沙棘果肉发育期油脂合成积累的源汇基因协同表达
丁 健,阮成江*,关 莹,管文柯,单金友,吴雨蹊,吴天忠
(1.大连民族大学 资源植物研究所,辽宁 大连 116600; 2.黑龙江省农业科学院 浆果研究所,黑龙江 绥棱 152200;3.新疆林业科学院,新疆 乌鲁木齐 830064)
沙棘果肉发育期油脂合成积累的源汇基因协同表达
丁 健1,阮成江1*,关 莹2,管文柯3,单金友2,吴雨蹊2,吴天忠3
(1.大连民族大学 资源植物研究所,辽宁 大连 116600; 2.黑龙江省农业科学院 浆果研究所,黑龙江 绥棱 152200;3.新疆林业科学院,新疆 乌鲁木齐 830064)
目的探讨沙棘果肉油脂合成积累与源汇基因表达的关系。方法以8个不同发育时期的近缘高油品系‘TF2-36’和低油品系‘杂56’果肉为材料,利用氯仿甲醇法测定含油率,采用qRT-PCR技术分析油脂合成源基因(GPD1)和汇基因(DGAT1和DGAT2)在近缘高低油果肉间的表达差异及其对油脂合成积累的影响。结果研究表明:(1)沙棘果肉含油率呈先上升后稳定趋势,‘TF2-36’的果肉含油率一直高于‘杂56’;(2)GPD1、DGAT1和DGAT2基因在‘TF2-36’果肉发育期间中均有明显高于‘杂56’的表达量峰值,但GPD1表达量峰值出现在油脂快速合成期,DGAT1和DGAT2表达量峰值出现在油脂稳定积累期。GPD1在发育前期高表达,促进合成更多的TAG前体G3P,而DGAT1和DGAT2在发育后期高表达,则促进了TAG的高积累。结论沙棘果肉高油脂积累源于源基因“GPD1”和汇基因“DGAT1和DGAT2”的协同高表达,研究结果为理解沙棘非种子组织(果肉)油脂合成机理提供了理论依据。
沙棘;油脂合成;源基因;汇基因;基因表达


阮成江等[18]研究表明TAG的生物合成与源基因“GPD1”和汇基因“DGAT”相关。GPD1基因是甘油酯合成通路中的关键限速酶基因,是油脂合成的“源基因”,具有调控TAG合成底物3-磷酸甘油含量的作用[9,18];DGAT为甘油二酯酰基转移酶,是催化合成TAG的关键限速酶,也是植物油脂合成的“汇基因”[12,18]。同时调控源汇基因的表达可提高植物种子含油率,解析二者的相互作用机制对提高油脂产量和品质具有重要作用。随后有学者将脂肪酸的生物合成形容为“推”,将脂肪酸组装合成TAG形容为“拉”[19],共同表达“推”和“拉”基因可明显提高油脂含量。因此,分析沙棘果肉油脂合成积累的源基因“GPD1”和汇基因“DGAT1和DGAT2”的表达与含油率的关系,对提高沙棘果肉油脂具有重要意义。
本研究以近缘高油品系‘TF2-36’和低油品系‘杂56’果肉为材料,分析不同时期的果肉含油率,利用qRT-PCR研究源基因“GPD1”和汇基因“DGAT1和DGAT2”的表达模式及其在高低油果肉中的表达差异,揭示高低油果肉的油脂合成积累与源汇基因表达的关系,为深入理解沙棘非种子组织(果肉)油脂合成提供科学依据。
1 材料与方法
1.1 实验材料
以‘TF2-36’(蒙古沙棘亚种)和‘杂56’(蒙古沙棘和中国沙棘杂交种)为试验材料,二者间基于ISSR标记分析的遗传相似系数为0.752[20],果实分别于2015年6月25日、7月6日、7月17日、7月28日、8月8日、8月19日、8月30日和9月10日采自黑龙江省农业科学院浆果研究所。各品系样品采自3株无性繁殖植株的多个部位,同植株果实混合,用锡纸包裹后置于液氮中速冻。样品运抵大连民族大学资源植物研究所,保存于-80℃冰箱备用。
