苯并咪唑-5,6-二羧酸Mnギ和Cdギ混配配合物的合成、结构及性质
王永利 魏 珍 罗永华 张朋美 庚丽丽 王立华 廖 波
(1燕山大学亚稳材料制备技术与科学国家重点实验室,秦皇岛 066004)
(2河北北方学院,张家口 075000)
苯并咪唑-5,6-二羧酸Mnギ和Cdギ混配配合物的合成、结构及性质
王永利1,2魏 珍*,2罗永华2张朋美2庚丽丽2王立华1,2廖 波*,1
(1燕山大学亚稳材料制备技术与科学国家重点实验室,秦皇岛 066004)
(2河北北方学院,张家口 075000)
在水热条件下,合成了 2 个配位聚合物{[Mn(Hbidc)(2,2′-bpy)(H2O)2]·1.5H2O}n(1)和{[Cd(Hbidc)(phen)][Cd(phen)2Cl2]}n(2)(H3bidc=苯并咪唑-5,6-二羧酸,2,2′-bpy=2,2′-联吡啶,phen=菲咯啉),并通过 X 射线单晶衍射、红外、元素分析、X 射线粉末衍射和热重对配合物结构进行了表征。配合物1是一维无限zig-zag链结构,可以通过O-H…O和N-H…O氢键的相互作用形成三维超分子结构。配合物2也是一维无限链结构。此外,测试了配合物1和2的固体紫外吸收光谱和研究了配合物2的固体荧光性能。
苯并咪唑-5,6-二羧酸;N-供体辅助配体;晶体结构;荧光性能
There is great current interest in the synthesis of novel coordination polymers,not only due to their fascinating variety of architectures and topologies,but also due to their potential applications in functional materials,such as gas adsorption[1],separation[2],catalysis[3],magnetism[4]and photoluminescent properties[5-6].Until now,a number of novel coordination polymers with outstanding properties have been obtained[7-9].
It is well-known that rational selection of a ligand with suitable shape,functionality,flexibility,and symmetry makes a pivotal role in constructing and tailoring metal-organic architectures with desirable properties[10-11].Recently,metal complexes for heterocyclic carboxylic acid benzimidazole-5,6-dicarboxylic acid(H3bidc)have attracted particular attention,owing to providing various coordination modes and multidimensional structures[12].It can act as a multidentate ligand with both N-and O-donor atoms,and easily coordinate with metal ions to form multidimensional complexes;the benzimidazole ring might contribute much to form π…π stacking interactions,and the NH group on the imidazole ring can act as proton-donor to form hydrogen bonds,which also play important roles in the formation of coordination complexes.
At the same time,N-donor ligands are frequently used as auxiliary ligands to design and synthesis of novel structural motifs.N-donor ligands,such as 2,2′-bipyridine[13],4,4′-bipyridine[14]and 1,10-phenanthroline[15],which were usually selected to construct interesting supramolecular architectures as the auxiliary co-ligands,can provide some insight in the construction of novel structures.
Considering all these above-mentioned,we think that the simultaneous use of the N-donor auxiliary ligand and benzimidazole-5,6-dicarboxylic acid may generate diverse supramolecular structures and potential applications.However,only a few examples of the metal complexes containing the mixed ligands of benzimidazole-5,6-dicarboxylic acid and 1,10-phenanthroline have been reported in coordination chemistry until now[16].In this paper,two coordination polymers from metal ions,benzimidazole-5,6-dicar-boxylic acid,and N-donor auxiliary ligand,namely{[Mn(Hbidc)(2,2′-bpy)(H2O)2]·1.5H2O}n(1),and{[Cd(Hbidc)(phen)][Cd(phen)2Cl2]}n(2)are reported.In addition,the UVVis absorption spectra of 1 and 2 were measured at room temperature and the fluorescent property of 2 was studied in the solid at room temperature in detail.
1 Experimental
1.1 Materials and general methods
All chemicals were commercially available and used as received without further purification.Elemental analyses(C,H,N)were carried out on an Elementar Vario ELⅢanalyzer.The infrared spectra were recorded from KBr pellets in the 4 000~400 cm-1range on a FTIR-650 spectrometer.The powder XRD data were collected on a Bruker D8 Advance X-ray diffractometer using Cu Kα radiation(λ=0.1548 nm)generated at 40 kV and 40 mA in a 2θ range of 5°~50°.Thermogravimetry analyses(TGA)were performed with a PTC-10ATG-DTA instrument in flowing N2at a heating rate of 10 ℃·min-1from 25~800 ℃.The UVVis absorption spectra were obtained using a Hitachi UV-3010 UV-Vis spectrophotometer.The luminescence spectra were detected on a Hitachi F-4500 fluorescence spectrophotometer.
