Spectrally adapted red flare tracers with superior spectral performance
Ramy Sadek,Mohamed Kassem,Mohamed Abdo,Sherif Elbasuney
School of Chemical Engineering,Military Technical College,Kobry Elkoba,Cairo,Egypt
Spectrally adapted red flare tracers with superior spectral performance
Ramy Sadek,Mohamed Kassem,Mohamed Abdo,Sherif Elbasuney*
School of Chemical Engineering,Military Technical College,Kobry Elkoba,Cairo,Egypt
Pyrotechnics Colored flames Tracers Luminous intensity Molecular spectroscopy
The production of bright light,with vivid color,is the primary purpose of signaling,illuminating devices,and fire control purposes.This study,reports on the development of red flame compositions with enhanced performance in terms of luminous intensity,and color quality.The light intensity and the imprint spectra of developed red flame compositions to standard NATO red tracer(R-284 NATO)were measured using digital luxmeter,and UV-Vis.spectrometer.The main giving of this study is that the light intensity of standard NATO red tracer was increased by 72%,the color quality was also improved by 60%(over the red band from 650 to 780 nm).This enhanced spectral performance was achieved by means of deriving the combustion process to maximize the formation of red color emitting species in the combustion flame.Thanks to the optimum ratio of color source to color intensifier using aluminum metal fuel;this approach offered the highest intensity and color quality.Upon combustion,aluminum was found to maximize the formation SrCL(the main reactive red color emitting species)and to minimize the interfering incandescent emission resulted from MgO and SrO.Quantification of active red color emitting species in the combustion flame was conducted using chemical equilibrium thermodynamic code named ICT.The improvement in red flare performance,established the rule that the color intensifier should be in the range from 10 to 15 Wt%of the total composition.
1.Introduction
The production of bright light,with vivid color,is the primary purpose for many pyrotechnic compositions[1-13].Certain elements and compounds when heated to high temperature have the unique property of emitting lines or narrow bands of light in the visible region(380-780 nm)[14-16].These elements are called the color source,for instance strontium(red),barium(green),copper(green or blue)[17-20].Strontium,barium,copper emits color by forming their halides;this type of emission is known as molecular emission[21-23].Such emitting species is targeted to be effectively produced during pyrotechnic combustion for colored flame production[24,25].Chlorine was found to be an essential element to create different molecular emitting species;it has been used as color intensifier to enhance the production of colored flames in the visible band.Without Chlorine good colors would be difficult to be achieved[26,27].
Light emission has a variety of applications,ranging from military signals,tracking,illuminating devices,highway distress flares,and spectacular aerial fireworks[28].Nothing in pyrotechnics is as difficult as the creation of reproducible visual effects[29,30].The production of a vividly colored flame is a challenging problem than creating white light.To obtain high quality colored flame,a delicate balance between different factors is required;these factors including:
❖An atomic or molecular species that can emit the desired wavelength.
❖The emitting species must be sufficiently volatile.
❖Sufficient heat should be generated to produce the excited emitter.
❖Heat should not exceed the dissociation temperature of the emitter.
Magnesium is the most commonly used metal fuel in many colored light formulas.In an oxidizing flame,the magnesium is converted to magnesium oxide(MgO).Magnesium oxide,with high-melting point,is an excellent white light emitter by incandescence.Also,the high heat output aids in achieving high flame temperatures but this may affect the color quality[31-33].Any interfering atomic and molecular emitters must be avoided or at least minimized[34-37].This is why aluminum fuel was investigated as a new metal fuel for colored flame compositions[38,39].
Usually,colored flames are produced by excitation of metallic atomic emitting species(atomic emission).Other metal atoms may combine with other radicals in the flame forming a color emitting molecule(molecular emission).The vapors of emitting species are then excited by the thermal energy of the flame[40].The excited levels of atoms,or molecules relaxed to the ground state with the emission of the visible light.Strontium chloride is the main emitting species of red color.The sequence of emission phenomena occurs in rapid succession[40].Fig.1 demonstrates the entire sequence of emission phenomena for strontium chloride[29].
