The effect of wax coating,aluminum and ammonium perchlorate on impact sensitivity of HMX
Yu-bin LI,Li-ping PAN,Zhi-jian YANG,Fei-yan GONG,Xue ZHENG,Guan-song HE
Institute of Chemical Materials,China Academy of Engineering Physics,Mianyang 621900,China
The effect of wax coating,aluminum and ammonium perchlorate on impact sensitivity of HMX
Yu-bin LI,Li-ping PAN,Zhi-jian YANG,Fei-yan GONG,Xue ZHENG*,Guan-song HE
Institute of Chemical Materials,China Academy of Engineering Physics,Mianyang 621900,China
Interaction HMX/AP/Al mixtures Impact sensitivity Thermal decomposition
Interaction of 1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX)/ammonium perchlorate(AP)and its effect on mechanical sensitivity may result in some restrictions for the application of AP/HMX system in high energetic weapon system.In this work,impact sensitivity test is used to study the effects of wax coating ofHMX,APand aluminum (Al)powderon sensitivitypropertiesofHMX/AP/Almixtures.Thermogravimetry-differential scanning calorimetry(TG-DSC)analysis has been developed to investigate the mechanism of interaction between HMX and AP during the course of thermal decomposition of HMX/AP/Al mixtures.The results show that severe interaction effect exists between AP and HMX,which causes the impact sensitivity(H50)to become smaller.The impact energy(E50)of mixture can be improved under the circumstances of effective separating HMX from AP by surface coating with Wax.AP may firstly engender low-temperature decomposition under the circumstance of external heat or mechanical impact,which causes the exothermic peak of HMX forward shift about 28°C.The gaseous product releasing from thermal decomposition of HMX accelerates further decomposition of AP.For HMX/AP composite system,the interactive catalysis effect between AP and HMX can be eliminated mostly by adding a great deal of Al powder(i.e.above 30%).
1.Introduction
Research on high energetic materials(HEM)recently mainly focuses on fabrication of novel composite explosive with high explosion energy combined with insensitivity to hazardous stimuli[1].The most effective way to improve the energy content is to increase the content of high explosives components in formulations such as nitramines[2].As typical representatives,1,3,5-trinitro-1,3,5-triazinane (RDX), 1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX)can greatly enhance the explosion power of the formulations.However,addition of these high explosives would raise many problems that need tobe studied and solved,such as the higher mechanical sensitivity,worse oxygen balance and mechanical strength[3,4],which may limit their application in powerful formulations.To obtain good oxygen balance of highenergy formulations,oxygen-rich oxidants are usually introduced.Ammonium perchlorate(AP)is a widely used oxidizing agent in composite propellants,and it has been extensively investigated in various propellant and pyrotechnic ammunitions[5].Hence,HMX/AP based explosive compositions have been extensively prepared and used,such as PBXN-110,PBXN-111 and PBXW-114[6].Since the thermal decomposition characteristics of HMX/AP plays a key role not only in combustion behavior of composite propellants,but also in detonation processes of composite explosives,many researches have been conducted on thermal decomposition behavior of HMX/AP.Li et al.[7]studied the interactions of AP and HMX in NEPE propellants from molecular structure and chemical reaction perspectives.The addition of AP to HMX enhanced its exothermic reaction rate with lower peak temperature.Jiao et al.[8]obtained the same results using TG-derivative thermogravimetry(DTG),highpressure differential scanning calorimetry(PDSC),and DSC-TGFourier transform infrared spectroscopy(FTIR).Interactions between AP and HMX playan important role in mechanical sensitivity of HMX/AP mixtures.Zhu et al.[9]investigated the sensitivity of HMX/AP systems at various concentrations and temperature via moleculardynamics simulations.They proposed thatthe maximum bond length of pyrogenation trigger bond N-NO2in HMX/AP mixtures increases first and then decreases,and the peak of the parabola is at the AP/HMX mass ratio of 1:1,which agrees well with the experimental fact that the sensitivity value was thehighest at the mass ratio of 1:1.However,experimental results about interaction affecting the sensitivity of mixtures were seldom reported,and hence it is necessary to make a comprehensive study on interaction between HMX and AP on the sensitivity of HMX/AP mixture, and fully understand the underlying catalytic mechanisms.