1.2 Synthesis of complex{[Mn(Hbidc)(2,2′-bpy)(H2O)2]·1.5H2O}n(1)
A mixture of MnSO4·H2O(0.2 mmol),H3bidc(0.2 mmol),2,2′-bpy(0.2 mmol),H2O (8 mL),and an aqueous solution of NaOH(2.0 mol·L-1,0.25 mL)was sealed in a 25 mL Teflon-lined reactor and heated at 170℃for 3 days.After slow cooling to room temperature,yellow bulk crystals of the complexes were obtained.Yield:65%based on MnSO4·H2O.Anal.Calcd.for C19H19MnN4O7.50(%):C,47.71,H,4.00,N,11.71.Found(%):C,47.32,H,4.06,N,11.65.IR data(KBr pellets,cm-1,Fig.1):3 150(vs),1 600(s),1 561(vs),1 490(s),1 473(s),1 440(s),1 402(vs),1 361(s),1 336(m),1 313(m),1 280(w),1 267(w),1 245(w),1 182(w),1 157(w),1058(vw),1016(w),937(w),885(w),811(w),761(s),736(m),666(m),647(w),616(m),449(vw),406(ww).
1.3 Synthesis of complex{[Cd(Hbidc)(phen)][Cd(phen)2Cl2]}n(2)
A solution of H3bidc(0.5 mmol)in 6 mL H2O was added dropwise with stirring at room temperature to a solution of Cd(NO3)2·4H2O(1.0 mmol)and phen(0.5 mmol)in the mixture of 10 mL water and 5 mL ethanol.Then an aqueous solution of hydrochloric acid was added dropwise with stirring to adjust the pH value of the solution being 4.The resulting mixture was sealed in a 25 mL Teflon-lined stainless reactor,kept under autogenous pressure at 160℃for 3 days,and then slowly cooled to room temperature at a rate of 10℃·h-1.The colorless block crystals for X-ray diffraction were isolated directly,washed with ethanol and dried in air.Yield:62% based on Cd(NO3)2·4H2O.Anal.Calcd.for C57H36Cd2Cl2N10O4(%):C,56.08,H,2.97,N,11.47.Found(%):C,56.02,H,2.65,N,11.55.IR data (KBr pellets,cm-1,Fig.1):3 432(vs),1 621(s),1 554(vs),1 515(vs),1 467(s),1 421(s),1 392(s),1 357(m),1 338(m),1 220(w),1 182(w),1 139(w),1 009(vw),944(w),862(w),844(w),781(s),727(m),636(m),418(ww).
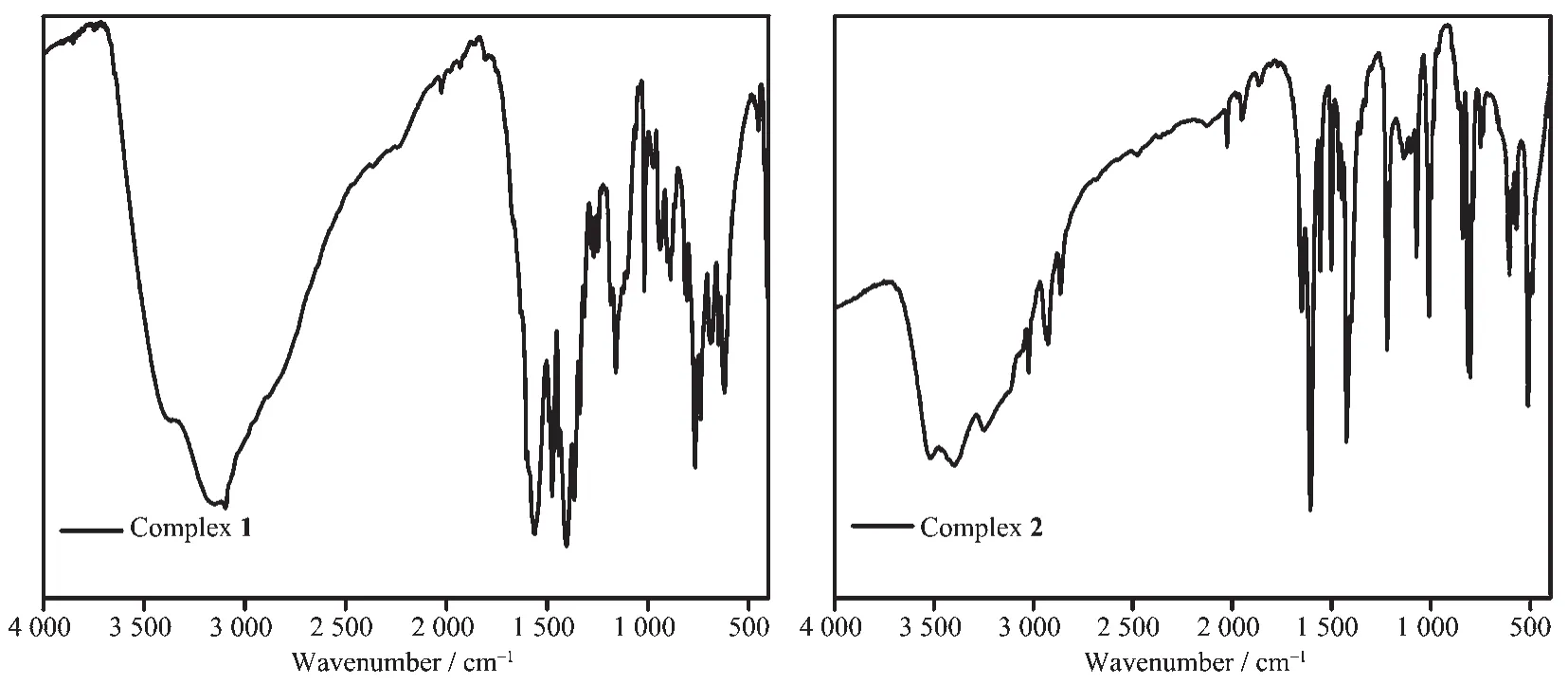
Fig.1 FT-IR spectra of 1 and 2
1.4 Structure determination
