Structurally modified RDX-A DFT study
Lemi Türker,Serhat Varis¸
Middle East Technical University,Department of Chemistry,06800,Ankara,Turkey
Structurally modified RDX-A DFT study
Lemi Türker*,Serhat Varis¸
Middle East Technical University,Department of Chemistry,06800,Ankara,Turkey
RDX Insensitivity Molecular structural modification Kamlet-Jacobs equations
RDX is a nitramine type explosive which is widely employed in military and industrial applications.A hot topic in military area is lowering the sensitivity of explosives.Along this direction,one approach,which is still being applied,is to use coatings or additives for explosives,as in the example ofi-RDX(reduced sensitivity RDX).Another attitude would be to make some slight molecular level chemical modifications in the explosive structure that cause a diminished sensitivity without substantial loss in explosive impact.RDX has three nitro groups.We assumed that by the conversion of these nitro groups to nitroso and amino groups,it might be possible to lower the sensitivity somewhat.We have correlated the bond dissociation energies with impact sensitivity.Additionally,the ballistic properties,i.e.detonation velocity(D),and detonation pressure(P)have been examined by using Kamlet-Jacobs equations.We have shown that the above mentioned molecular modifications are a successful way of lowering the sensitivity of RDX.
1.Introduction
RDX,known as Research Department explosive,is a nitramine type explosive so long extensively employed in army and industrial applications[1].It was synthesized as an explosive more influential thanTNT,and it was broadlyused in World War II.RDX is also called cyclonite,hexogen and T4[2].Its chemical name is hexahydro-1,3,5-trinitro-1,3,5-triazine,(cyclotrimethylenetrinitramine).RDX is a white,crystalline solid in its pure synthesized state.RDX is accepted as one of the most powerful military high explosives and quite stable under storage conditions[3].It is frequently used as a component in mixtures including supplementary explosives and plasticizers,phlegmatizers or desensitizers.RDX is vital in the formulations of many well-known military explosives such as Composition A,B and C,CH-6,Torpex and particularly plastic bonded explosive(PBX)[4-9].
Desensitization of explosives(reduction of their sensitivity)is a hot research topic in military area.One approach covers the usage of additives and coatings of explosives where surface may play a significant role.There are many examples in the literature,especially on RDX[10].
Another approach,which is also adopted in the present study,is to apply some molecular level changes in the molecule structure of an explosive providing a decrease in impact sensitivityetc.,without notable decrease of power.RDX has three nitro(-NO2)groups in the form of nitramine.Presently,it is assumed that conversion of these nitro(-NO2)groups to nitroso(-NO)and amino(-NH2)groups(Fig.1)might lessen the sensitivity.Moreover,a comprehension of the tendency in energetic character in going from RDX to derivatives discloses the factors which can be used in altering the sensitivity of explosives via structural modification.Desensitization produces much safer explosives in comparison to their parental molecules and prevents some unintentional detonations initiated by several factors like thermal and/or mechanical shock,static electric discharging,etc.
In the current article,some computational studies have been done on RDX itself and five dissimilar RDX derivatives.
2.Theoretical methods
The initial structure optimizations of RDX and further structures were accomplished following the basis set order:MM2 method,PM3 method,STO and HF/6-31G(d,p)and finally DFT,B3LYP/6-31G(d,p).There exists no imaginary frequency in the normal mode analysis of the molecules carried out at the same level of theory.The total electronic energies were calculated considering zero point vibrational energies(ZPE).The heats of formation of all the molecules were calculated by a T1 Thermochemical Recipe[11]implemented in Spartan’08.The computational calculations were done using Spartan’08 software in standard conditions(298.15 K and 1.00 atm)[12].Furthermore,the geometry optimizations and the single point calculations of all the structures were performed at UB3LYP/6-31G(d,p)theoretical level for bond dissociation energy(BDE)calculations.The basis set superposition error(BSSE)was achieved using the Boys and Bernardi counterpoise method in Gaussian 03 software package[13,14].