Nowadays,attention has been paid to improvement of sensitivity properties of composite explosives with maintained high energy density.An effective method is to coat sensitive energetic fillers with low-sensitive or inert materials[10].Up to now,coating with wax is still the most impactful and convenient desensitizing mean for high explosive though it is not new.Manning and Thelma[11]used graphite to coat RDX fillers by solvent-evaporation and investigated its application in composite explosives.After such surface coating,drop height(H50)of RDX and RDX-containing composite explosives increased by 40%and 115%,respectively.For nitroamine explosive used in a composite,best coating material should be selected from its ingredients[12].For instance,An et al.[13]used nitrocellulose(NC)to coat nitroamine explosive by a new slurry emulsion coating method.Compared to propellants made with raw RDX,the safety and processing technology of propellants using coated RDX are all improved.However,there are still two limitations for this coating method:1)energy will decrease greatly if coating materials is higher than 5%,and 2)coated particles can easily form agglomerates.Thereby,some newcoating materials and new coating techniques need to be developed to obtain better desensitizing effect with less coating agent[14].
The sensitivity of composite explosives was demonstrated to be strongly dependent on the nature of the ingredients,in particular their physiomechanical and thermophysical properties[15-18].For example,there are many open literature with regard to the sensitivity and stability of the HMX based explosives[19-21].Besides,if some of the ingredients have high thermal conductivity or lubricating effect,sensitivity of composites would be relative low.Gogulya[22]confirmed that impact sensitivity of composite explosive was decreased visibly along with the addition of Al powder.However,their attention was not further focus on the effect of Al powder on nitroamine explosive/oxidant system.How to eliminate interactional effect between explosive and oxidant,harmonize contradiction between high energy of ammunition and its excellent security,becomes a key issue which must be solved for designing of high energy but insensitive ammunition.
In this paper,safety characteristics of key ingredients in composite explosives,interaction among these ingredients(HMX,AP and Al)and its effect on safety were investigated in detail.The mechanism of interaction among these ingredients is elucidated from a view of thermal decomposition arose by the creation of hot spots during impact-loading.The effect of waxcoating and addition of Al on the sensitivity of HMX/AP mixture were also explored sequentially from the point of eliminating the interactive catalysis effect.The results of this paper may provide a useful information and guidance for researchers in designing powerful insensitive ammunitions.
2.Experimental section
2.1.Materials
HMX(Gansu Yinguang Chemical Industry Group Co.,Ltd.,Baiyin,China)used was of industrial grade with an average particle size of 30μm.AP(Dalian Potassium Chlorate Factory,Dalian,China)used was of industrial grade with a mean size of 120μm.Average particle sizes of Al powders used were 13μm(Southwest of China Aluminum Industry Co.,Ltd.,China,98.8%).Wax was provided in refined mode by Fushun Petrochemical Co.,Ltd.Other chemicals and reagents used in this study were commercially purchased and used without further purification.
2.2.Preparation
A precipitation technique was used to coat HMX,AP or Al by using Wax as coating agent via a water-suspension solvent-evaporation process.98.0 g HMX particles were firstly added into distilled water to get a suspension under mechanical agitation(450 rpm).At this time,2.0 g Wax was dissolved to acquire uniform dispersion by using petroleum ether as a good solvent.Then,the solution containing desensitizing agent was added to HMX slurry under stirring at 54°C,and the vacuum(200 mbar)was introduced for several minutes.Finally,the Coated HMX products were filtered,washed by distilled water and dried under vacuum for 6 h.Coated AP and Al samples were prepared similarly via solvent suspension process by using a non-aqueous liquid as suspension media.Coated samples were marked as HMXW,APW and AlW,respectively.