Excited SrCl species emit a series of bands in the region 620-680 nm,the deep red portion of the visible spectrum[40].The main aim is to maximize the formation of reactive red color emitting species in the flame zone.The emission takes place in the red region of the visible spectrum(650-780 nm).The main emitting species for red color are strontium mono-chloride(SrCl),strontium hydroxide(SrOH),and strontium hydride(SrH).This active species are formed only during combustion of red flare formulation.Sr(NO3)2is a good oxidizing species;it is often used as an oxidizer and color source[41-43].The aim of this study is to optimize different approaches to maximize the formation of active red color emitting species in the combustion flame.Red flares with enhanced performance,in terms of luminous intensity,and color quality were developed.Quantification of combustion temperatureand red color emitting species in the combustion flame was conducted using chemical equilibrium computer program named ICT Thermodynamic Code(Institute of Chemical Technology in Germany,virgin 2008).Spectral performance of developed flares was evaluated using Ocean Optics USB 4000 spectrometer and a Miltronics DL 1076 digital lux-meter.Light intensity,and color quality of reference red flare(R-284 NATO)was enhanced by 72%and 60%respectively.This was achieved mainly by optimizing the ratio of color intensifier poly vinyl chloride(PVC)to color source Sr(NO3)2using aluminum as a fuel.
2.Experimental work
2.1.Chemicals and materials
The main constituents for red flare manufacture include:oxidizer,metal fuel,color source,color intensifier,and binder.One constituent can have a dual function;Sr(NO3)2can act as a color source and an oxidizer.Poly vinyl chloride(PVC)can act as a binder and as color intensifier.The metal fuel can be Mg or Al.Table 1 tabulates a list of chemicals used in this study.
2.2.Red flare formulation
Artillery red tracer,which gives a deep red color,known as R-284 NATO was employed as a reference.The chemical composition of such tracer was 55 Wt%Sr(NO3)2,28 Wt%Mg and 17 Wt%PVC[44].A systematic study to develop enhanced red flare was conducted;this study includes the following main steps:
❖Fuel rich/stoichiometric formulations(Fo-F1).
❖Type of binder(F2-F3).
❖Type of fuel(F4).
❖Fuel to oxidizer ratio(F5-F8).
❖Color intensifier to color source at different fuel types(F9-F12).
Table 2 summarizes the chemical composition of different investigated formulations.
Red flare development should emphasize mixing of different ingredients to the molecular level,good homogenization,and accepted mechanical properties[6].In this study,the red flares were manufactured through eight main steps including:sieving of solid particles to fine powder,intimate mixing,granulation,filling,and pressing.The employed equipments in red flare preparation and spectral testing are scheduled in Table 3.
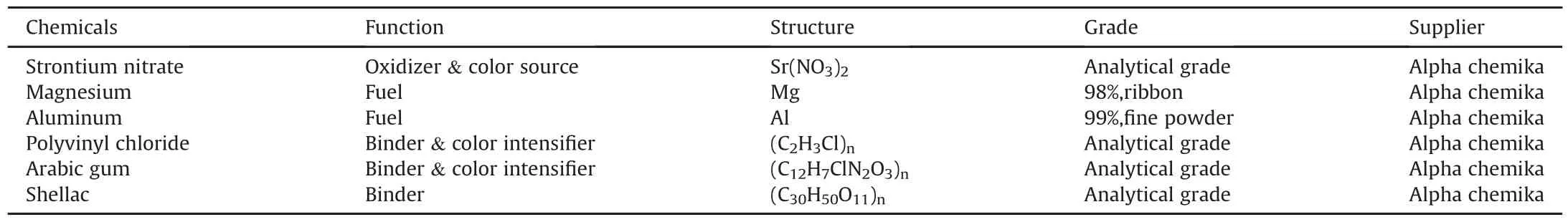
Table 1The function and structure of employed chemicals.