Abbreviations
B3LYP Becke,3-parameter,Lee-Yang-Parr
BDE Bond Dissociation Energy
BSSE Basis Set Superposition Error
DFT Density Functional Theory
HF Hartree Fock
HMX Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine PBX Plastic Bonded Explosive
PM3 Parametric Method
RDX Research Department Explosive;1,3,5-Trinitroperhydro-1,3,5-triazine
STO Slater-type orbital
TNT 2-Methyl-1,3,5-trinitrobenzene
T1 Thermochemical Recipe
QCISD Quadratic configuration interaction
3.Results and discussion
3.1.The geometries
All the structures considered in Fig.1 have been supposed to be the potential nominees of explosives.The geometry optimizations of the molecules in Fig.1 have been done at the B3LYP/6-31G(d,p)level.In the optimized structure,RDX has one nitro group in the axial and the other two in the equatorial positions.In structures 2-5,the conformational pattern of RDX is preserved,namely one axial and two equatorial positions occupied by the substituents.In structure-2 the nitro group is in the axial position.In structures-4 and-5 the axial position is occupied by the amino and nitro groups,respectively.Whereas in structure-6,the nitroso groups occupy the axial positions in that respect its conformation pattern is different from RDX.The amino and nitroso groups present in structure-3 prefer the equatorial positions.The calculated bond length data for the optimized geometries are shown in Table 1.The subscript numbers showing the bonds in Table 1 is arranged considering Fig.2.The experimental X-ray diffraction data for RDX[15]are shown in Table 1 as well.The similarity between the experimental and theoretical bond length data for RDX and the absence of imaginary frequency in the potential energy diagrams assure the true geometry optimization of the molecules.This compatibility also assures that bond length data of all the molecules are close to the real values.
Overall,the experimental and theoretical bond length results for RDX are almostequivalent.Thereare slight differences between the experimental and calculated data.For instance,the X-ray bond length data for RDX has shorter nitramine N-NO2bonds approximately 0.01 Å than the computed values.These slight differences stem from the solid-state effect including the intermolecular interactions.These interactions are not available in the DFT computations employed presently[16].
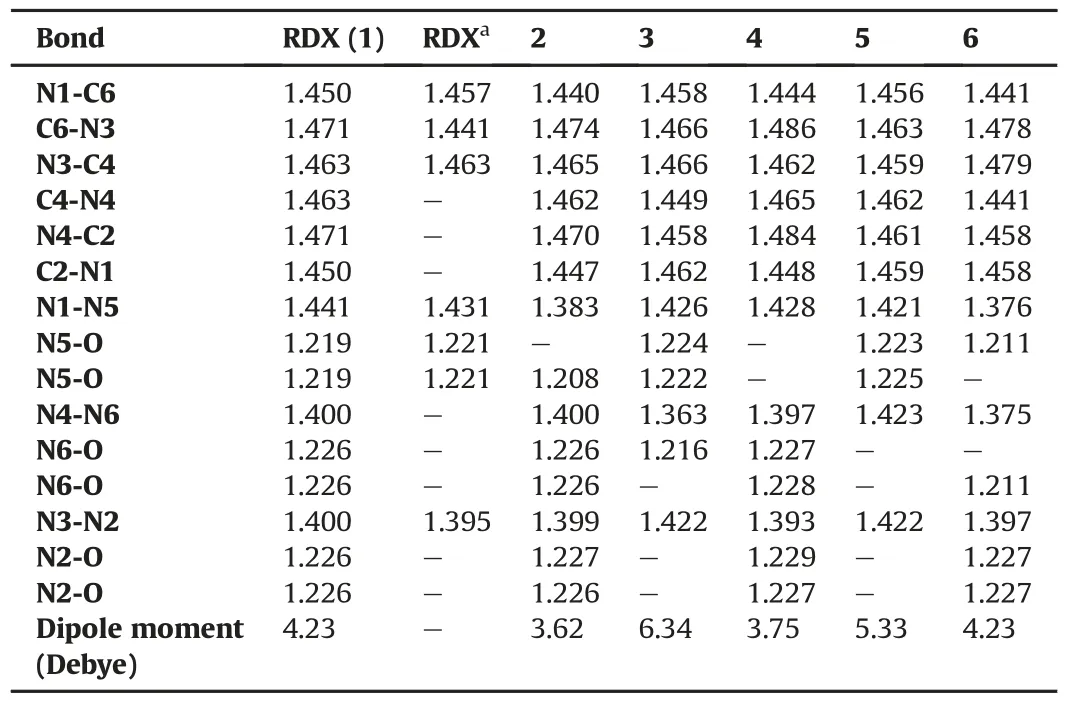
Table 1The bond length data(Å)of the optimized RDX and its derivatives computed at(DFT)B3LYP/6-31G(d,p)level.