In addition,physical mixtures of HMX,AP and Al or their coated samples with different mass ratio were prepared by mixing two or three ingredients in a carnelian mortar for 10 min,and then used to test.
2.3.Characterization
The morphologies of HMX,Al,AP and their coated samples were observed by scanning electron microscopy(SEM,Hitachi TM-1000,Japan),shown in Fig.1.Obviously,the surface of coated HMX(Fig.1(b))and coated AP(Fig.1(f))are sleeker than the uncoated sample(Fig.1(a),e).As for the global Al powder,the surface differentia of coated sample(Fig.1(d))is not distinct in comparison with the original sample(Fig.1(c)),ascribing to its lesser particle size.
Impact sensitivity was tested by a WL-1 type drop-hammer apparatus.Each sample(35 mg)was subjected to an impact of a 5 kg hammer at various heights using a widely used“up-anddown”method.If sample initiates/does not initiate,next trial will be performed one step lower/higher.Based on 25 go/no-go trials,impact sensitivity of each sample was expressed by drop height of 50%explosion probability(H50)and impact energy(E50).In this way,higherE50orH50value represents lower impact sensitivity.
Thermogravimetry(TG)test of sample was recorded on a TAQ 600 instrument(TA,USA),and differential scanning calorimeter(DSC)test was recorded on a TAQ100 instrument(TA,USA),from 30°C to 500°C with a temperature rise rate of 10°C min-1,in a nitrogen atmosphere(50 mL min-1).The alumina crucible was selected as the sample pan.
3.Results and discussion
3.1.Effect of wax coating on sensitivity of HMX/AP mixture
Fig.2 displays the results of impact sensitivity for pure or coated HMX,AP and their physically mixed samples(mass ratio 50/50).It is clear that pure HMX is very sensitive towards impact,which E50value is only 8.2 J.After coated by wax,the sensitivity of HMX can be visibly reduced,E50value increases to 40.1 J.Pure AP is moderate sensitive towards impact,and after coated by wax its sensitivity is slightly declined,E50value increased from 24.9 J to 35.4 J.Formixed sample,there is a wondrous phenomenon.Whether original or coated samples,all E50value of the mixtures descend markedly compared with that of single ingredient.For instance,E50of HMX/AP is only 5.3 J,far less than that of pure HMX(8.2 J)or AP(24.9 J).Similarly,E50of HMX/APW(7.3 J)is also lower than that of pure HMX or APW(35.4 J).E50of HMXW/AP(9.7 J)is as well lower than of pure HMXW(40.1 J)or AP.All these results imply that the interaction effect between HMX and AP is very intense.In general,impact sensitivity of mixed explosives can be obviously reduced after HMX was coated by Wax,that is to say,the interaction effect between HMX and AP can be weakened to a certain extent by coating HMX with desensitizer.As can be seen in Fig.2,E50of both HMXW/AP(9.7 J)and HMX/APW(7.3 J)are both higher than that of HMX/AP(5.3 J),especially,E50of HMXW/APW increases markedly to 21.9 J in comparison with HMX/AP.In brief,to eliminate the intense interaction effect between AP and HMX,it needs to effectively separate HMX from AP by using enough coating materials,i.e.desensitizer,polymeric binder or other inert matter.
3.2.Effect of Al powder on sensitivity of AP,HMX and their mixture
3.2.1.Effect of Al powder on sensitivity of HMX
Impact sensitivity results of uncoated or coated HMX,Al and physically mixed HMX/Al samples are summarized in Table 1.For mixed samples,no matter adding pure Al or coated Al,all E50value increase compared with that of coated HMX,implying that the addition of micro-sized aluminum powder doesn't make HMX more sensitive.It can be deduced that there is no interaction effect between HMX and Al while Al content is 50%,probably because micro-sized Al has no effect on initial thermal decomposition step of HMX[3].This result indicates that reaction rate of deflagrationto-detonation of HMX reduces as a great deal of Al powder mixes into HMX,a plentiful of Al decentralizes the impact stress on explosive,accordingly diminishes the probability of hot-spots formation.Simultaneously,a vast of Al can improve heat transmit which hamper explosion propagation from hot-spots.
