新型顺-1,3-二芳基螺[吡唑-4,2′-吡唑并[1,2-a]吡唑]衍生物的非对映选择性合成
朱美军, 张 瑜, 陆玉玲, 孙 晶, 颜朝国*
(1. 江海职业技术学院,江苏 扬州 225101; 2. 扬州大学 化学化工学院,江苏 扬州 225002)
新型顺-1,3-二芳基螺[吡唑-4,2′-吡唑并[1,2-a]吡唑]衍生物的非对映选择性合成
朱美军1, 张 瑜2, 陆玉玲2, 孙 晶2, 颜朝国2*
(1. 江海职业技术学院,江苏 扬州 225101; 2. 扬州大学 化学化工学院,江苏 扬州 225002)
在三乙胺催化下,环偶氮甲亚胺与4-芳亚基-5-甲基-2-苯基吡唑-3-酮在乙腈中回流反应,经1,3-偶极环加成反应合成了13个新型的1,3-二芳基取代的螺[吡唑-4,2′-吡唑并[1,2-a]吡唑]衍生物(3a~3m),其结构经1H NMR,13C NMR, IR和HR-MS(ESI)表征。采用X-射线单晶衍射研究了3d,3h,3j和3l的单晶结构。结果表明:3为特殊的顺式-1,3-二芳基构型。
偶氮甲亚胺; 吡唑酮; 螺环化合物; 1,3-偶极环加成; 非对映选择性合成
吡唑及其衍生物是多种天然产物和合成化合物的重要结构单元。吡唑类化合物大多具有良好的生物活性,在新药和农药开发中有诸多应用[1]。其中,吡唑并[1,2-a]吡唑是应用最为广泛的吡唑类化合物之一[2-3]。如吡唑并[1,2-a]吡唑啉酮可用作除草剂,也可用作乙酰辅酶A羧化酶和肌醇(内肽)胞质Ca2+-ATPase的抑制剂[4-5]。此外,还对有氧和厌氧细菌有显著的广谱杀灭活性[6]。γ-内酰胺类抗生素是基于肽模拟物的吡唑并[1,2-α]吡唑啉酮类化合物[7],药理性质独特,药用价值较高,其合成方法的研究一直是该领域的热点之一[8-9]。
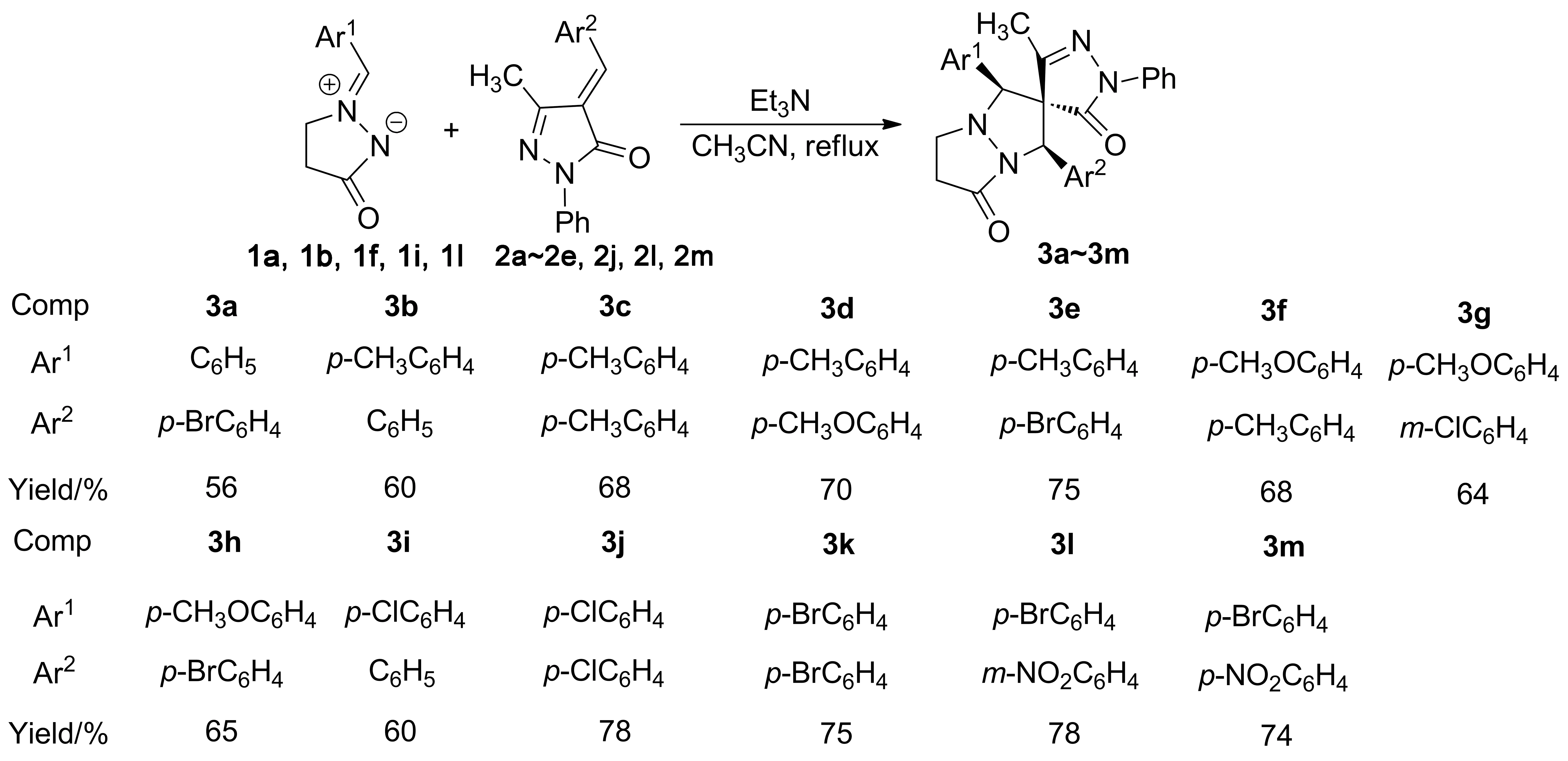
Scheme1
1,3-偶极子偶氮甲亚胺和含双键、三键的亲偶极体的1,3-偶极环加成反应,由于具有底物多样性,区域选择性和立体选择性,成为合成吡唑并[1,2-a]吡唑啉酮衍生物的高效方法之一[10-19]。本课题组持续开展了螺杂环化合物的高效合成方法的研究工作,取得诸多成果[20-23]。但使用环状偶氮甲亚胺进行1,3-偶极环加成反应合成螺吡唑并[1,2-a]吡唑啉酮衍生物的报道较少[24-26]。最近,我们成功地通过环状偶氮甲亚胺进行1,3-偶极环加成反应,合成了螺[茚-2,2′-吡唑并[1,2-a]吡唑]和螺[二氢吲哚-3,2′-吡唑并[1,2-a]吡唑]衍生物[27]。为进一步拓展环状偶氮甲亚胺在1,3-偶极环加成反应中的应用范围,我们继续展开了环状偶氮甲亚胺与4-亚芳基-5-甲基-2-苯基吡唑-3-酮的1,3-偶极环加成反应的研究。
本文在三乙胺催化下,环偶氮甲亚胺(1a,1b,1f,1i,1l)与4-芳亚基-5-甲基-2-苯基吡唑-3-酮(2a~2e,2j,2l,2m)在乙腈中回流反应,经1,3-偶极环加成反应合成了13个新型的1,3-二芳基取代的螺[吡唑-4,2′-吡唑并[1,2-a]吡唑]衍生物(3a~3m, Scheme 1),其结构经1H NMR,13C NMR, IR和HR-MS(ESI)表征。采用X-射线单晶衍射研究了3d,3h,3j和3l的单晶结构。
