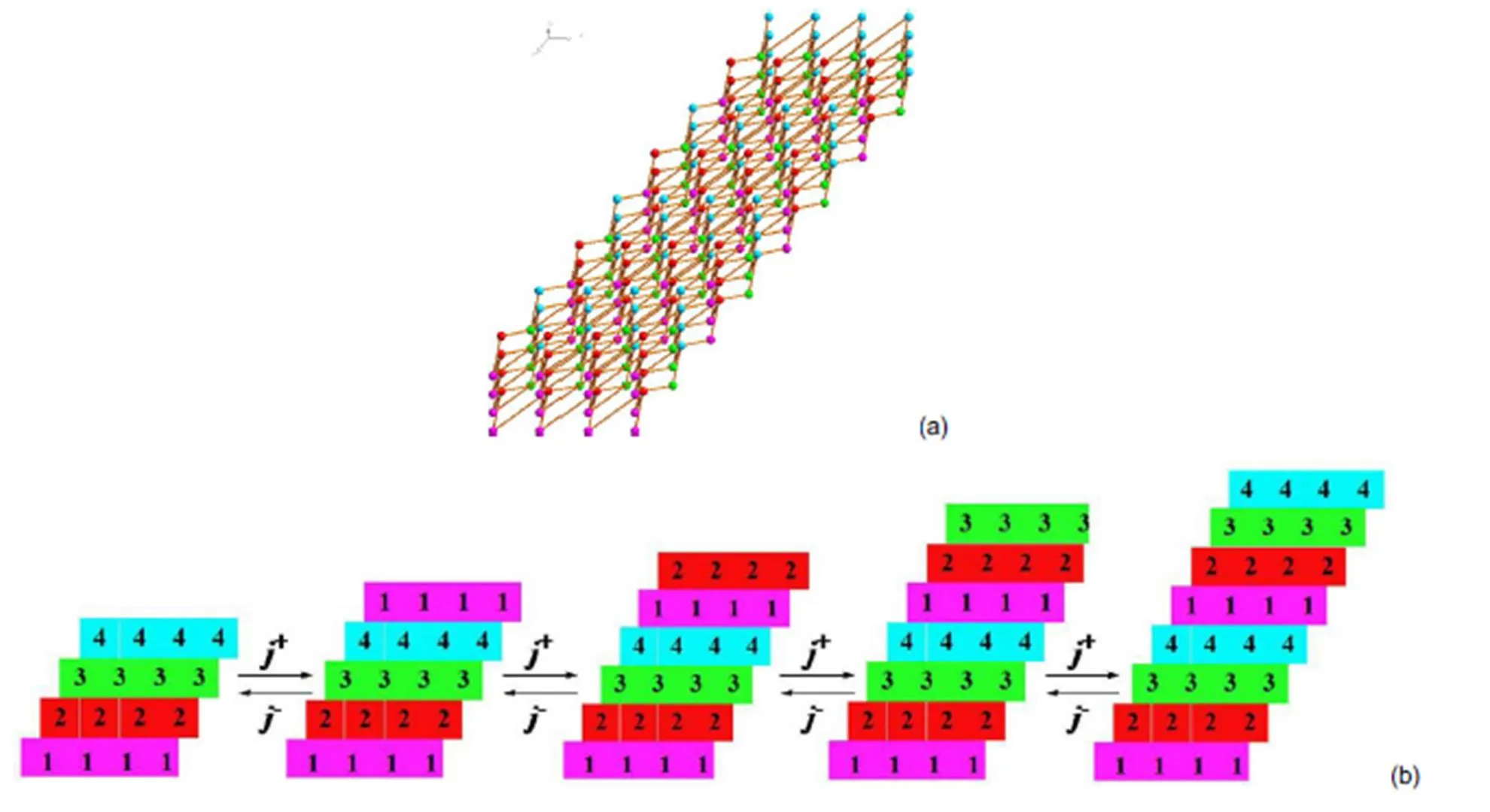
Fig.14 The bonding network of the (011) face; (a) top view of the face, (b) schematic image of incorporation of growth units.