3.2.2.Effect of Al powder on sensitivity of AP
Impact sensitivity of the samples mixed coated AP with pure Al orcoated Al is summarized inTable 2.For whether APW/Al or APW/AlW sample,the E50value increases visibly in comparison with the single coated AP.This result also implies no interaction effect between AP and Al while Al content is 50%.Actually,many research results confirmed that micro-sized Al powder had little catalyzing effect on thermal decomposition of AP[23,24].Moreover,Al powder has adsorption and inhibition effect to gaseous product sublimed of AP,thereby it can suppress low-temperature thermal decomposition of AP[25],and then decrease impact sensitivity of AP/Al mixture.
3.2.3.Effect of Al powder on sensitivity of HMX/AP mixture
From previous results,it is not difficult to find out the rule of interaction effect on the sensitivity between two ingredients(with 50/50 ratio).In fact,the performance of composite explosives or propellants is highly relevant to the interaction between HMX,AP and Al powder[26].However,for HMX/AP/Al ternary composite system,the effect of different ingredient content on its sensitivity is still not clear.As 30%Al is usually a critical value for high energy composite propellants or explosives,such as AFX-757[27],it is selected to study the effect of Al powder on E50of HMX/AP mixture.By keeping Al content as steady,HMX content of increases as 10%step from 15%to 55%,meanwhile AP content decreases from 55%to 15%,a series of compositions can be achieved for HMX/Al/AP ternary composite system.E50of each composition are listed in Table 3.It is clear that E50of HMX/Al/AP ternary composite descends along with HMX content increasing and AP content decreasing.However,all E50of HMX/Al/AP ternary composite system are larger than that of coated HMX(40.1 J),shows that when Al content in ternary composite system keeps as 30%,effective isolation between AP and HMX is achieved,and the interaction effect between HMX and AP is basically eliminated.
While AP content keeps steady,such as 30%,HMX content increases from 15%to 55%,meanwhile Al content decreases from 55%to 15%,a series of mixtures can be also achieved for HMX/Al/AP ternary composite system.As can be seen in Table 4,E50value of HMX/Al/AP ternary composite declines as the content of HMX increase and AP decrease.However,if Al content is less than 30%,like sample B4 and B5,E50value of HMX/Al/AP ternary composite system are lower than that of coated HMX(which E50value is 40.1 J).This data seems to show that effective separation between AP and HMX is not achieved,and the synergistic interaction between HMX and AP still exists when Al content in ternary composite system is far less 30%.In fact,research shows that E50value of composite explosive descends early,then ascends while Al content increases gradually and the inflexion corresponding to the content of Al is about 10%[28].The impact sensitivity of composite explosive declines along with the addition of Al because a great deal of Al decentralizes the impact stress on explosive and doesn't make energy localize on explosive particles,thereby diminishes the probability of hot-spots formation.Moreover,a vast of Al can improve heat transmission and this also prevents the formation and buildup of hot-spots.

Table 1E50and H50of Al,HMX and HMX-Al mixture.

Table 2E50and H50of Al,AP and AP-Al mixture.