Single-crystal X-ray analysis of the complexes 1 and 2 were carried out on a Bruker SMART APEX CCD diffract meter using graphite-monochromatized Mo Kα radiation (λ=0.071 073 nm)at room temperature using the ω-scan technique[17].Crystal size of complexes 1 and 2 are 0.20 mm×0.20 mm×0.16 mm and 0.24 mm×0.20 mm×0.18 mm,respectively.The structure was solved by direct methods with SHELXS-97[18]and refined with the full-matrix least-squares technique using the SHELXL-97 program[19].All nonhydrogen atoms were refined anisotropically and the hydrogen atoms of organic ligands were generated geometrically.The crystallographic data are listed in Table 1.Selected bond lengths and angles are listed in Table 2.
CCDC:1040339,1;1472092,2.

Table1 Crystallographic data for complexes 1 and 2
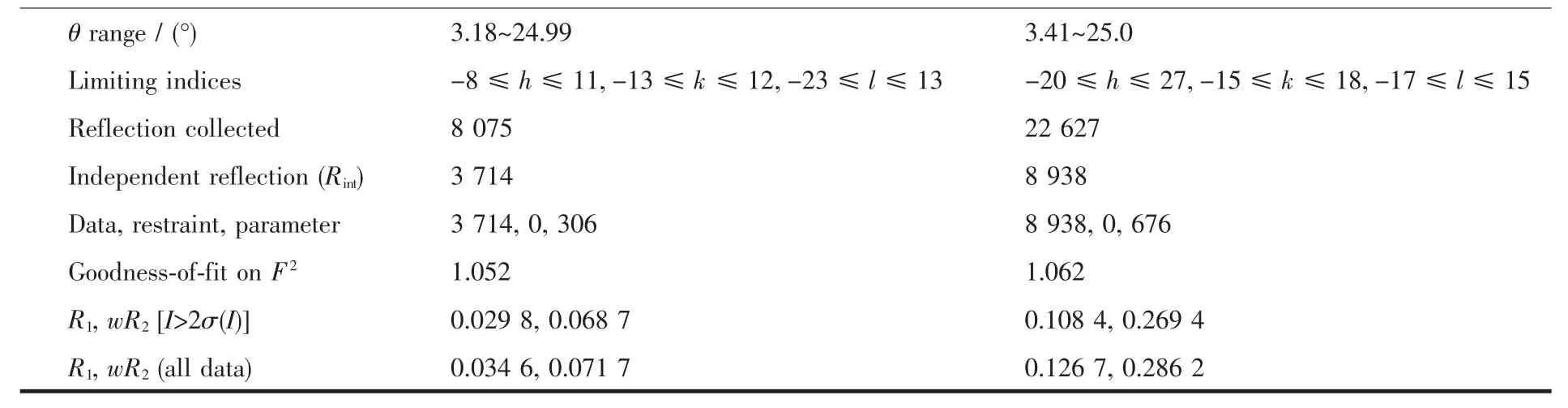
Continued Table 1

Table2 Selected bond lengths(nm)and angles(°)for complexes 1 and 2
2 Results and discussion
2.1 Structure description of 1
Single-crystal X-ray diffraction study reveals that complex 1 belongs to P21/n space group.The asymmetric unit of complex 1 consists of one Mnギion,two Hbidc2-ligands,one 2,2′-bpy ligand,two coordinated water molecules.As shown in Fig.2a,each Mnギion is six-coordinated in a distorted octahedron arrangement by two atoms(N3,O4)from two Hbidc2-ligands,two nitrogen atoms(N1,N2)from one 2,2′-bpy ligand,and two oxygen atoms(O5,O6)from two coordinated water molecules.The Mn-O bond lengths are in the range of 0.213 3(13)~0.225 3(12)nm,while the Mn-N bond lengths are slightly longer,0.213 6(15)~0.225 1(16)nm.The bond angles vary from 88.60(5)°to 176.94(5)°,all of which are similar to those found in other six coordinated Mnギcompounds with O-and N-donating complexes[20].The two oxygen atoms(O4,O6)occupy the axial positions,and the other nitrogen atoms(N1,N2,N3)and oxygen atom (O5)comprise the equatorial plane.