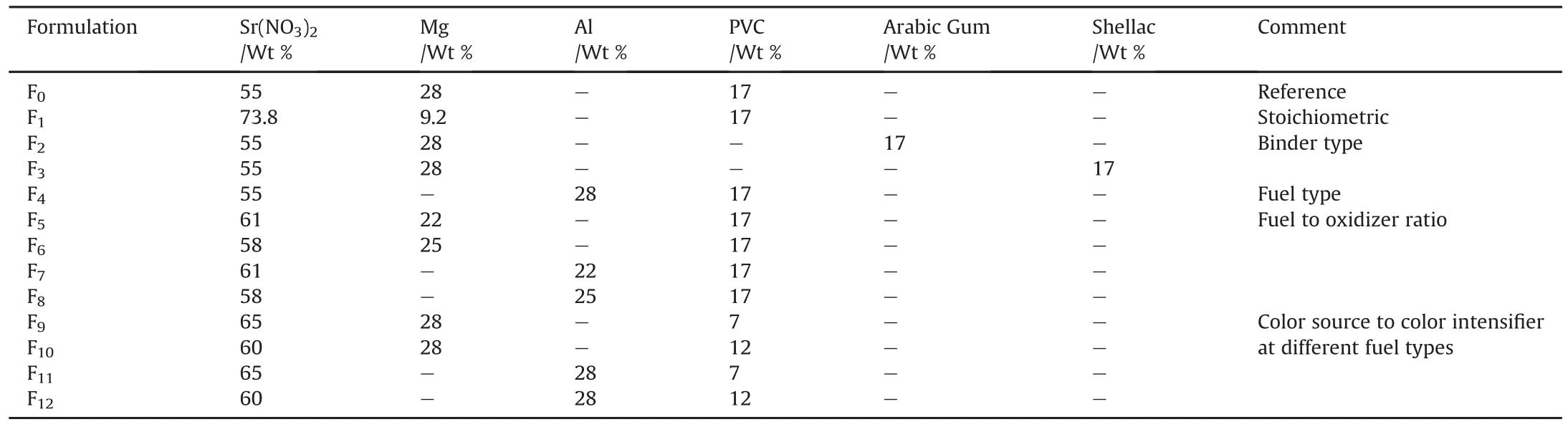
Table 2Chemical composition of developed red flares.
Table 3Equipment used in red flare preparation and spectral measurements.
2.3.Spectral measurements of red f l ares
Photometric tunnels are widely used to measure the imprint spectra of different pyrotechnic devices including:flares,signal,tracer,etc.The employed tunnel dimensions were 8 m(L)X 2 m(H)X 0.5 m(W).A schematic of the employed photometric dark tunnel is represented in Fig.2.
The Miltronics DL 1076 digital luxmeter measured the illuminance(E)in lux(lx).The illuminace(E)was converted into luminous intensity(I)in candela(cd)using Equation(1)[45].
Where d is the distance between the light source and detector.The average luminous intensity(cd/s)was calculated by measuring the summation of the area under the curve of the total luminous intensity(I)in candela(cd)divided by the burning time.The main drawback of such measurement is that it cannot offer information about color quality.In order to judge the color quality,ocean optics USB 4000 spectrometer was employed.The detector was adapted for the measurement over the red band from 650 to 780 nm[17].The average luminous intensity(cd/s)and spectrometer response(counts/s)were measured over the red band(620-750 nm)for the developed flares to reference flare[44].
3.Results and discussion
3.1.Effect of binder type
The type of binder could have a significant impact on red flare performance.The binder itself can act as color intensifier(as a source of chlorine).Arabic gum(F2),Shellac(F3)didn't improve the light intensity of standard red flare(F0)based on PVC(Fig.3).
Fig.4 demonstrated that intense color was achieved using PVC as a binder.PVC,with its high chlorine content,can have dual functions as a binder and color intensifier(source of chlorine)[17,41].
3.2.Effect of fuel type
Magnesium is the most commonly used fuel in pyrotechnics particularly colored flame compositions[13].However the formation of MgO as black body emitter can broaden the generated spectra,and deteriorate the colorquality[41].This is why,the metal fuel of reference red flare(Mg)was replaced byAl(F4)in an attempt to improve the color quality[35].The employed binder was PVC which was found to be effective binder and color intensifier.F4offered an imprint spectrum with sharp intense peaks corresponds to SrCl as indicated by green circle(Fig.4).