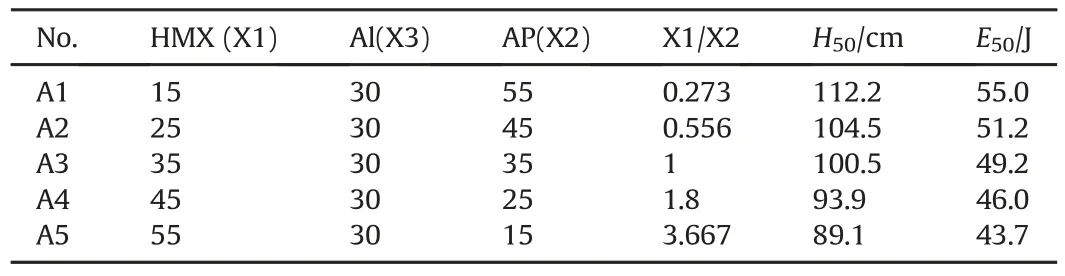
Table 3E50and H50of HMX-Al-AP mixture(30%Al).
To validate these experimental results,empirical function of sensitivities(E50)of HMX/AP/Al mixtures versus HMX/AP or HMX/Al ratio is fitted to exponential equation from experimental data,depicted in Fig.3 and Fig.4,respectively.On one hand,from the fitted equation(depicted as Fig.3),it can deduce that if HMX content increases to 70%,AP content decreases to 0%,the HMX/Al ratio is 70/30,then the E50is 43.3 J.As can be seen in Table 1,E50of HMX/Al mixture(50/50)is 51.9 J.Hence,when Al content increases from 30%to 50%,it can diminish the probability of hot-spot formation from impact[22],makes E50increase.On the other hand,from the fitted function depicted in Fig.4,it can also deduce that if HMX content is 70%,Al content is 0%,i.e.HMX/AP is 70/30,E50of HMX/Al/AP ternary mixtures would be 35.8 J.According to fitted equation depicted in Fig.3,when HMX/AP ratio keeping as 70/30,E50of HMX/AP/Al mixtures(49/21/30)would be 44.8 J.70HMX/30AP system is more sensitive than 49HMX/21AP/30Al,which further clarifies that more Al adding into HMX/AP mixture can effectively eliminate the interaction effect between HMX and AP.
3.3.Thermal mechanism of interaction effect for HMX/AP mixture
As a key energetic material,ammonium perchlorate(AP)continues to inspire new research efforts to better understand its thermal decomposition.Among many peculiar features,the most striking is the discovery of low-and high-temperature modes of decomposition[29].The low-temperature decomposition(LTD)occurs belowapproximately300°C.Actually,pure AP is quitestable compound at room temperature,nothing has happened exceptwater loss at 150°C.However,slight thermal decomposition of AP begins to appear above 150°C,leading up to form a porous product[30].When heated to about 240°C,pure AP undergoes a polymorphous transition(from orthorhombic into cubic crystalline form)[29,31]with an endothermic peak(about 243.7°C)in DSC curve(see Fig.5).As can be seen,the first exothermic peak at 301.7°C is accompanied by a weight loss of 30.1%,which corresponds to partial decomposition(LTD)of AP and the formation of an intermediate product[32].The second main exothermic peak appears at higher temperature of 392.1°C,which is associated with 69.9%weight loss,indicating the further and complete decomposition(high-temperature decomposition,HTD)of the intermediate products.
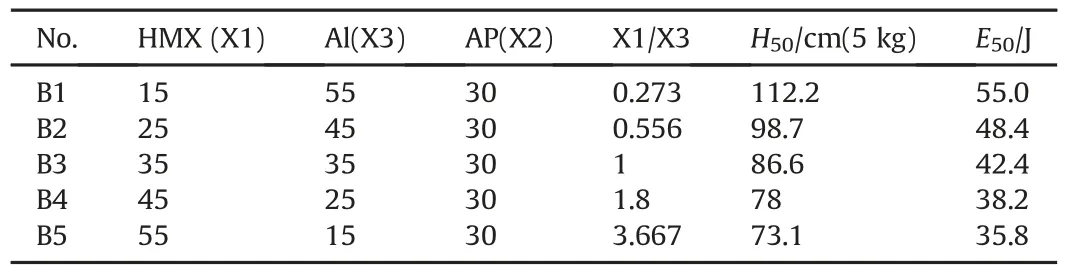
Table 4E50and H50of HMX-Al-AP mixture(30%AP).