In this manner,a zig-zag chain is formed by direct connection of Mnギatoms bridged by Hbidc2-ligands along the b-axis,with the Mn…Mn separation being 1.037(5)nm(Fig.2b).Adjacent 1D chains connect to form a 2D layer network through intermolecular O1W-H1WB…O6 and O6-H6B…O1 hydrogen bonds(Table 3).Furthermore,the 2D layer network is extended into a 3D supramolecular network by O2WH2WA…O1,O2W-H2WB…O3,O5-H5B…O3 and O6-H6A…O3W hydrogen bonds(Fig.2c).Therefore,the O-H…O and N-H…O hydrogen bonds play an important role in stabilizing the crystal structure.

Fig.2 (a)Molecular structure of 1 with numbering scheme;(b)View of an infinite chain of complex 1;(c)Hydrogen-bonding interactions linking layers into a 3D supramolecular network of complex 1
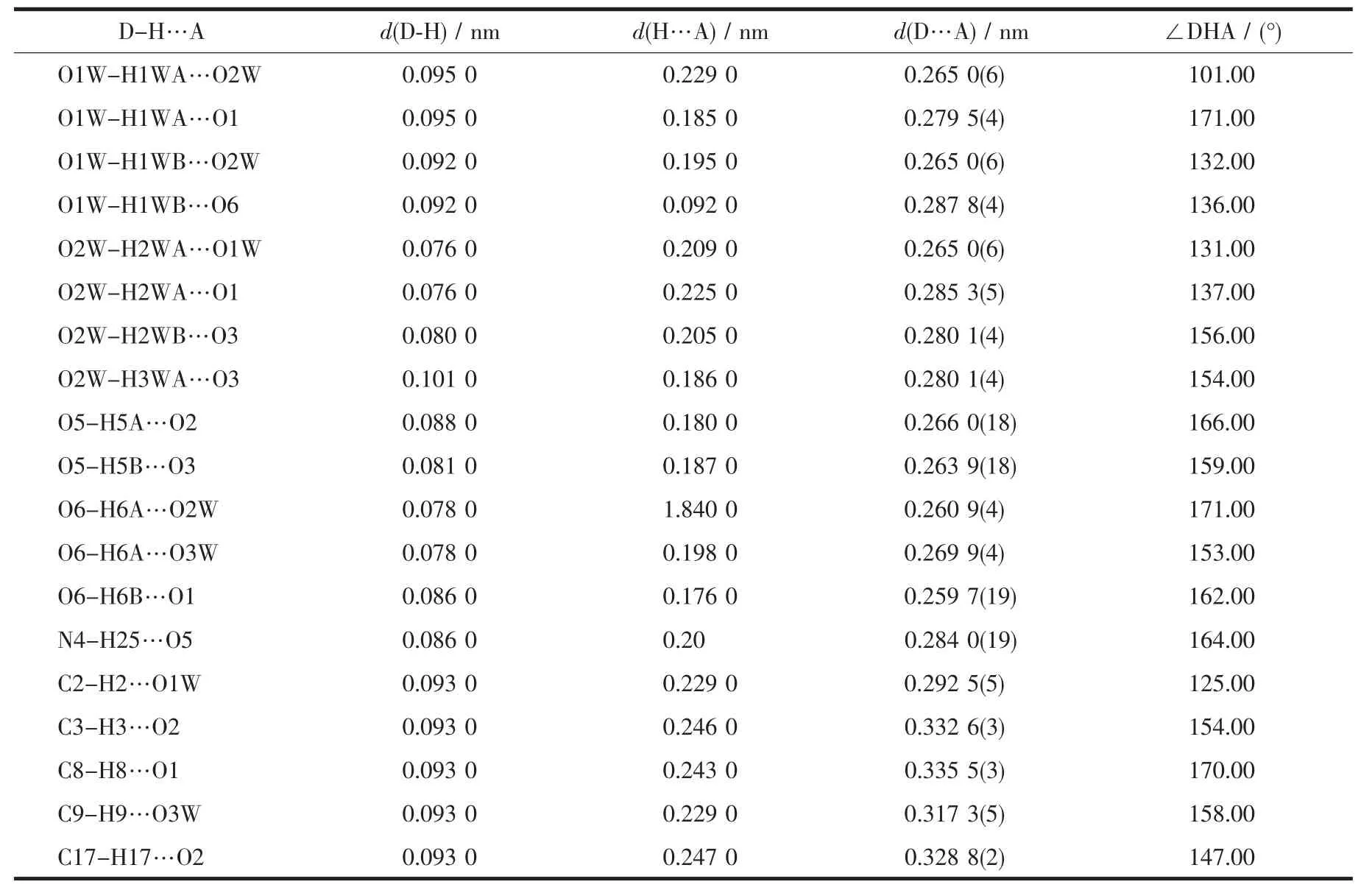
Table3 Hydrogen-bonding geometry for 1
2.2 Structure description of 2
Single-crystal X-ray diffraction analysis reveals that complex 2 crystallizes in the P21/c space group.The asymmetric unit contains two Cdギions,one Hbidc2-ligand,four phen ligands,and two chloride ions.The Cd1 ion adopts a slightly distorted octahedral geometry via coordinating to one N atom(N4)of phen and one O atom (O1)of Hbidc2-in the axial position,two N atoms(N1 and N2)of one phen ligand,one N atom (N3)of phen and one O atom (O4)of Hbidc2-in the equatorial