1.2 方法
1.2.1 果肉含油率测定 采用氯仿甲醇法[2,21-22]测定不同时期沙棘果肉含油率:冷冻干燥的果肉粉末转移至玻璃试管中,加入甲醇和氯仿(均为色谱纯,Honeywell公司)漩涡混匀后超声30 min,上清液转移到新试管中,残渣用氯仿甲醇(体积浓度百分比2∶1)再次提取,合并的上清液加入其1/4体积的氯化钾溶液(质量浓度0.88%),收集下层液至玻璃样品瓶中,挥发至恒质量。含油率(%)=(m1-m2)/m×100;m1为油脂和玻璃样品瓶的质量/g;m2为玻璃样品瓶的质量/g;m为干燥样品粉末的质量/g,实验设3次生物学重复。
1.2.2 qRT-PCR检测 参照柱式植物总RNA提取试剂盒(上海生物工程有限公司)方法提取沙棘果肉总RNA,根据PrimeScriptTMRT reagent Kit with gDNA Eraser试剂盒(大连宝生物公司)方法合成第一链cDNA[23]。本研究前期构建了沙棘种子、果肉、叶、茎和根转录组,获得了大量的功能基因注释以及差异表达基因信息。利用筛选获得的目的基因片段和PrimerQuest在线软件设计特异引物(表1)。参照SYBR Premix Ex TaqTMII(Tli RNaseH Plus)试剂盒(大连宝生物公司)方法和ABI7500 Real time PCR仪(美国Applied Biosystems公司)推荐程序进行qRT-PCR[11],以沙棘UBQ5为内参基因[4],采用2-ΔΔCt方法分析目的基因相对表达量[24]。实验设3次生物学重复。

表1 基因名称及qRT-PCR引物
1.2.3 数据统计分析 利用SPSS 20.0软件进行单因素方差分析和LSD法进行差异性检验,采用EXCEL2010进行作图。
2 结果与分析
2.1 沙棘果肉发育过程中含油率的动态变化
随着果实的增大,果肉颜色由绿色转为黄绿色,再转为黄色(‘杂56’)或桔黄色(‘TF2-36’,图1)。果肉含油率总体呈上升趋势,‘TF2-36’干果肉含油率一直高于‘杂56’(图2),最大增幅均发生在7月28日至8月8日期间,但‘TF2-36’干果肉含油率增速明显大于‘杂56’。8月30日‘TF2-36’干果肉含油率达高峰(37.19%),约为‘杂56’的3.9倍(图2)。

图1 品系‘TF2-36’和‘杂56’果实形态和颜色变化Fig.1 Morphology and color of fruits of lines ‘TF2-36’ and ‘Za56’
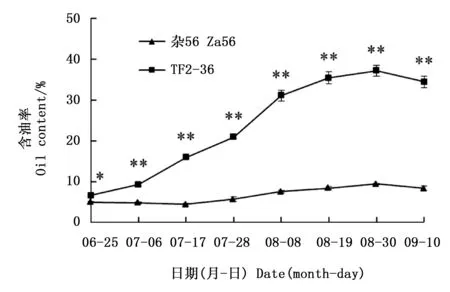
注:*和**分别表示同一时期两个品系间含油率在0.05和0.01水平上的显著差异Note: * and ** indicated significant differences of oil content between two lines at the same harvest time at the level of 0.05 and 0.01, respectively图2 品系‘TF2-36’和‘杂56’果肉发育期间的含油率变化Fig.2 Changes of oil contents in fruit pulp of lines ‘TF2-36’ and ‘Za56’
2.2 GPD1、DGAT1和DGAT2基因表达分析