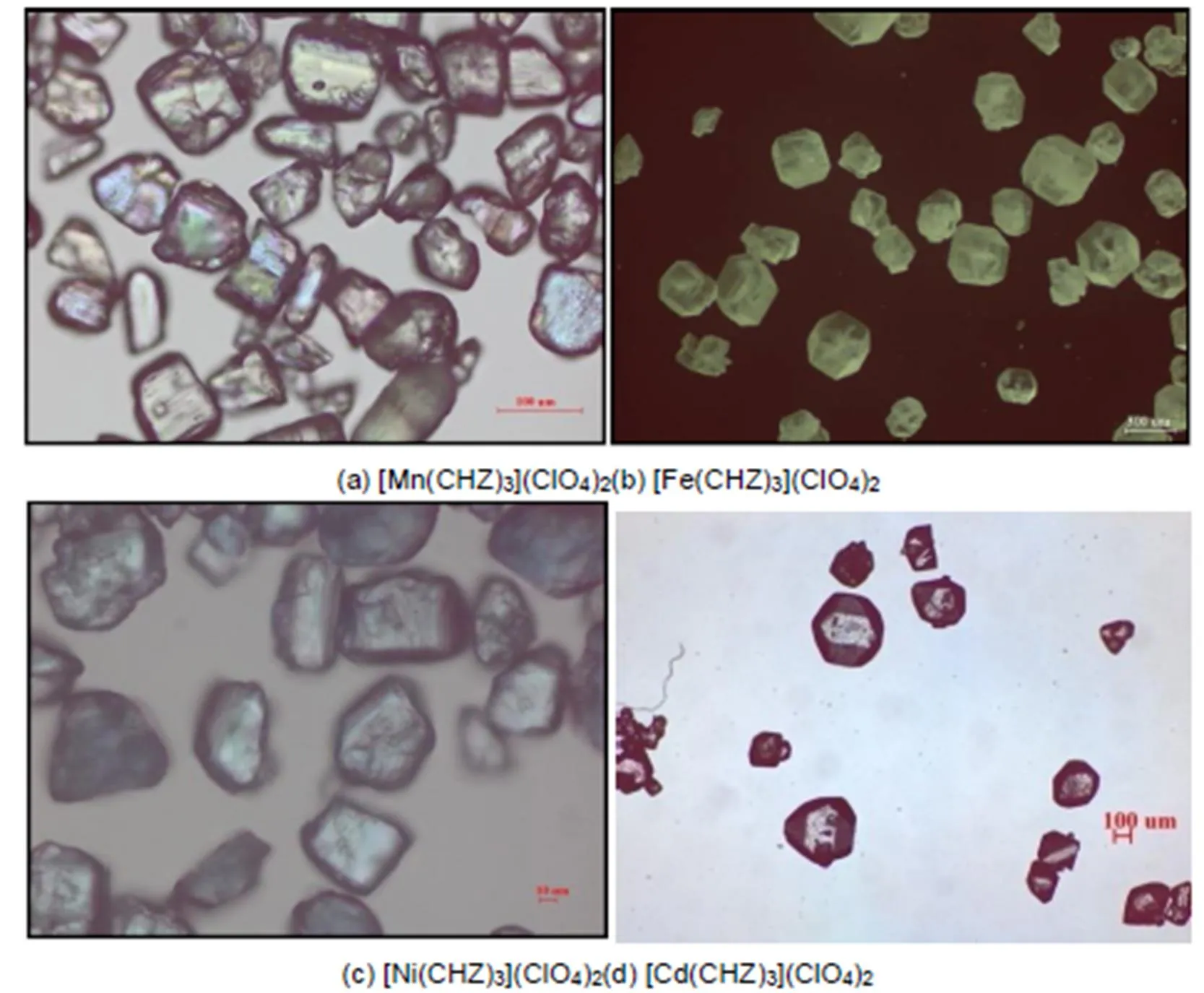
Fig.15 Crystal-morphology of [Mn(CHZ)3](ClO4)2, [Fe(CHZ)3](ClO4)2, [Ni(CHZ)3](ClO4)2 and [Cd(CHZ)3](ClO4)2 without crystal-control reagent.