plane.The Cd2 center also adopts a distorted octahedral geometry,which is provided by one Cl ion (Cl1)and one phen N atom(N9)in the axial position,as well as three N atoms from two phen ligands(N7,N8,N10),one Cl ion(Cl2)in the equatorial plane(Fig.3a).The Cd-N,Cd-O bond lengths and O-Cd-O,O-Cd-N,N-Cd-N angels,all of which are within the range of those observed for analogous Cd complexes[21];e.g.,0.225 6(9)~0.252 2(4)nm,and 68.1(3)°~175.3(3)°.In the complex 2,Hbidc2-chelats in a monodentate manner,whereas the phen ligand displays a chelating bidentate coordination modes to link two Cdギions.The Hbidc2-ligands link the Cd1 ions to form a one-dimensional chain with a Cd…Cd distance of 0.740 8(16)nm along the c axis(Fig.3b).

Fig.3 (a)Molecular structure of 2 with numbering scheme;(b)View of an infinite chain of complex 2
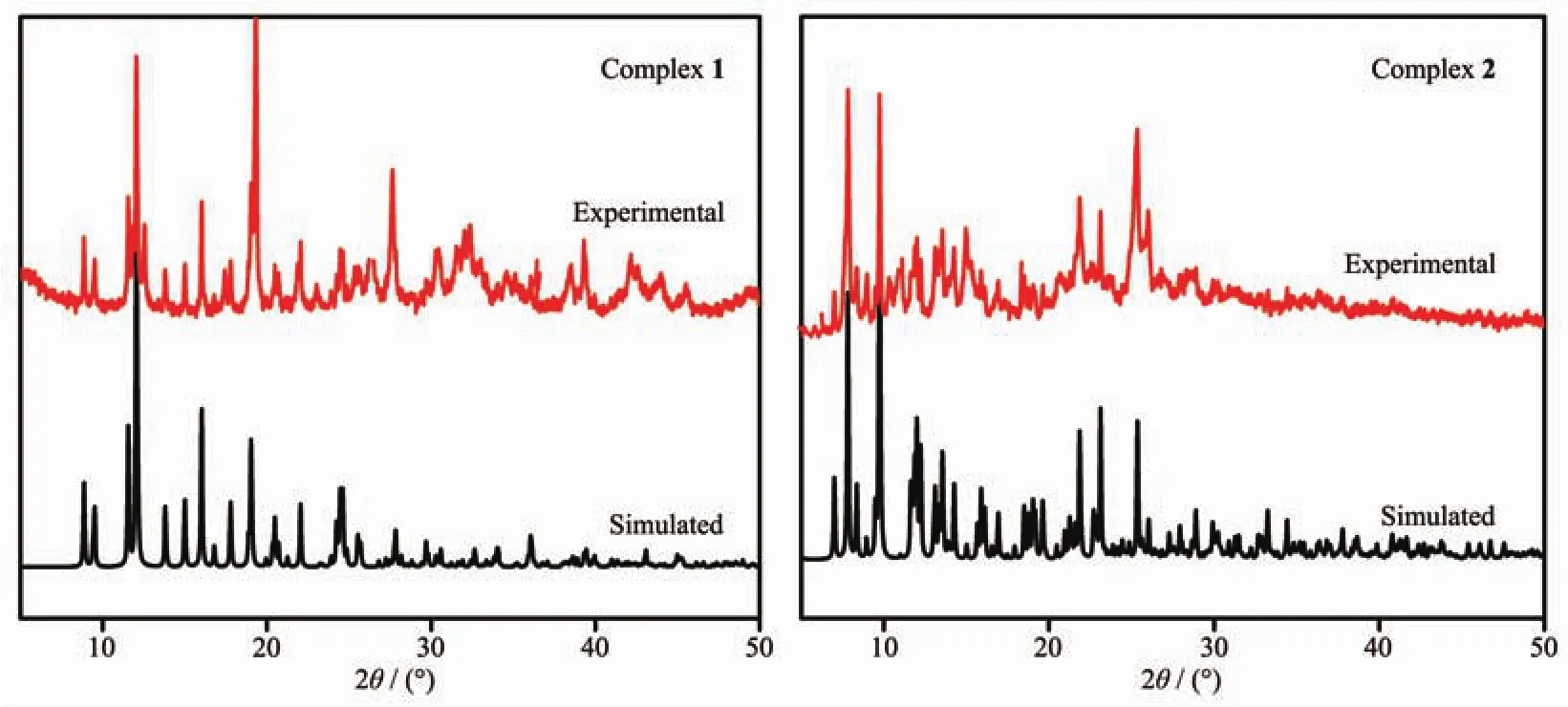
Fig.4 PXRD patterns of 1 and 2
2.3 PXRD patterns
To confirm the mass identities and phase purity of 1 and 2,powder X-ray diffraction (PXRD)experiments were carried out for complexes 1 and 2.The PXRD experimental and computer-simulated patterns of the corresponding complexes are shown in Fig.4.The PXRD patterns of as-synthesized complexes 1 and 2 are coincident with the simulated ones from the single-crystal data,indicative of its high purity and homogeneity.