The imprint spectrum of F4demonstrated the formation of intense sharp peaks at 625.41 and 637.15 nm which are characteristic peaks of SrCl[41].The main red color emitter(SrCl)was increased by 58%and the incandescent emissions from MgO and SrO were no longer present in the reaction products using ICTcode(Table 4).
MgO and SrO emits incandescent emission which increases the light intensity but could deteriorate the color quality[46].Thus Al fuel could be the fuel of choice in red flare formulations with high color quality.
3.3.Optimization of fuel to oxidizer
Red flare formulations based on different fuel to oxidizer ratios weredeveloped.Fuel lading levels were 22,25 and 28 Wt%for both fuel types(Mg and Al)using PVC as a binder and color intensifier.F7(22 wt%Al)offered the most intense red color peaks resulting from SrCL emission as indicated by the red arrows(Fig.5).The discrete emission of SrCl at 625.41,637.15,661.83 and 675.53 nm can secure high quality of red light.
F7demonstrated the highest formation of SrCL(the main red color emitter)using ICT thermodynamic code.The increase in SrCL formation was found to be 184%compared with F0(Table 5).

Table 4Thermochemical reaction products for different fuel types.
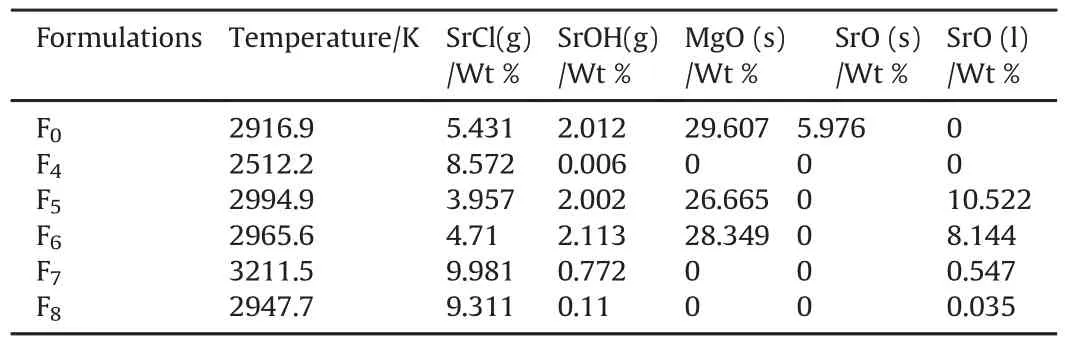
Table 5Decomposition products for different fuel type/content to oxidizer.
Even though F6exhibited the highest average luminous intensity(cd/s)(Fig.6);this could be ascribed to black emission resulted from MgO.MgO is a good white light emitter by incandesce emission this effect could be detrimental to color quality.
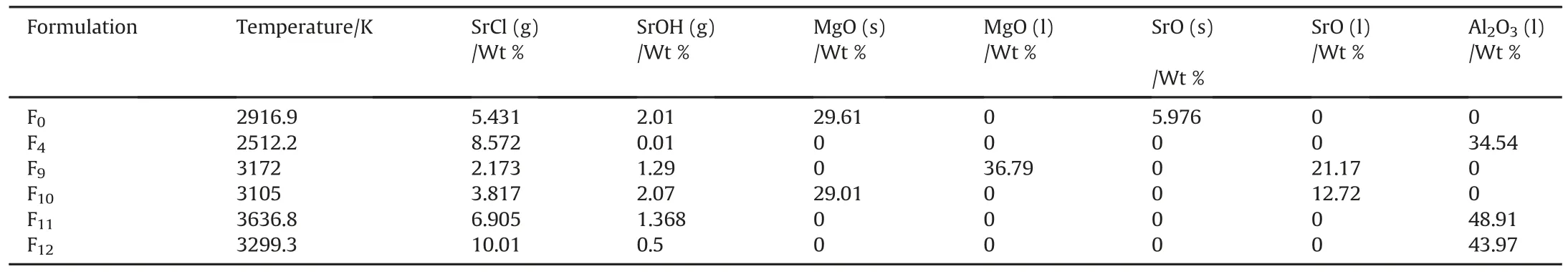
Table 6Impact of color intensifier to color source on red color emitting species.