3.2.Charges
Fig.3 represents the electrostatic charge(in esu)development of the system computed at the B3LYP/6-31G(d,p)level and acquired in the direct output of the software.Moreover,the electrostatic potential VS(r)computed on the 0.001 au isodensity surface[17]has been superimposed on each structure(property range spans over-200 to 200 kJ/mol).As seen in Figs.1 and 3 the dipole moments in all the structures except 4 are oriented upward.Note that the dipole moments of structures 1,2,4 are inclined over the equatorial groups(in structure-4 it is almost in the ring plane)whereas in 5 its inclination is towards the axial group.Whereas,in 3 and 6 the dipole moment is almost vertical to the ring plane.The charges and bond lengths in the optimized geometries are arranged such that order of dipole moments (see Table 1) is 2<4<1=6<5<3.
The orientation of the dipole moments indicate that the conformation of the substituents makes the bottom side of the molecules electron deficient contrary to the upper side.In structures-3 and-5 the region around the axial nitro group becomesrelativelyelectron rich(see Fig.3).In4 the relativelyelectron rich region is around one of the equatorial nitro groups.The electrostatic potential VS(r)on the isodensitysurface of 2 resembles the respective plot of RDX.Therefore it is not unexpected that most properties of 1 and 2 should be comparable.
3.3.The bond dissociation energies(BDE)vs.impact sensitivity
Numerous experimental studies show that nitramine(N-NO2)bond homolysis is the first step of thermal decomposition of RDX and other nitramines[18-21].In this article,for the comparison of the nitramine bond strengths of the compounds,homolytic bond dissociation energy(BDE)calculations considering the removal of nitrogen dioxidemoietyfrom theoriginalgeometrywere computed at UB3LYP/6-31G(d,p)level.The equalities for the homolytic nitro bond(nitramine)break and calculation of the BDE are shown below
R-NO2stands for the neutral molecule and R·and NO2·stands for the radicals occurring after the nitramine bond dissociation;BDE(R-NO2)denotes the bond dissociation energy of nitro bond;E(R-NO2),ER·,and ENO2·are the ZPE added total energies of the compounds and the radicals,respectively[22-24].Additionally,the basis set superposition error(BSSE)analyses were carried out.
The sensitivity behavior of an explosive under different heat,impact,friction conditions may vary.In this work,the “sensitivity”term designates the “impact sensitivity”of the focused explosive.Impact sensitivity behavior of explosives can be determined experimentally by the drop height test.The impact sensitivity can also be examined by theoretical methods.Murray et al.[25]have put out an association between the BDEs of the nitro bonds and the electrostatic potentials on the molecular surfaces of some energetic molecules.The several appreciated studies in the references[26-29]have indicated that there is a parallel correlation between the BDE for the weakest nitro bond of the molecule and its impact sensitivity.The typical tendency is that the larger the BDE data of nitro bond,the lower the sensitivity is.
Desensitization of explosives(reducing their vulnerability)becomes more of an issue in military applications.Our methodology in the current work is to apply small structural changes in the explosive that lead to the probable decrease in impact sensitivity without substantial loss of explosive power.RDX has three nitro groups.We foresaw that the conversion of nitro(-NO2)groups to nitroso(-NO)and amino(-NH2)groups might enable a decrease in the impact sensitivity.The lowest sensitivity has been attributed to the highest nitramine(N-NO2)bond dissociation energy.Also,a comprehension of the tendency of energetic character in going from RDX to the derivatives might reveal the features which can be used in changing the impact sensitivity of explosives via structural modification.Table 2 shows the computed BDE data.The consistency of our BDE value(without BSSE)and the calculated literature data(the datum excerpted from the reference is without BSSE)[30]for RDX increases the reliability of the technique adopted in the current article(see Table 2).
We have compared the impact sensitivity of explosives in the present study on the basis of BDE values such that attributing the highest sensitivity to the lowest nitramine bond dissociation energy value.We have changed the nitro groups with amino and/or nitroso group(s)and examined the nitramine BDE values.An increase in the nitro BDE has been interpreted as a more insensitive explosive,and a decrease in nitro BDE has been evaluated as a more sensitive explosive.When a nitro group of RDX is changed with nitroso group(structure-2),the BDE for nitramine bond decreases by 2.69 kJ/mol,resulting in a more sensitive explosive.However,replacement of both of the nitro groups by nitroso groups(structure-6)creates a more insensitive explosive compared to RDX that is,BDE value of nitramine bond increases by 7.92 kJ/mol.