2.4 Thermogravimetric analysis
To investigate their thermal stabilities,thermogravimetric analyses(TGA)of complexes 1 and 2 were carried out at a heating rate of 10℃·min-1.TGA traces for complexes 1 and 2 are depicted in Fig.5.For complex 1,the first weight loss of 8.17%in the range of 102~135 ℃ corresponds to the loss of the release ofthe free water molecules and one coordinated water molecule(Calcd.9.4%).The TGA curve displays that it was stable up to about 351℃.The other weight loss in a temperature range of 352~434℃were attributed to the remaining coordinated water molecule and the decomposition of all organic ligands.For complex 2,a weight loss of 14.5%between 243 and 400℃is in accordance with the release of two chloride ions and two phen water molecules in complex 2(Calcd.17.6%).Further heating above401℃causesthenetworktocollapse accompanied by the decomposition of the organic ligands,but this degradation does not end upon 800℃.It suggests that complex 2 is rather stable on heating,which shows higher thermal stability.

Fig.5 TGA curves of complexes 1 and 2
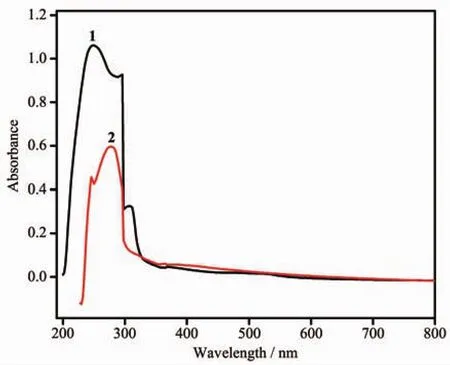
Fig.6 UV-Vis spectra of 1 and 2 in the solid state at room temperature
2.5 Semiconductor properties and optical band gaps
Severalcoordination architectureshave been certified to be promising semiconductors,so the UVVis absorption spectra of 1 and 2 were measured in the solid state at room temperature.The UV-Vis absorption spectra of 1 and 2 show a broad absorption bands in the UV regions at 200~300 nm as shown in Fig.6,which can be principally ascribed to the π-π*or n-π*transitions of ligands[22].Meanwhile,the band gap energy of 1 and 2 can be calculated according to the formula:Eg=1 240/λg(eV),where Egis the band gap energy(eV)and λgis the absorption edge wavelength (nm).The Egvalues are 4.12 eV for 1 and 4.04 eV for 2,as shown in Fig.7,which indicates that 1 and 2 may be beneficial to catalysis and that 1 and 2 have potential semiconductor properties.
2.6 Luminescent properties
Luminescence properties of Znギcomplexes have attracted considerable attention because oftheir potentialapplicationsasvaluableluminescentmaterials.The solid-state luminescence properties of complex 2 as well as the benzimidazole-5,6-dicarboxylic acid ligand and 1,10-phenanthroline auxiliary ligand were investigated at room temperature.According to the reported literature[16],upon excitation at 365 nm for benzimidazole-5,6-dicarboxylic acid and 378 nm for 1,10-phenanthroline,the pure ligands display fluorescence spectra with emission maxima bands at 430 nm for benzimidazole-5,6-dicarboxylic acid and 438 nm for 1,10-phenanthroline.The emission spectrum of complex 2 is depicted in Fig.8.Complex 2 exhibits an intense blue luminescentproperty with emission maxima at 416 nm under excitation at 370 nm,which indicates that this emission band of 2 is neither ligand-to-metal charge transfer(LMCT)nor metal-toligand charge transfer(MLCT)because Znギion is difficult to reduce or oxidize,but rather could be attributed to intraligand transition.The luminescent emission band in the blue area demonstrates that complex 2 may be potential emitting material.