GPD1基因在‘TF2-36’果肉中先上调表达,在7月28日到达峰值;随后,表达下调,并逐渐稳定。GPD1基因在‘杂56’果肉中呈总体下调表达模式。‘TF2-36’果肉的GPD1基因表达量一直显著高于‘杂56’,且在7月28日达峰值(图3A),两个品系的GPD1基因表达量峰值约相差3.4倍,与含油率峰值相差倍数相似。
DGAT1基因在‘TF2-36’果肉中先下调表达后上调再微量下调,而在‘杂56’果肉中呈先下调再上调表达模式。‘TF2-36’果肉的DGAT1基因表达量一直显著高于‘杂56’(图3B),且与‘TF2-36’干果肉含油率显著高于‘杂56’相一致。DGAT2基因在‘TF2-36’果肉中先稳定表达,在8月30日达峰值且显著高于‘杂56’。‘TF2-36’果肉的DGAT2基因表达量和干果肉含油率达峰值的时间相同(图2)。
与低果肉油品系‘杂56’相比,源基因GPD1在高果肉油品系‘TF2-36’发育初期的高表达(图3A),为TAG合成提供了更多的前体G3P;且汇基因DGAT1在GPD1高表达后,仍维持高表达水平(图3B),加速TAG组装。可见,源汇基因的协同高表达促进了TAG合成积累。
3 讨论
沙棘非种子组织(果肉)高积累棕榈油酸的特性在自然界中非常罕见,它是我国唯一高积累棕榈油酸的植物,但果肉较低的含油率严重限制了其有效开发利用。
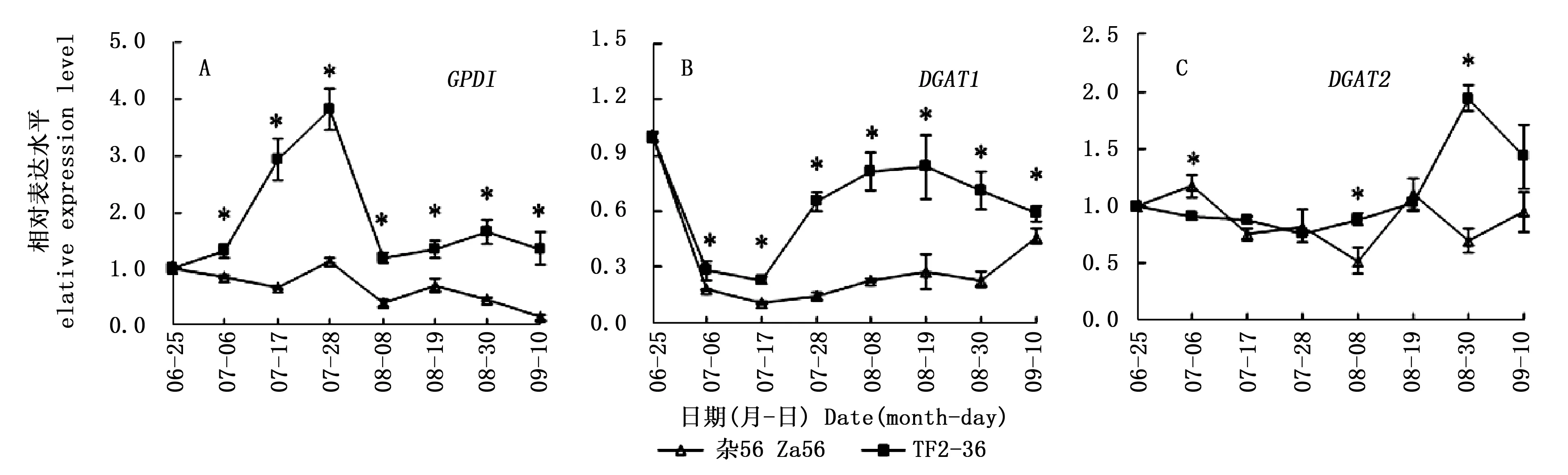
注:*表示同一采收期两个品系间的基因相对表达量在0.05水平上的显著差异Note: * indicated significant differences of gene relative expression level between two lines at the same harvest time at the level of 0.05图3 GPD1、DGAT1和DGAT2基因在‘TF2-36’和‘杂56’果肉中的表达差异Fig.3 Differences of GPD1, DGAT1 and DGAT2 genes expression in fruit pulp between the lines ‘TF2-36’ and ‘Za56’
‘TF2-36’果肉的GPD1基因表达量一直显著高于‘杂56’,且于7月28日达峰值,促使‘TF2-36’的干果肉含油率在8月30日达峰值且显著高于‘杂56’,含油率的峰值滞后于GPD1基因表达量的峰值,而且两个品系的干果肉含油率峰值相差倍数(3.9倍)与GPD1基因表达量峰值相差倍数(3.4倍)相近。可见,GPD1基因在‘TF2-36’果肉发育前期的高表达,为油脂合成积累了更多的G3P[7],进而提升其果肉含油率。近年来,在麻疯树(JatrophacurcasL.)和印加果(PlukenetiavolubilisL.)种子中也发现GPD基因表达量与TAG含量变化相关[23,25],且注射外源甘油的油菜种子油脂含量高峰也滞后于G3P高峰[10]。