Likewise,the replacement of one nitro group with an amino group(structure-4)induces an increase in nitramine BDE by 10.83 kJ/mol.The substitution of both nitro groups byamino groups(structure-5)increases the BDE value by 26.89 kJ/mol.The results showed that amino replacements produce the most insensitive explosives of all.The amino groups balance the electron demand of nitro groups existing in the system.
The introduction of one nitroso and one amino groups instead of nitro groups of RDX accomplishes structure-3.Meanwhile,the BDE increases by 15.55 kJ/mol in going from RDX to structure-3,resulting in a more insensitive explosive.
All the conversions considered,except for structure-2,cause a notable increase in BDEs,therefore an effective decrease in impact sensitivity is expected theoretically.This type of variation of functional groups of RDX is very supportive for decreasing the sensitivity.
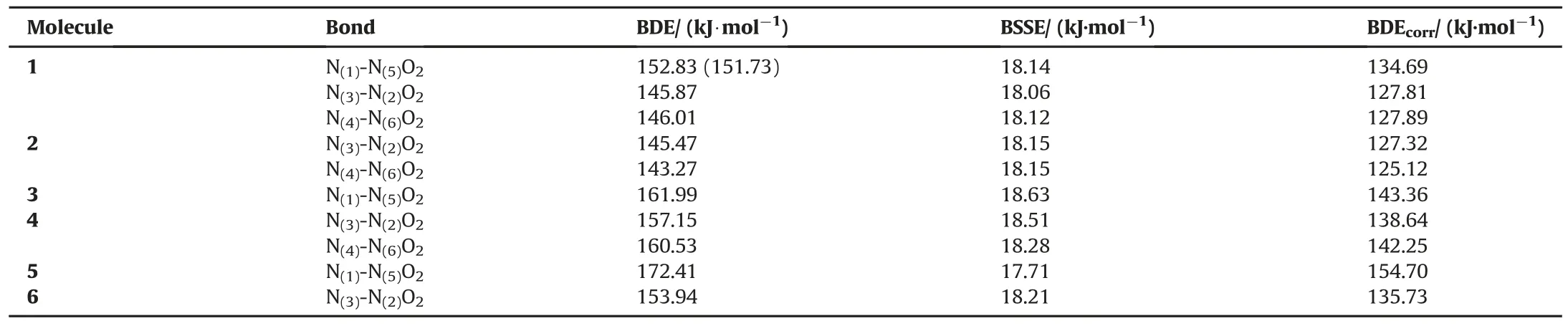
Table 2The homolytic bond dissociation energies,BSSE and corrected BDEs of N-NO2bonds of RDX and other derivatives calculated at(DFT)UB3LYP/6-31G(d,p)theoretical level.
Insensitivityconcept has been correlated with Bond dissociation energy(BDE).The higher the BDE,the more insensitive the explosive is.Since the BDE values are in the following order:2<1<6<4<3<5(as seen from the chart in Fig.3 and Table 2);the insensitivity is in the same order.For structures 2-6,there exists an inverse relationship between the BDE and detonation velocity.The structure-5 has been assigned as the most insensitive RDX derivative and the structure-2 has been identified as the most sensitive RDX derivative.
3.4.Explosive properties
Explosive effects of energetic materials can be assessed by the determination of the ballistic properties,especially detonation velocity(D)and detonation pressure(P).The use of empirical Kamlet-Jacobs[31-34]equations enables one to calculate these properties by means of Eqs.(3)and(4).