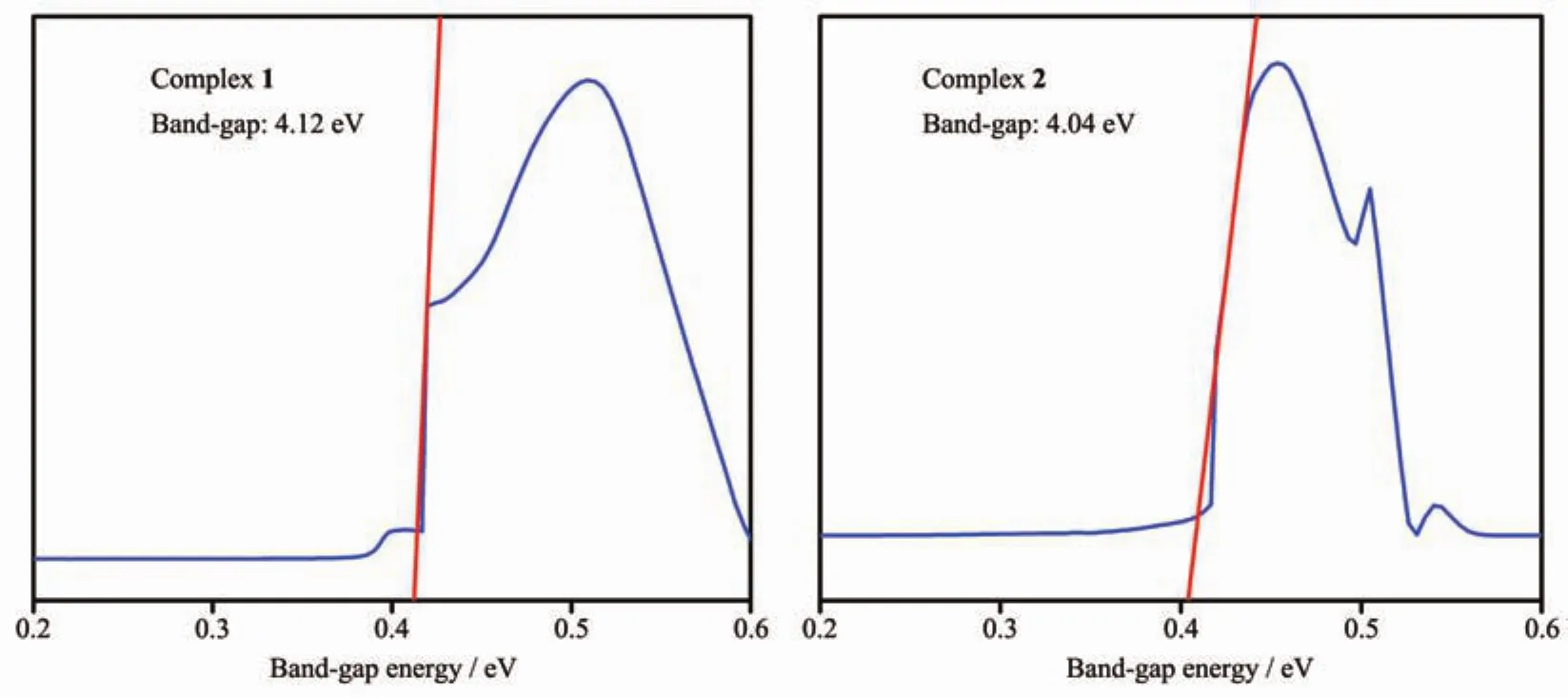
Fig.7 Diffuse reflectance spectra of K-M functions versus band-gap energy of complexes 1 and 2 at room temperature

Fig.8 Emission spectrum of 2 in the solid state at room temperature
3 Conclusions
In summary,two new coordination polymers with benzimidazole-5,6-dicarboxylic acid,2,2′-bipyridine or 1,10-phenanthroline ligands have been synthesized and structurally characterized by single-crystal X-ray diffraction,IR spectroscopy,elemental analysis,powder X-ray diffraction (PXRD)and thermogravimetric analysis (TGA).Complexes 1 and 2 consist of different 1D chain structures.The one-dimensional zig-zag chain is formed by the Hbidc2-ligands and the Mnギions,which are extended into a 3D supramolecular framework via O-H…O and N-H…O hydrogen bonding interactions for complex 1.In addition,the UV-Vis absorption spectra indicate that complexes 1 and 2 show a broad absorption band in the UV regions of 200~300 nm and 1 and 2 have the Egvalues of 4.12 eV for 1 and 4.04 eV for 2,which demonstrates that 1 and 2 have potential semiconductor properties.The emission spectrum of complex 2 exhibits an intense blue fluorescent emission with emission maxima at 416 nm under excitation at 370 nm,indicating that complex 2 may be potential emitting material.