高油品系‘TF2-36’果肉的汇基因DGAT1和DGAT2在源基因GPD1的前期高表达后出现表达量高峰,且显著高于低果肉油品系‘杂56’,表明DGAT1和DGAT2基因在果肉发育期间的高表达也促进了油脂高积累。发育期的麻疯树和蓖麻(RicinuscommunisL.)DGAT1基因以及印加果DGAT2基因表达量呈明显的单峰曲线变化规律,且与油脂合成相关[23,25-26]。发育期文冠果胚的油脂积累与XsDGAT1和XsDGAT2基因表达模式相关,与非转基因植株相比,异源表达文冠果XsDGAT1和XsDGAT2基因的拟南芥种子油脂含量分别提高71.6 μg·mg-1(20.3%)和30.9 μg·mg-1(8.8%)[11]。不同物种和组织对DGAT1和DGAT2基因的响应程度存在一定差异,而它们对沙棘果肉含油率的影响程度还需要进一步验证。吴永美等[27]将猫爪草(Doxanthaunguis-cati)的Δ9D(delta-9 desaturase)基因(催化棕榈酸去饱和为棕榈油酸)和对棕榈油酸有特异选择性的DGAT酶基因在大豆中共同表达发现,大豆体细胞胚的棕榈油酸含量上升到19%,比单独转化Δ9D基因高7%。Li等[16]发现油桐中有催化桐油酸(十八碳三烯酸)与TAG特异结合的DGAT2基因。因此在后续研究中获得对棕榈油酸有特异选择性的DGAT基因对进一步解析沙棘果肉油脂合成积累机理具有重要意义。
与低果肉油品系‘杂56’相比,高果肉油品系‘TF2-36’的源基因GPD1在发育前期高表达,而汇基因DGAT1和DGAT2在发育后期出现高表达,源和汇基因的协同高表达促进了沙棘果肉油脂的高积累。在烟草中共表达拟南芥的WRI1和DGAT1基因可产生协同作用,转基因烟草的干叶TAG含量可提高约100倍(2.48%),是转化单一基因的5倍左右[19]。Chen等[17]将油桐的VfFAD2和VfDGAT2基因融合导入红酵母和拟南芥中,发现它们具有协同促进不饱和脂肪酸积累的作用,且可获得更高含量的C18:3(红酵母中提高174%,拟南芥中提高14.6%)。非种子组织(果肉)的油脂生物合成途径复杂,同时涉及到种子油脂的合成,这些结构基因和调节基因对沙棘果肉油脂积累的影响正在进一步的研究验证之中。
4 结论
(1)品系‘TF2-36’的果肉含油率一直显著高于‘杂56’,两个品系的果肉油脂积累模式相似;(2)GPD1、DGAT1和DGAT2基因在‘TF2-36’果肉中均有明显高于‘杂56’的表达量峰值,但GPD1表达量峰值出现在油脂快速合成期,DGAT1和DGAT2表达量峰值出现在油脂稳定积累期,源基因“GPD1”在发育前期高表达,促进合成更多的TAG前体G3P,而汇基因“DGAT1和DGAT2”在发育后期高表达,则促进了TAG的高积累。(3)源基因“GPD1”和汇基因“DGAT1和DGAT2”的协同高表达在促进TAG合成底物G3P积累的同时加速acyl-CoA脂肪酸与DAG组装成TAG。这不仅首次揭示了非种子组织(果肉)的油脂合成积累的源汇基因协同表达机制,而且为提高沙棘果肉含油量提供理论依据。
[1] Cenkowski S, Yakimishen R, Przybylski R,etal. Quality of extracted sea buckthorn seed and pulp oil[J]. Canadian Biosystems Engineering, 2006, 48(3): 9-16.
[2] Yang B R, Kallio H. Fatty acid composition of lipids in sea buckthorn (HippophaerhamnoidesL.) berries of different origins[J]. Journal of Agricultural and Food Chemistry, 2001, 49(4): 1939-1947.