The terms in Kamlet Jacobs Equations(3)and(4)are shown as:D,detonation velocity(km/s);P,detonation pressure(GPa);ρ,density of a compound(g/cm3);N,moles of gaseous detonation products per gram of explosive;Mav,average molecular weight of gaseous products;Q,chemical energy of detonation(kcal/g).The parametersN,Mav,andQare calculated according to the chemical composition of each explosive as revealed in Ref.[16].For a CaH-bOcNdtype explosive,if 2a+b/2>c≥b/2 equality is fulfilled,N is calculated using (b+2c+2d)/4M,Mavis calculated as(56d+88c-8b)/(b+2c+2d),andQis calculated as[28.9b+94.05(c/2-b/4)+0.239ΔHof]/M.Ifb/2>cequality is satisfied,Nis calculated as(b+d/2M),Mavis calculated as(2b+28d+32c)/(b+d),Qis calculated as[(57.8c+0.239ΔHof)/M].The coefficient(0.239)of ΔHofin the relations is a conversion factor from kJ/mol to kcal/mol.“M”is the molecular weight of the compound(in g/mol);ΔHofis the standard heat of formation of the compound(in kJ/mol).The standard heat of formation(ΔHof)was calculated usingT1 Thermochemical Recipe.This recipe follows the G3(MP2)recipe,by replacing HF/6-31G(d)for the MP2/6-31G*geometry,omitting both the HF/6-31G(d)frequency and QCISD(T)/6-31G(d)energy and approximating the MP2/G3MP2 large energy using dual basis set RI-MP2 techniques[35].The density(ρ)of each compound is calculated by ratio of molecular weight to the molar volume.The statistical average of hundred single-point molar volume calculations results in the molecular volume of each optimized molecule.The Monte Carlo integration in the Gaussian 03 software package was used for molar volume calculations[14].The density of each compound was anticipated from the molecular volume divided by molecular weight.Structures 1,2,4,and 6 are in accordance with 2a+b/2>c≥b/2 relation,whereas,structures-3 and-5 are in accordancewithb/2>crelation.The calculatedoxygen balance(Ω),heat of formation(ΔHof),the chemical energy of detonation(Q),density(ρ)and detonation velocity(D)and pressure(P)are listed in Table 3.It also includes experimentalvalues of RDX[16,36,37]taken from the literature.
Table 3 shows the oxygen balance(Ω%)values of the explosives considered in the present study.For a CaHbOcNdtype explosive,%Ω is calculated as 1600(2a+b/2-c)/M.It is an expression that indicates the degree to which an explosive can be oxidized.If an explosive molecule has sufficient amount of oxygen to produce CO2from carbon,H2O from hydrogen molecules,sulfur dioxide from all of its sulfur,and all metal oxides from metals with no excess,the molecule is supposed to have a zero oxygen balance.The molecule is supposed to have a positive oxygen balance if it has more oxygen than is required and a negative oxygen balance if it contains less oxygen than needed.In this work,all the considered molecules have negative oxygen balances,like a well-known explosive,TNT.
The heat of formation(ΔHof)values of all molecules was calculated with a thermochemical recipe,T1.The comparability of the calculated(ΔHof)value of RDX and the literature value[16]raises the credibilityof the method.When density(ρ)data are considered,replacement of the nitro groups with amine groups(structures 4 and 5)causes a dramatic decrease in the density.The nitroso group substitutions(structures 2 and 6)also cause a slight decrease in the density.
The detonation velocity and detonation pressure values for RDX both agree well with the experimental literature data[2,16,36].When Table 3 is considered,it is obvious that the explosive performances of all molecules are superior to the well-known explosive,TNT.The performances of RDX and other derivatives are in the following manner(See Fig.4):TNT<5<3<4<6<2<RDX.The replacement of nitro groups with nitroso groups(on going from RDX to structures 2 and 6)slightly decreases the ballistic properties.Similarly,the amino group substitutions(from RDX to structures 4 and 5)lead to an average decrease in detonation velocity and pressure.The structure-3 has one nitro,one nitroso and one amino group.The presence of the nitro and nitroso groups instead of nitro groups also lowers the detonationproperties inappreciably.
The explosive properties and sensitivity characteristics of the structures considered should be evaluated together.Amino group replacement(structures 4 and 5)is an effective method in decreasing sensitivity;however,these replacements deteriorate the detonation properties.Also,nitroso substitutions(structures 2 and 6)bring about higher BDEs than RDX(more insensitive compounds)with lower detonation velocity values.Similarly,conversion of RDX to structure-3 results in decreasing the sensitivity with worsedetonation properties.Amongthem,structure-6(themolecule having two nitroso and one nitro group)is the optimum.It is not only more insensitive than RDX,but also as good as RDX in performance.