[1]Rowsell J L C,Yaghi O M.Angew.Chem.Int.Ed.,2005,44:4670-4679
[2]Sudik A C,Millward A R,Ockwig N W,et al.J.Am.Chem.Soc.,2005,127:7110-7118
[3]Leng J D,Liu J L,Zheng Y Z,et al.Chem.Commun.,2013,9:158-160
[4]Kurmoo M.Chem.Soc.Rev.,2009,38:1353-1379
[5]Pasatoiu T D,Tiseanu C,Madalan A M,et al.Inorg.Chem.,2011,50:5879-5889
[6]ZHANG Xiao-Ge(张潇戈),GAO Lou-Jun(高楼军),CHEN Xiao-Li(陈小莉),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(4):739-748
[7]WANG Hui-Sheng(王 会 生),YUE Lin(乐 琳),PAN Min(潘敏),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,32(1):153-160
[8]Pattanayak P,Pratihar J L,Patra D,et al.Polyhedron,2014,79:43-51
[9]Appelhans L N,Kosa M,Radha A V,et al.J.Am.Chem.Soc.,2009,131:15375-15386
[10]Zheng R,Zhou Q Z,Gu H N,et al.Tetrahedron Lett.,2014,55:5671-5675
[11]Hong X J,Wang M F,Jin H G,et al.CrystEngComm,2013,15:5606-5611
[12]Sun Y G,Gu X F,Ding F,et al.Cryst.Growth Des.,2010,10:1059-1067
[13]Ji B M,Deng D S,He X,et al.Inorg.Chim.Acta,2014,416:102-108
[14]Yang M Z,Shi Y,Wang S,et al.Inorg.Chem.Commmun.,2014,48:86-89
[15]Jing B Q,Dong J F,Wei Q,et al.Transition Met.Chem.,2014,39:605-611
[16]Xu T,Li L Z,Ma X,et al.Cryst.Growth Des.,2012,12:5227-5232
[17]SMART and SAINT,Siemens Analytical X-Ray Systems Inc,Madison,WI,1996.
[18]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structures,University of Göttingen,Germany,1997.
[19]Sheldrick G M.SHELXL-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[20]Qi Y,Wang Y.Polyhedron,2014,73:133-138
[21]Li B,Yu K,Wang C M,et al.J.Inorg.Organomet.Polym.,2014,24:525-530
[22]Dong Y W,Fan R Q,Wang X M,et al.Cryst.Growth Des.,2016,16:3366-3378
Manganeseギ and Cadmiumギ Mixed-Ligand Complexes Constructed from Benzimidazole-5,6-dicarboxylic Acid:Syntheses,Crystal Structures and Properties
WANG Yong-Li1,2WEI Zhen*,2LUO Yong-Hua2ZHANG Peng-Mei2GENG Li-Li2WANG Li-Hua1,2LIAO Bo*,1
(1State Key Laboratory of Metastable Materials Science and Technology,Yanshan University,Qinhuangdao,Hebei 066004,China)
(2Hebei North University,Zhangjiakou,Hebei 075000,China)
Two coordination polymer,{[Mn(Hbidc)(2,2′-bpy)(H2O)2]·1.5H2O}n(1)and{[Cd(Hbidc)(phen)][Cd(phen)2Cl2]}n(2)(H3bidc=benzimidazole-5,6-dicarboxylic acid,2,2′-bpy=2,2′-bipyridine and phen=1,10-phenanthroline)have been synthesized under hydrothermal method.They were characterized by single-crystal X-ray diffraction,IR spectroscopy,elemental analysis,powder X-ray diffraction (PXRD)and thermogravimetric analysis(TGA).Complex 1 exhibits one-dimensional infinite zig-zag chain,which is extended into a three dimensional supramolecular network via O-H…O and N-H…O hydrogen bonding interactions.Complex 2 also possesses one dimensional chain structure.In addition,the UV-Vis absorption spectra of 1 and 2 were measured at room temperature and the solid fluorescent property of 2 was studied at room temperature.CCDC:1040339,1;1472092,2.
benzimidazole-5,6-dicarboxylic acid;N-donor ancillary ligand;crystal structures;luminescent properties
O614.71+1;O614.24+2
A
1001-4861(2018)01-0170-09
10.11862/CJIC.2018.027
2017-05-31。收修改稿日期:2017-11-19。
河北省人才工程培养项目(No.A201400233)、河北省高等学校青年拔尖人才项目(No.BJ2017035)、河北省卫生厅研究项目(No.20160030)和河北北方学院博士基金项目(No.220703)资助。
*通信联系人。 E-mail:wz0209119@163.com,frxiao@ysu.edu.cn