4 Conclusions

(1) Duan, X.; Wei,C.; Liu,Y.; Pei,C.. 2010, 174. doi: 10.1016/j.jhazmat.2009.09.03
(2) Czerski, H.; Proud, W.. 2007,, 113515. doi: 10.1063/1.2818106
(3) Taylor, G.; Thomas, A. T.1968,, 391. doi: 10.1016/0022-0248(68)90181-4
(4) Baer, M. R.2002,, 351. doi: 10.1016/S0040-6031(01)00794-8
(5) Fabbiani, F. P.; Pulham, C. R.. 2006,, 932. doi: 10.1039/B517780B
(6) Kröber, H.; Teipel, U.. 2008,, 33. doi: 10.1002/prep.200800205
(7) Kishore, K.; Sunitha, M. R.. 1979,, 1118. doi: 10.2514/3.61286
(8) Akiyoshi, M.; Nakamura, H.; Hara, Y.. 2000,, 41. doi: 10.1002/(SICI)1521-4087(200001)25:1<41:: AID-PREP41>3.0.CO;2-X
(9) Schoyer, H. F. R.; Welland-Veltmans, W. H. M.; Louwers, J.; Korting, P. A. O. G.; vander Heijden, A. E. D. M.; Keizers, H. L. J.; vanden Berg, R. P.2002,, 138.
(10) Dutta, R. L.; Sarkar, A. K. J.. 1981,, 2557. doi: 10.1016/0022-1902(81)80302
(11) Mansour, A. K.; Eid, M. M.; Khalil, N. S.2003,, 744. doi: 10.3390/81000744
(12) Akiyoshi, M.; Hirata, N. ;Nakamura, H.; Hara, Y..1996,, 238.
(13) Bustos, C.; Burckhardt, O.; Schrebler, R.; Carrillo, D.; Arif, A.; Cowley, A.; Nunn, C.. 1990,, 3996. doi: 0020-1669/90/1329-3996$02.50/0
(14) Bushuyev, O. S.; Arguelles, F. A.; Brown, P.; Weeks, B. L;. Hope-Weeks, L. J.. 2011, 4622. doi: 10.1002/ejic.201100465
(15) Rahn, P. C.; Siggia, S.. 1973,, 2336. doi: 10.1021/ac60336a012
(16) Akiyoshi, M.; Hirata, N.; Nakamura, H.; Hara, Y..1997,, 68.