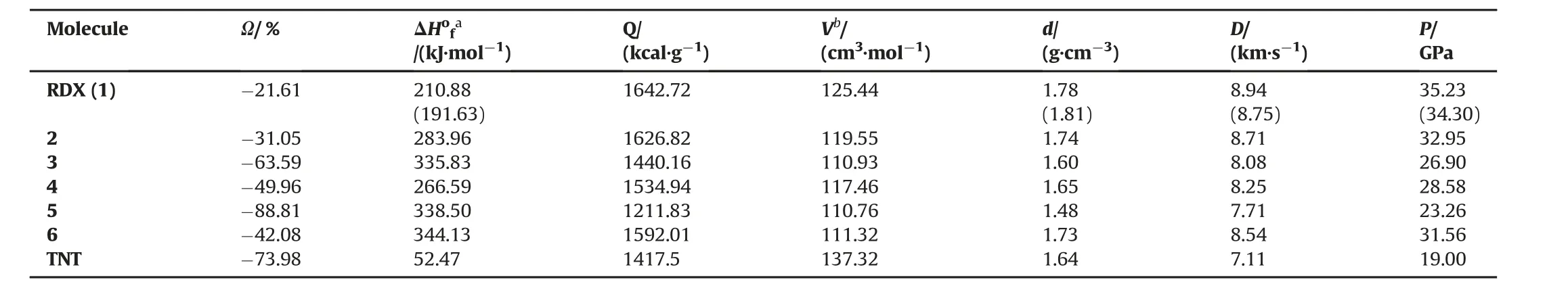
Table 3Anticipated density data and detonation properties of RDX and its derivatives at B3LYP/6-31G(d,p)level.
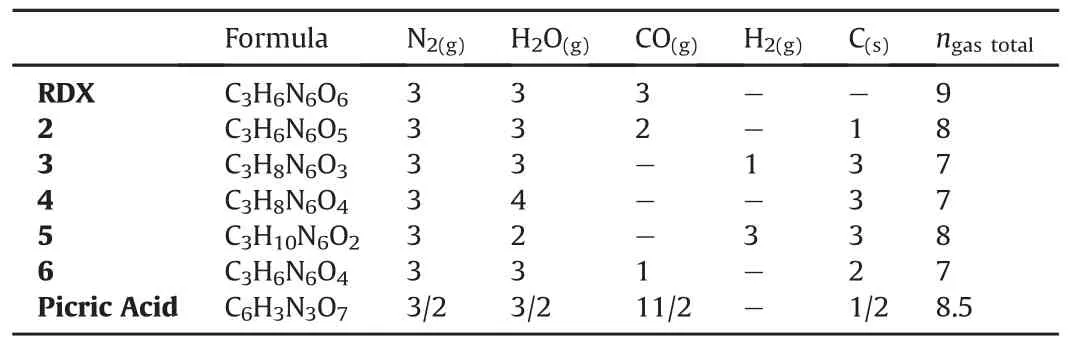
Table 4The number of moles of gaseous decomposition products of RDX and its derivatives using the Kistiakowsky-Wilson Rules.
3.5.Detonation products and explosive power
The small molecules,i.e.,CO2,CO,H2O,etc.are the main detonation products of CaHbOcNdtype explosives.In order to elucidate the number and type of the decomposition products,a series of rules was proposed by Kistiakowsky and Wilson[37,38].Table 4 displays the moles of detonation products of the compounds investigated in this article.
The total quantity of gas produced upon detonation has been found by adding the moles of H2O,N2,CO and H2,except for the moles of solid C.When total amount of gas is measured,the structures 2-6 produce 7-8 mol of gas upon detonation.
The main products of an explosive reaction are heat and hot gases.The volume of produced gas gives information on how much work done by the energetic materials.Standard conditions should be adopted to calculate the volume of generated gas,because the volume of gas changes with the changing temperature.The standard conditions(273 K,1 atm)also enable the scientist to make comparisons among different explosives.Division of the value of total volume of gas produced upon detonation by the molecular weight gives an idea of how much gas is released per gram of explosive[39-42].