[3] Ruan C J, Rumpunen K, Nybom H. Advances in improvement of quality and resistance in a multipurpose crop: sea buckthorn[J]. Critical Reviews in Biotechnology, 2013, 33(2): 126-144.
[4] Fatima T, Snyder C L, Schroeder W R,etal. Fatty acid composition of developing sea buckthorn (HippophaerhamnoidesL.) berry and the transcriptome of the mature seed[J]. Plos One, 2012, 7(4): e34099.
[5] Yang B R, Kallio H. Analysis of Triacylglycerols of seeds and berries of sea buckthorn (Hippophaerhamnoides) of different origins by mass spectrometry and tandem mass spectrometry[J]. Lipid, 2006, 41(4): 381-392.
[6] Ramli U S, Baker D S, Quant P A,etal. Use of control analysis to study the regulation of plant lipid biosynthesis[J]. Biochemical Society Transactions, 2002, 30(6): 1043-1046.
[7] Vigeolas H, Waldeck P, Zank T,etal. Increasing seed oil content in oil-seed rape (BrassicanapusL.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter[J]. Plant Biotechnology Journal, 2007, 5(3): 431-441.
[8] 张 霞, 张 桦, 张富春. 盐胁迫下盐穗木甘油醛-3-磷酸脱氢酶基因的表达与亚细胞定位分析[J]. 西北植物学报, 2015, 35(7): 1283-1288.
[9] Remize F, Barnavon L, Dequin S. Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production inSaccharomycescerevisiae[J]. Metabolic Engineering, 2001, 3(4): 301-312.
[10] Vigeolas H, Geigenberger P. Increased levels of glycerol-3-phosphate lead to a stimulation of flux into triacylglycerol synthesis after supplying glycerol to developing seeds ofBrassicanapusL. in planta[J]. Planta, 2004, 219(5): 827-835.
[11] Guo H H, Wang T T, Li Q Q,etal. Two novel diacylglycerol acyltransferase genes fromXanthocerassorbifoliaare responsible for its seed oil content[J]. Gene, 2013, 527(1): 266-274.
[12] Zheng P, Allen W B, Roesler K,etal. A phenylalanine in DGAT is a key determinant of oil content and composition in maize[J]. Nature Genetics, 2008, 40(3): 367-372.
[13] Jako C, Kumar A, Wei Y D,etal. Seed-specific over-expression of anArabidopsiscDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight[J]. Plant Physiology, 2001, 126(2): 861-874.
[14] Cases S, Smith S J, Zheng Y,etal. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis[J]. Proceedings of National Academy of Sciences of the United States of America, 1998, 95(22): 13018-13023.
[15] Kroon J T M, Wei W X, Simon W J,etal. Identification and functional expression of a type 2 acyl-CoA: diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals[J]. Phytochemistry, 2006, 67(23): 254-259.
[16] Li R, Yu K, Hildebrand D F.DGAT1,DGAT2 andPDATexpression in seeds and other tissues of epoxy and hydroxy fatty acid accumulating plants[J]. Lipids, 2010, 45(2): 145-157.
[17] Chen Y C, Cui Q Q, Xu Y J,etal. Effects of tung oilseed FAD2 and DGAT2 genes on unsaturated fatty acid accumulation inRhodotorulaglutinisandArabidopsisthaliana[J]. Molecular Genetics and Genomics, 2015, 290(4): 1605-1613.
[18] 阮成江, 李 群. 基因调控种子含油量研究进展及生物柴油用海滨锦葵遗传改良策略[J]. 可再生能源, 2008, 26(4): 35-40.
[19] Vanhercke T, EI Tahchy A, Shrestha P,etal. Synergistic effect ofWRI1 andDGAT1 coexpression on triacylglycerol biosynthesis in plants[J]. FEBS Letters, 2013, 587(4): 364-369.
[20] Ding J, Ruan C J, Bao Y H,etal. Analysis of genetic relationships in sea buckthorn (Hippophaerhamnoides) germplasm from China and other countries using ISSR markers[J]. Journal of Horticultural Science & Biotechnology, 2015, 90(6): 599-606.
[21] Christie W W. Fatty acids and lipids: Structures, extraction and fractionation into classes[M]// Christie W W. Gas Chromatography and Lipids. Glasgow, UK: The Oily Press Ltd., 1989: 11-42.