As it is known,the exothermic peak of pure HMX is at about 280°C.From DSC curve in Fig.5(a),it is clear that large difference was observed in HMX/AP mixture where the two exothermic peaks occur at about 298°C and 375°C,in contrast with two exothermic peak of pure AP at 302°C and 392°C,respectively.From TG curve in Fig.5(b),the loss in first step exceeds 50%,which indicates that except for the decomposition of HMX,a small quantity of AP has also participated in decomposition at low temperature(below 240°C).This change can be explained as follows.When HMX/AP mixtures undergo external heat or mechanical action,AP engenders low-temperature decomposition firstly and releases some gaseous products,such as oxidative HClO4[30,31],which urges HMX to decompose ahead of schedule and makes exothermic peak of HMX shift from 275°C to 247°C.Similar to the decomposition of HMX/AN mixture[33],the acidic intermediate product(perchloric acid)resulting from dissociation of AP attacks the nitrogen atoms of HMX,then enormous gaseous product NO2is released from the decomposition of HMX,which in reverse oxidizes gaseous products NH3of AP in first decomposition step,therefore the second decomposition characteristic of AP would also be affected.In fact,this process is also accompanied by a simultaneous dissociative sublimation.Moreover,when AP mixes with HMX,apparently unlike the decomposition characteristic of pure AP[31],the loss of AP in first decomposition step is almost the same as that of the second decomposition step,which is about 18.0%and 21.9%,respectively.This interactional catalyzing effect between HMX and AP on thermal decomposition was also found in slow cook-off test ofHMX/AP/hydroxylterminatedpolybutadiene(HTPB)propellants,which causes a violent reaction[34].All results imply that there is a synergistic interaction of HMX with AP during thermal decomposition while the ratio of HMX/AP in mixture is 50/50.
To validate the effect of different Al content on synergistic interaction of HMX/AP/Al system,two composite samples are selected to study their thermal decomposition characteristics.TG curves of two samples are shown in Fig.6.As for sample with 10%Al,HMX in ternary mixture(10Al/50HMX/40AP)decomposes acutely at about 245°C,which is accompanied with partial thermal decomposition of AP.The loss rate in the first step was 70.6%,which exceeds the content of HMX(50%),and about half of AP participates in decomposition,indicating that an interaction with catalyzing effect between HMX and AP still exists.The mass residue of sample(b)is over 10%,partly ascribing to the oxidation of Al during the thermal decomposition of strong oxidizer.However,while Al content adds to 30%,the decomposition peak of HMX in ternary mixture(30Al/40HMX/30AP)is about 275°C,almost the same as pure HMX,and decomposition peak of AP is about 302°C,also like pure AP.The first step loss rate was 42.4%,almost consistent with HMX content in mixture.This result validates that enough Al powder adds to HMX/AP mixture can weaken or eliminate the interactional catalyzing effect between HMX and AP.
4.Conclusions
In this paper,interaction among the ingredients(AP,HMX and Al)of HMX/AP/Al based composites and its effect on impact sensitivity of mixtures are first investigated by using impact sensitivity test and TG-DSC analysis.Violent interaction effect exists in HMX/AP mixtures,which cause the impact sensitivity(E50)of mixture less than pure AP or HMX.Whereas,the impact sensitivity of HMX/AP mixture can be decreased on the condition of effective separation HMX from AP,i.e.surface coating of HMX or AP with 2 wt-%wax.No interaction effect on safety was found when high content(50%)Al blends with HMX or AP.Impact-induced heat causes AP low-temperature decompose firstly,then the acidic gaseous products of AP decomposition oxidizes HMX and cause its exothermic peak temperature occur in advance about 28°C.Conversely,the gaseous product produced by HMX decomposition oxidizes the pyrolytic gaseous product of AP and accelerates further decomposition of AP.This synergistic interaction causes HMX/AP mixture become more sensitive.Moreover,A large amount of Al(such as 30 wt-%)can separate effectively HMX from AP,and spread around theheatfrom hot-spots,then suppressthelowtemperature thermal decomposition of AP,sequentially eliminate interactive catalysis effect between AP and HMX.Understand fully the interaction of ingredients and their effect is of important particularly for designing explosive formulations for their later potential use in insensitive applications.