The heat of explosion “Q”and the volume of produced gas“V”can be combined to obtain the explosive power data as indicated below[36]
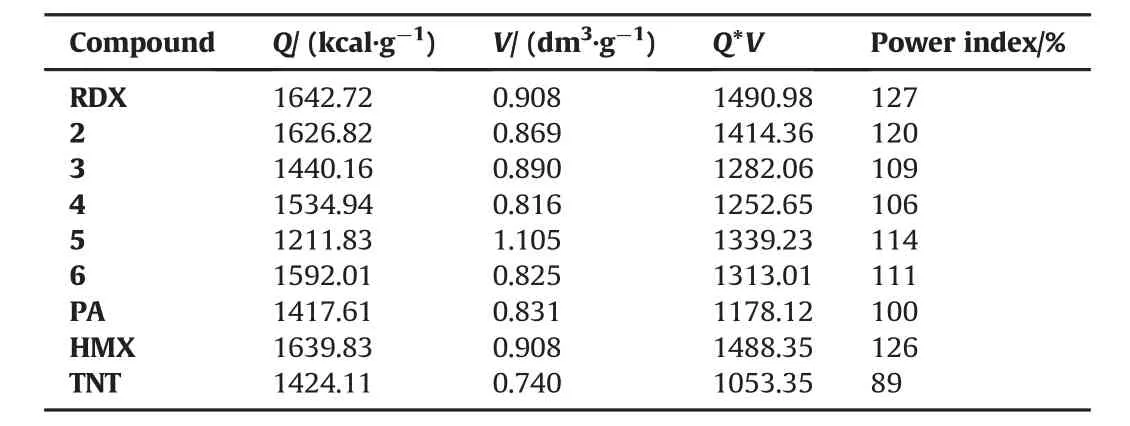
Table 5The power index data of RDX,its derivatives,Picric Acid,TNT and HMX.
The value for the explosive power is then compared with the explosive power of a standard explosive,namely Picric Acid(PA),to obtain power index,as shown in the following equation
Table 5 displays the power index values of RDX,the other RDX derivatives,Picric Acid,TNT,and HMX.The power index values of RDX and RDX derivatives presently considered are in between 106 and 127% and in the subsequent manner:HMX~RDX>2>5>6>3>4>PA>TNT.The structure-2 has the highest power index value after RDX.All the structures(2-6)are better in terms of the power index than the well-known explosives PA and TNT.All the molecules in the present study can easily be employed when higher amount of gas is required as an alternate to RDX.
4.Conclusion
Currently,computational studies have been completed on RDX itself and five different RDX derivatives.The bond length data of the structurally optimized(at the theoretical level of DFT B3LYP/6-31G(d,p))RDX were compared to experimental values quite satisfactorily.The lowest bond dissociation energies of the nitramine bond were associated with impact sensitivity concept.The possibility of decreasing the sensitivity of an explosive without signif icant loss in power by the conversion of nitro groups of RDX to nitroso and amino groups has been proven.All the compounds were evaluated as a better explosive than TNT.The conversion of nitro groups to amino groups creates more insensitive explosive when compared to nitroso conversions.All the compounds examined in this article showed better explosive properties than TNT.They are all possible nominees for insensitive high explosives.Thereby,they are all alternative to renowned explosive RDX whenever lower sensitivity applications are required.This study has shown that molecular modification is an operative method in desensitization of RDX.
[1]Partnership MacDonald Mack.Finalpropertiesreport:newportarmy ammunition plant.AD-A175818.National Park Service;1984.
[2]Meyer R,K¨ohler J,Homburg A.Explosives.6th ed.Weinheim:Wiley-VCH;2007.
[3]Agrawal JP,Hodgson R.Organic chemistry of explosives.New York:Wiley-VCH;2007.p.234-9.
[4]Davis TL.The chemistry of powder and explosives II.New York:John Wiley&Sons Inc.;1943.p.396.
[5]Superintendent AMPC,Sterling TS.Can J Chem Eng 1958;36:82-4.
[6]Pesce-Rodriguez RA,Piraino SM.Characterization of cyclohexanone inclusions in class 1 RDX.ARL-TR-6962.Army National Laboratory;2014.
[7]AD No:317-974 Development of RDX composition CH-6.Maryland:US Naval Ordnance Laboratory;1960.
[8]Ravi P,Badgujar DM,Gore GM,Tewari SP,Sikder AK.Propellants Explos Pyrotech 2011;36:393-403.
[9]Jadhav PM,Sarangapani R,Ghule VD,Prasanth H,Pandey RK.J Mol Model 2013;19:3027-33.
[10]Nicolich S,Niles J,Ferlazzo P,Doll D,Braithwaite P,Rausmussen N,Ray M,Gunger M,Spencer A.Recent developments in reduced sensitivity melt pour explosives,In:34th Int.Annual Conference of ICT,Karlsruhe,Germany,24-27 June 2003.