3.4.Optimization of color source to color intensifier
PVC can act as a binder and color intensifier;the optimum ratio of color source SrNO3to color intensifier(PVC)was optimized at different fuel types(Mg&Al).The dramatic and superior change in red flare performance was achieved with PVC as binder and color intensifier,using Al fuel at 12 Wt%.Red flare(F12)exhibited the highest color quality(Fig.7).
The imprint spectra of F12and F11confirmed the finding that Al fuel could be the metal of choice for new generation of red flame compositions with superior color quality.Thanks to the optimum ratio of color source to color intensifier,which offered this high intensity.F12exhibited the highest content of main red color emitting species(SrCl)in combustion gaseous products;SrCl was increased by 90%compared to reference red flare(Table 6).The absence of MgO and SrO could secure high spectrum quality.F11also showed similar behavior,the main advantage of F11is the formation of SrOH which another red color emitter.
F12and F11also demonstrated superior optical performance in terms of luminous intensity(cd/s)as demonstrated in Fig.8.
F12demonstrated the highest average luminous intensity(cd/s);this was correlated to the high color quality as well due to the intense formation of SrCL and the elimination of any incandescent emission due to MgO.
Aluminum offered not only high spectral performance,but it also could offer extended surface life.Aluminum has a high chemical stability compared with magnesium fuel.Aluminum surfaces are readily oxidized by the oxygen in the air,and a tight surface coating of aluminum oxide(Al2O3)is formed that protects the inner metal from further oxidation.Hence,aluminum powder can be stored for extended periods with little loss of reactivity due to air oxidation[47].On the other hand,magnesium is a very reactive metal.It is oxidized by moist air to form magnesium hydroxide,Mg(OH)2,and it readily reacts with all acids[48].The reactions of magnesium with water and acid(HX)are demonstrated by equations(2)and(3).
Consequently,red flare formulations based on aluminum can exhibit enhanced performance as well extended service life without loss of reactivity.The optimum chemical composition of red flare was found to be 60 Wt%Sr(NO3)2as both oxidizer and color source,28 Wt%Al as fuel,and 12 Wt%PVC as binderand color intensifier.This composition can secure red light with high intensity and high quality.Fig.9 demonstrates digital photos of emitted red light for the developed red flare(F12)to reference flare.It is apparently clear that higher intensity and color quality were achieved via the optimized chemical composition.For developed red flare,the color broadening by incandescent MgO and SrO incandescent black body was eliminated.
The tailored red flare F12was developed via the optimization of the ratio between the color intensifier(PVC)to color source(Sr(NO3)2)using Al as a fuel.This flare exhibited an increase in the luminous intensity by 72%;the color quality over the red band(650-780 nm)was improved by 60%to the reference.Fig.10 summarized the average luminous intensity as well as the detector response over the red band for all investigated formulation.
From Fig.10 it is apparently clear that F11and F12achieved enhanced spectral performance compared to reference red flare.
4.Conclusions
PVC was found tobe the binderof choice for redflares;as it has a dual function as a binder and color intensifier(source of chlorine).The released chlorine exhibited an effective rule to support the formation of SrCl(the main red color emitter).Aluminum fuel was found to eliminate the formation of incandescent emitters(MgO and SrO).Furthermore aluminum supported the formation of SrcL;consequently high quality color was achieved.The optimization between the color intensifier(PVC)and the color source(Sr(NO3)2)with aluminum fuel was found to have the greatest impact for enhance luminous intensity and color quality.These parameters were optimized to develop red flare with enhanced spectral performance(F12).F12exhibited an increases the luminous intensity by 72%;the red band spectrum(650-750 nm)was enhanced by60%to the standard reference flare.This enhanced spectral performance supported the general rule that the color intensifier should be in the range from 10 to 15 Wt%of the total composition.Thanks to the optimum ratio of color source to color intensifier using aluminum metal fuel which offered this advanced spectral performance.