[22] Vuorinen A L, Markkinen N, Kalpio M,etal. Effect of growth environment on the gene expression and lipids related to triacylglycerol biosynthesis in sea buckthorn (Hippophaerhamnoides) berries[J]. Food Research International, 2015, 77(3): 608-619.
[23] Wang X J, Liu A Z, Expression of genes controlling unsaturated fatty acids biosynthesis and oil deposition in developing seeds of Sacha Inchi (PlukenetiavolubilisL.)[J]. Lipids, 2014, 49(10): 1019-1031.
[24] Schmittgen T D, Livak K J. Analyzing real-time PCR data by the comparative CT method[J]. Nature Protocols, 2008, 3(6): 1101-1108.
[25] Xu R H, Wang R L, Liu A Z. Expression profiles of genes involved in fatty acid and triacylglycerol synthesis in developing seeds of Jatropha (JatrophacurcasL.)[J]. Biomass and Bioenergy, 2011, 35(5): 1683-1692.
[26] Chen G Q, Turner C, He X H,etal. Expression profiles of genes involved in fatty acid and triacylglycerol synthesis in castor bean (RicinuscommunisL.)[J]. Lipids, 2007, 42(3): 263-274.
[27] 吴永美, 毛 雪, 王书建, 等. 植物ω-7脂肪酸的系统代谢工程[J]. 植物学报, 2011, 46(5): 575-585.
CoordinatedExpressionofSourceandSinkGenesInvolvedinLipidBiosynthesisandAccumulationDuringSeabuckthornPulpDevelopment
DINGJian1,RUANCheng-jiang1,GUANYing2,GUANWen-ke3,SHANJin-you2,WUYu-xi2,WUTian-zhong3
(1.Institute of Plant Resources, Dalian Nationalities University, Dalian 116600, Liaoning, China; 2.Institute of Berries, Heilongjiang Academy of Agricultural Sciences, Suiling 152200, Heilongjiang, China; 3.Xinjiang Academy of Forestry Sciences, Urumqi 830064, Xinjiang, China)
ObjectiveThe objective of this study is to explore the relationship between lipid biosynthesis and source and sink genes’ expression in seabuckthorn (HippophaeL.) pulp.MethodTwo close-related trains ‘TF2-36’(with higher oil content) and ‘Za 56’ (with lower oil content) were selected as test samples. Their pulps were harvested in eight developmental stages. The oil content in pulp was tested by the method of chloroform methanol, and the differential expression of source gene ‘GPD1’ and sink genes ‘DGAT1 andDGAT2’ involved in lipid biosynthesis between high and low oil content lines were determined using qRT-PCR, and the effects of the three genes on lipid biosynthesis and accumulation were analyzed.Result(1) The oil contents in pulp of ‘TF2-36’ were higher than that of ‘Za 56’ at all stages, but it first increased, and then kept stable for two lines; (2) the peak values ofGPD1,DGAT1 andDGAT2 expression in pulp of TF2-36 were significantly higher than that in ‘Za 56’ during pulp development. The peaks ofGPD1 gene appeared in the period of rapid lipid biosynthesis, and the peaks ofDGAT1 andDGAT2 genes appeared in the period of stable lipid accumulation. The high expression of source gene (GPD1) contributed to synthesis more G3P of TAG precursor in early stages of pulp development, but the high expression of sink genes (DGAT1 andDGAT2) accelerated high TAG accumulation in later stages of pulp development.ConclusionThe high coordinated expression of source gene ‘GPD1’ and sink gene ‘DGAT1 andDGAT2’ resulted in the high lipid biosynthesis and accumulation in seabuckthorn pulp. These results provided basis for understanding lipid biosynthesis mechanism in seabuckthorn non-seed (pulp) tissue.
HippophaeL.; lipid biosynthesis; source gene; sink gene; gene expression
10.13275/j.cnki.lykxyj.2017.06.003
2016-09-02
国家自然科学基金(31570681)
丁 健(1983—),男,博士,讲师,主要从事资源植物开发利用与遗传育种.
* 通讯作者:阮成江(1972—),博士,教授,主要从事木本油料资源高效培育与利用研究。E-mail:ruan@dlnu.edu.cn
S793.6
A
1001-1498(2017)06-0902-06
张 研)