[11]Ohlinger WS,Klunzinger PE,Deppmeier BJ,Hehre WJ.J Phys Chem A 2009;113:2165-75.
[12]SPARTAN’08.Irvine CA,USA:Wavefunction Inc.;2008.
[13]Boys SF,Bernardi F.Mol Phys 1970;19:553-62.
[14]Inc.,Wallingford CT Gaussian 03,revision C.02.2004.
[15]Choi CS,Prince E.Acta Crystallogr 1972;B28:2857-62.
[16]Qiu L,Xiao H,Gong X,Ju X,Zhu W.J Phys Chem 2006;A110:3797-807.
[17]Politzer P,Murray JS,Koppes WM,Concha MC,Lane P.Cent Eur J Energ.Mater 2009;6(2):167-82.
[18]Adams GF,Shaw Jr RW.Annu Rev Phys Chem 1992;43:311-40.
[19]a)Zhao X,Hintsa EJ,Lee YT.J Chem Phys 1988;88:801-10.b)Chambers CC,Thompson DL.J Phys Chem 1995;99:15881-9.
[20]Botcher TR,Wight CA.J Phys Chem 1994;98:5441-4.
[21]Oxley JC,Kooh AB,Szekeres R,Zheng W.J Phys Chem 1994;98:7004-8.
[22]Rice BM,Sahu S,Owens FJ.J Mol Struct(THEOCHEM)2002;583:69-72.
[23]Shao J,Cheng X,Yang X.J Mol Struct(THEOCHEM)2005;755:127-30.
[24]Young DC.Computational chemistry:a practical guide for applying techniques to real World problems.John Wiley&Sons.Inc;2001.
[25]Murray JS,Concha MC,Politzer P.Mol Phys 2009;107:89-97.
[26]Owens FJ.J Mol Struct(THEOCHEM)1996;370:11-6.
[27]Politzer P,Murray JS.J Mol Struct(THEOCHEM)1996;37:419-24.
[28]Politzer P,Lane PJ.Mol Struct(THEOCHEM)1996;388:51-5.
[29]Harris NJ,Lammertsma K.J Am Chem Soc 1997;119:6583-9.
[30]Song X,Cheng X,Yang X.Propellants Explos Pyrotech 2006;31:306-10.
[31]Kamlet MJ,Jacobs SF.J Chem Phys 1968;48:23-5.
[32]Kamlet MJ,Ablard JE.J Chem Phys 1968;48:36-42.
[33]Kamlet MJ,Dickenson C.J Chem Phys 1968;48:43-51.
[34]Kamlet MJ,Hurwitz HJ.J Chem Phys 1968;48:3685-92.
[35]Ohlinger WS,Klunzinger PE,Deppmeier BJ,Hehre WJ.J Phys Chem A 2009;113(10):2165-75.
[36]Akhavan J.The chemistry of explosives.2nd ed.Cambridge:The Royal Society of Chemistry;1998.
[37]Cowan RD,Fickett W.J Chem Phys 1956;24:932-9.
[38]Muthurajan H,Sivabalan R,Talawar MB,Asthana SN.J Hazard Mater 2004;A112:17-33.
[39]Martin AR,Yallop HJ.J Appl Chem 1959;9:310-5.
[40]Zel'dovich Y,Kompaneets AS.Theory of detonation.Academic Press;1960.p.208-10.
[41]Hougen OA,Watson K,Ragatz R.Chemical process principles.John Wiley&Sons;1954.p.66-7.
[42]Anderson HV.Chemical calculations.McGraw-Hill;1955.p.206.
18 November 2016
in revised form 9 February 2017 Accepted 20 February 2017 Available online 22 February 2017
©2017 Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
*Correspondingauthor.MiddleEastTechnicalUniversity,Departmentof Chemistry,06531,Ankara,Turkey.
E-mail address:lturker@metu.edu.tr(L.Türker).
Peer review under responsibility of China Ordnance Society
- Defence Technology的其它文章
- Influence of electric current intensity on the performance of electroformed copper liner for shaped charge application
- Micro-seeding and soft template effects on the control of polymorph and morphology of HMX micro particles in solvent-antisolvent process
- Control system design of flying-wing UAV based on nonlinear methodology
- Spectrally adapted red flare tracers with superior spectral performance
- Microstructural observations on the terminal penetration of long rod projectile
- The effect of wax coating,aluminum and ammonium perchlorate on impact sensitivity of HMX