(17) Akiyoshi, M.; Nakamura, H.; Hara, Y.. 2000,, 224. doi: 10.1002/1521-4087(200011)25:5<224: AID-PREP224>3.0.CO;2-O
(18) Talawar, M. B.; Agrawal, A. P.; Chhabra, J. S.; Asthana, S. N.. 2004,, 57. doi:10.1016/j.jhazmat.2004.07.001
(19) Qi, S. Y.; Li, Z. M.; Zhang, T. L.; Zhou, Z. N.; Yang, L.; Zhang, J. G.; Qiao, X. J. ;Yu, K. B.. 2011,, 987. doi: http://sioc-journal.cn/Jwk_hxxb/CN/Y2011/V69/I08/987
(20) Mi, Z.H.; Chen, S. T.; Jing, Z.; Yang, L.; Zhang, T. L.. 2016,, 3978. doi: 10.1002/ejic.201600479
(21) Mi, Z.H.; Zhang, T. L.; Zhang, J. G.; Zhou, Z. N.; Yang, L.. 2016,, 46828. doi: 10.1039/C6RA07277A
(22) Joas, M.; Klapotke, T. M.. 2015,, 246. doi: 10.1002/prep.201400142
(23) Casewit, C. J.; Colwell, K. S.; Rappe, A. K.. 1992,, 10035. doi: 0002-7863/92/1514-10035$03.00/0
(24) Casewit, C. J.; Colwell, K. S.; Rappe, A. K.. 1992,, 10046. doi: 0002-7863/92/1514-10046$03.0
(25) Rappe, A. K.; Colwell, K. S.; Casewit, C. J.. 1993,, 3438. doi: 0020-16691931 1332-3438%04.0
(26) Kern, A.; Nather, C.; Studt, F.; Tuczek, F.. 2004,, 5003. doi: 10.1021/ic030347d
(27) Bureekaew, S.; Amirjalayer, S.; Tafipolsky, M.; Spickermann, C.;Roy, T. K.; Schmid, R.2013,, 1128.doi: 10.1002/pssb.201248460
(28) Ogawa, T.; Kurita, N.; Sekino, H.; Kitao, O.; Tanaka, S.. 2003,, 271. doi: 10.1016/S0009-2614(03)00720-6
(29) Rappe, A. K.; Casewit, C. J.; Colwell, K. S.; Goddard, W. A.; Skiff, W. M.. 1992,, 10024. doi: 10.1021/ja00051a040
(30) Fischer, T. H.; Almlof, J.. 1992,, 9768. doi: 10.1021/j100203a036
(31) Perdew, J. P. Chevary, J. Vosko, S. Jackson, K. A. Pederson, M. R. Singh, D. Fiolhais, C..1992,, 6671. doi: 10.1103/PhysRevB.46.6671
(32) Perdew, J. P.; Wang, Y.1992,, 12947. doi: 10.1103/PhysRevB.46.12947.
(33) Docherty, R.; Clydesdale, G.; Roberts, K. J.; Bennema, P.. 1991,, 89. doi: 10.1088/0022-3727/24/2/001
(34) Hartman, P.; Perdok, W. G. I.. 1955,, 49. doi: 10.1107/S0365110X55000121
(35) Bennema, P.; Meekes, H. ;Boerrigter, S.; Cuppen, H.; Deij, M.; Van Eupen, J.; Verwer, P.; Vlieg, E.. 2004,, 905. doi: 10.1021/cg034182v
(36) Berkovitch-Yellin, Z.. 1985,, 8239. doi: 0002-7863/8S/l507-8239$01.50/0
(37) Kawasaki, T.; Tanaka, H. P.. 2010,, 14036. doi: 10.1073/pnas.1001040107/-/DCSupplemental
(38) Chen, J. X.; Wang, J. K.; Zhang, Y.; Wu, H.; Chen, W.; Guo, Z. C.2004,, 266. doi: 10.1016/j.jcrysgro.2004.01.055
(39) Givand, J. C.; Rousseau, R. W.; Ludovice, P. J.1998,, 228. doi: 10.1016/S0022-0248(98)00535-1
(40) Lv, C. H.; Zhang, T. L.; Ren, L. B.; Yu, K. B.; Lu, Z.; Cai, R. J.. 2000,, 31. doi: 1007-7812( 2000D 01-0031-03
高氯酸碳酰肼过渡金属配合物晶体形态的理论和实验研究
杨 利*张国英 刘 影 张同来
(北京理工大学,爆炸科学与技术国家重点实验室,北京 100081)

晶体形貌;预测;附着能;生长速率
O641
10.3866/PKU.WHXB201706193
May 17, 2017;
June 13, 2017;
June 19, 2017.
Corresponding author. Email: yanglibit@bit.edu.cn; Tel: +86-10-68911682.
The project was supported bythe State Key Laboratory of Explosion Science and Technology, China (YB2016-17) and the National Natural Science Foundation of China (11672040).
爆炸科学与技术国家重点实验室基金(YB2016-17)及国家自然科学基金(11672040)资助项目