Acknowledgments
This work was supported by the National Nature Science Foundation of China(Nos.11402238,11502243 and 11502245).The authors thank Yu Chi and Shaojun Yu for their technical assistance in TG-DSC analysis and sensitivity test of mixed material.
[1]Agrawal JP.High energy materials.Wiley VCH;2010.
[2]Keshavarz MH.Prediction of detonation performance of CHNO and CHNOAl explosives through molecular structure.J Hazard Mater.2009;166:1296-301.
[3]Muravyev N,Frolov Y,Pivkina A,Monogarov K,Ordzhonikidze O,Bushmarinov I,Korlyukov A.Influence of particle size and mixing technology on combustion of HMX/Al compositions.Propell Explos Pyrot 2010;35(3):226-32.
[4]Gogulya MF,Makhov MN,Brazhnikov MA,Dolgoborodov AY,Arkhipov VI,Zhigach AN,Leipunskii IO,Kuskov ML.Explosive characteristics of aluminized HMX-based nanocomposites.Combust Explos Shock+2008;44(2):198-212.
[5]Rajiæ M,Suæeska M.Study of thermal decomposition kinetics of lowtemperature reaction of ammonium perchlorate by isothermal TG.J Therm Anal Calorim 2001;63:375-86.
[6]Sutherland GT,Brousard LJ,Leahy JF,Prickett S,Deiter JS.Composition choice and formulation of three HMX-based research explosives.J Energ.Mater.2004;22:181-97.
[7]Fang C,Li SF.Synergistic interaction between AP and HMX.J Energ.Mater.2002;20:329-34.
[8]Jiao Q-J,Zhu Y-L,Xing J-C,Ren H,Huang H.Thermal decomposition of RDX/AP by TG-DSC-MS-FTIR.J Therm Anal Calorim 2014;116:1125-31.
[9]Zhu W,Wang XJ,Xiao JJ,Zhu WH,Sun H,Xiao HM.Molecular dynamics simulations of AP/HMX composite with a modified force field.J Hazard Mater.2009;107:810-6.
[10]Highly energetic compositions based on functionalized carbon nanomaterials.Nanoscale 2016;8:4799-851.
[11]Manning TG,Strauss B.Reduction of energetic filler sensitivity in propellants through coating.U.S.Patent No.6,524,706,filed 27.March 2001.
[12]Li FS,Guo XD,Liu GP.Design and manufacture of advanced propellant.Beijing:National Defense Industry Press;2008.p.202-4.
[13]An CW,Li FS,Wang JY,Guo XD.Surface coating of nitroamine explosives and its effects on the performance of composite modified double-base propellants.J Propul Power 2012;28:444-8.
[14]Li YB,Huang H,Pan LP,Zhang JH,Li JS,Zheng BH.Desensitizing technology of AP by coating and its application.Chin.J Energ.Mater.2014;22:792-7.
[15]Teselkin VA.Characteristics of the impact explosion initiation of HMX/energetic additive mixtures.Combust Explo Shock+2010;4:748-54.
[16]Xiao JJ,Wang WR,Chen J,Ji GF,Zhu W,Xiao HM.Study on the relations of sensitivity with energy properties for HMX and HMX-based PBXs by molecular dynamics simulation.Phys B Condens Matter 2012;407:3504-9.
[17]Huang B,Cao M,Nie F,Huang H,Hu C.Construction and properties of structure and size-controlled micro/nano energetic materials.Def Technol 2013;9:59-79.