Acknowledgement
Military technical college is acknowledged for funding the research project entitled“Enhanced Visible Tracers for Illumination and Tracking”.
[1]Ledgard J.The preparatory manual of black powder and pyrotechnics.Lulu Enterprises Incorporated;2006.
[2]Johnston SF.A history of light and colour measurement:science in the shadows.CRC Press;2001.
[3]Shidlovskiy AA.Principles of pyrotechnics.3rd ed.1964.Moscow.
[4]Bebie,J.,Manual of explosives military pyrotechnics and chemical warfare agents.
[5]Kosanke KL,et alP.R.S.N.2,editor.Encyclopedic dictionary of pyrotechnics:(and related subjects).J Pyrotech 2012[Incorporated],6-1 to 7-49.
[6]Conkling JA,Mocella C.Chemistry of pyrotechnics:basic principles and theory.2nd ed.CRC Press;2010.
[7]Shidlovsky A.Principles of pyrotechnics.3rd ed.1974.
[8]Command USAM.Engineering design handbook:theory and application.In:Command USAM,editor.Military pyrotechnic series.Washington,DC:AMC Pamphlet;1967.706-185.
[9]Brock ASH.Pyrotechnics:the history and art of firework making.Daniel O'Connor;1922.
[10]Russell MS,Chemistry RSo.The chemistry of fireworks.Royal Society of Chemistry;2000.
[11]Ellern DH.Military and civilian pyrotechnics.United States of America:Chemical Publishing Company Inc;1968.
[12]Conkling JA,Mocella C.Introduction.In:Chemistry of pyrotechnics:basic principles and theory.2nd ed.CRC Press;2010.p.1-6.
[13]Shimizu T.Fireworks:the art,science,and technique.Pyrotechnica Publications;1996.
[14]Hardt AP,et al.Generation of light.In:Pyrotechnics.Pyrotechnica Publications;2001.p.277-98.
[15]Conkling JA,Mocella C.Basic chemical principles.In:Chemistry of pyrotechnics:basic principles and theory.CRC Press;2010.p.7-57.
[16]Ellern H.Modern pyrotechnics.Fundamentals of Applied Physical;1961.
[17]Conkling JA,Mocella C.Color and light production.In:Chemistry of pyrotechnics:basic principles and theory.CRC Press;2010.p.179-202.
[18]Ellern H.Colored lights.In:Military and civilian pyrotechnics.NY:CHEMICAL Publishing Company Incorporated;1968.p.122-30.
[19]Hardt AP,et al.Color creation.In:Pyrotechnics.Pyrotechnica Publications;2001.p.39-43.
[20]Weingart GW.Pyrotechnics.Survival Press;2001.
[21]Tro N.Chemistry in focus:a molecular view of our world.Cengage Learning;2015.
[22]Kosanke KL,Kosanke BJ.Selected pyrotechnic publications of KL and BJ Kosanke,Part 1:1981 through 1989.J Pyrotech 1995;(2):1-22.
[23]Meyerriecks W,Kosanke K.Color values and spectra of the principal emitters in colored flames.J Pyrotech 2003;18:1-22.
[24]Dillehay DR.Illuminants and illuminant research.J Pyrotech 2004:53-60.
[25]Ellern H.Underlying phenomena.In:Military and civilian pyrotechnics.NY:CHEMICAL Publishing Company Incorporated;1968.p.87-98.
[26]Army USDot.Military explosives.Headquarters,Department of the Army;1989.
[27]Tuukkanen IM,et al.Pyrotechnic and thermal studies on the magnesiumstrontium nitrate pyrotechnic system.Propellants,Explos Pyrotech 2006;31(2):110-5.
[28]Bailey A,Murray SG.Pyrotechnics.In:Explosives,propellants and pyrotechnics.Brassey's;2000.p.115-40.