[18]Kumar R,Siril PF,Soni P.Tuning the particle size and morphology of high energetic material nanocrystals.Def Technol 2015;11:382-9.
[19]Impact sensitivity in respect of the crystal lattice free volume and the characteristics of plasticity of some nitramine explosives.Chin J Energet Mater 2015;23(12):1186-91.
[20]Notes on the use of the vacuum stability test in the study of initiation reactivity of attractive cyclic nitramines in the C4 matrix.J Therm Anal Calorim 2013;112(3):1433-7.
[21]Recent advances in thermal analysis and stability evaluation of insensitive plastic bonded explosives(PBXs).Thermochim Acta 2012;537:1-12.
[22]Gogulya MF,Makhov MN,Yu Dolgoborodov A,Brazhnikov MA,Arkhipov VI,Shchetinin VG.Mechanical sensitivity and detonation parameters of aluminized explosives.Combust Explo Shock+2004;40:445-57.
[23]Liu L,Li F,Tan L.Effects of nanometer Ni,Cu,Al and NiCu powders on the thermal decomposition of ammonium perchlorate.Propell Explos Pyrot 2004;29:34-8.
[24]Zhi J,Wang T,Li S.Thermal behavior of ammonium perchlorate and metal powders of different grades.J Therm Anal Calorim 2006;85:315-20.
[25]Zhu Y,Jiao Q,Huang H,Ren H.Effect of aluminum particle size on thermal decomposition of AP.Chem J Chin Univ 2013;34:662-7.
[26]Zhu Y,Xiao Z,Jiao Q.Effects of aluminum on thermal decomposition of hexogen/ammonium perchlorate.Chem Res Chin Univ 2014;30:666-71.
[27]G.W.Brooks,Extremely insensitive detonating substance(EIDs)testing and qualification of AFX-757 explosive,Insensitive Munitions and Energetic Materials Technology Symposium,2001,203-223.
[28]Wang C,Chen S,Zhao S.Influence of Al powder on mechanical sensitivity of RDX.Initiators&Pyrotechnics 2010;1:32-4.
[29]Vyazovkin S,Wight CA.Kinetics of thermal decomposition of cubic ammonium perchlorate.Chem Mater.1999;11:3386-93.
[30]Mallick L,Kumar S,Chowdhury A.Thermal decomposition of ammonium perchlorate-a TGA-FTIR-MS study:Part I.Thermochim Acta 2015;610:57-68.
[31]Boldyrev VV.Thermal decomposition of ammonium perchlorate.Thermochim Acta 2006;443:1-36.
[32]Singh NB,Ojha AK.Formation of copper oxide through NaNO3/KNO3 eutectic melt and its catalytic activity in the decomposition of ammonium perchlorate.Thermochim Acta 2002;390:67-72.
[33]Zeman S,Shu Y,Friedl Z,agenknecht JV.Thermal reactivity of some nitro-and nitroso-compoundsderived from 1,3,5,7-tetraazabicyclo[3.3.1]nonane at contamination by ammonium nitrate.J Hazard Mater 2005;A121:11-21.
[34]Chen Z,Tang C,Zhao X.Relationship between slow cook-off behavior and thermal decomposition characteristics of solid propellant.Chinese J Energ.Mater.2005;13:393-6.
12 March 2017
in revised form 9 May 2017 Accepted 26 May 2017 Available online 28 May 2017
©2017 Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
*Corresponding author.
E-mail address:zhengxue@caep.cn(X.ZHENG).
Peer review under responsibility of China Ordnance Society.
- Defence Technology的其它文章
- Influence of electric current intensity on the performance of electroformed copper liner for shaped charge application
- Structurally modified RDX-A DFT study
- Micro-seeding and soft template effects on the control of polymorph and morphology of HMX micro particles in solvent-antisolvent process
- Control system design of flying-wing UAV based on nonlinear methodology
- Spectrally adapted red flare tracers with superior spectral performance
- Microstructural observations on the terminal penetration of long rod projectile