[29]Douda B,In NADC.Theory of colored flame production.Defense Technical Information Center;1964.
[30]Kosanke KLKaBJ.The chemistry of colored flames.J Pyrotech 2007;25:30-48.
[31]Conkling JA,Mocella C.Components of high-energy mixtures.In:Chemistry of pyrotechnics:basic principles and theory.CRC Press;2010.p.59-96.
[32]Wada Y,Foster N,Yoshida T.Safety of reactive chemicals and pyrotechnics.Elsevier Science;1995.
[33]Defense,U.S.O.DOD Contractor's safety manual for ammunition and explosives.2008.
[34]Shimizu T.Part 4.Pyrotechnics.In:Fireworks:the art,science,and technique.Pyrotechnica Publications;1996.p.85-177.
[35]Ellern H.Magnesium and aluminum.In:Military and civilian pyrotechnics.NY:CHEMICAL Publishing Company Incorporated;1968.p.328-31.
[36]Jackson Jr B,et al.Substitution of aluminum for magnesium as a fuel in flares.DTIC Document;1975.
[37]L˘az˘aroaie C,et al.Temperature measurements of magnesium-and aluminumbased flares.J Therm Anal Calorim 2014;115(2):1407-15.
[38]Crawford BL,Huggett C,McBrady JJ.The mechanism of the double base propellants.J Phys Colloid Chem 1950;54(6):854-62.
[39]Rice OK,Ginell R.Theory of burning of double-base rocket propellants.J t Phys Colloid Chem 1950;54(6):885-917.
[40]Conkling J,Mocella C,editors.Chemistry of pyrotechnics basic principles and theory.2nd ed.London:CRC;2012.
[41]Ernst-Christian Koch JJS,Poret Jay C,Moretti Jared D.New pyrotechnic signal flare compositions based on cheap established and environmentally acceptable ingredients.In:Energetic materials:performance,safety and system applications.Germany:Fraunhofer ICT;2015.p.1-8.
[42]Siekierski SC,Burgess J.Concise chemistry of the elements.Elsevier Science;2002.
[43]Kosanke K,et al.Pyrotechnic chemistry,pyrotechnic reference series,No.4.J Pyrotech Inc 2004;11:12-24.
[44]Ellern H.Formulas.In:Military and civilian pyrotechnics.NY:CHEMICAL Publishing Company Incorporated;1968.p.353-87.
[45]Online Scientific and Engineering Resource.Lux to candela calculator Rapidtables.2014.Available from:,http://www.rapidtables.com/calc/light/lux-tocandela-calculator.htm.
[46]Douda B.Spectral observations in illuminating flames(Spectrum analyses on illuminating flames caused by magnesium-sodium nitrate combustion).1968.
[47]Mohamed AK,Mostafa HE,Elbasuney S.Nanoscopic fuel-rich thermobaric formulations:chemical composition optimization and sustained secondary combustion shock wave modulation.J Hazard Mater 2016;301:492-503.
[48]Elbasuney S,Mostafa SF.Continuous flow formulation and functionalization of magnesium di-hydroxide nanorods as a clean nano-fire extinguisher.Powder Technol 2015;278:72-83.
20 January 2017
in revised form 16 April 2017 Accepted 24 May 2017 Available online 27 May 2017
©2017 The Authors.Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
*Corresponding author.
E-mailaddresses:s.elbasuney@mtc.edu.eg,sherif_basuney2000@yahoo.com(S.Elbasuney).
Peer review under responsibility of China Ordnance Society.
- Defence Technology的其它文章
- Influence of electric current intensity on the performance of electroformed copper liner for shaped charge application
- Structurally modified RDX-A DFT study
- Micro-seeding and soft template effects on the control of polymorph and morphology of HMX micro particles in solvent-antisolvent process
- Control system design of flying-wing UAV based on nonlinear methodology
- Microstructural observations on the terminal penetration of long rod projectile
- The effect of wax coating,aluminum and ammonium perchlorate on impact sensitivity of HMX