1 实验部分
1.1 仪器与试剂
XT-4型显微熔点仪(温度未校正);Agilent DD2 400 MHz型核磁共振仪(CDCl3为溶剂,TMS为内标);Bruker Tensor 27型红外光谱仪(KBr压片);Bruker MaXis型超高分辨质谱仪;Bruker Smart APEX-2型X-射线单晶衍射仪。
1和2a~2m按文献[14]方法合成;其余所用试剂和溶剂均为分析纯。
1.2 3a~3m的合成通法
在反应瓶中依次加入10.5 mmol,20.5 mmol,三乙胺0.1 mmol和乙腈15.0 mL,回流反应6 h。旋蒸除溶,残余物经硅胶柱层析(洗脱剂:石油醚/乙酸乙酯=1/1,V/V)纯化得3a~3m。
3a: 白色固体,产率56%, m.p.160~162 ℃;1H NMRδ: 7.80~7.77(m, 2H, ArH), 7.44~7.39(m, 4H, ArH), 7.28~7.29(m, 2H, ArH), 7.26~7.25(m, 1H, ArH), 7.24~7.22(m, 3H, ArH), 7.04~7.02(m, 2H, ArH), 5.66(s, 1H, CH), 4.41(s, 1H, CH), 3.95~3.88(m, 1H, CH), 3.27~3.19(m, 1H, CH), 3.08~2.99(m, 1H, CH), 2.97~2.90(m, 1H, CH), 1.56(s, 3H, CH3);13C NMRδ: 173.9, 170.5, 159.1, 137.3, 134.5, 132.0, 131.6, 129.1, 129.0, 128.9, 126.0, 125.8, 122.2, 119.2, 77.3, 72.7, 63.5, 47.8, 31.8, 17.2; IRν: 2 871, 1 702, 1 494, 1 397, 1 320, 1 246, 1 125, 1 081, 1 007, 854, 806, 725 cm-1; HR-MS(ESI)m/z: Calcd for C27H24N4O2Br{[M+H]+}515.107 7, found 515.108 4。
3b: 白色固体,产率60%, m.p.198~200 ℃;1H NMRδ: 7.82~7.79(m, 2H, ArH), 7.41(t,J=8.0 Hz, 2H, ArH), 7.30~7.27(m, 2H, ArH), 7.26~7.22(m, 2H, ArH), 7.14~7.10(m, 4H, ArH), 7.07~7.05(m, 2H, ArH), 5.74(s, 1H, CH), 4.38(s, 1H, CH), 3.93~3.86(m, 1H, CH), 3.24~3.16(m, 1H, CH), 3.07~3.00(m, 1H, CH), 2.96~2.90(m, 1H, CH), 2.27(s, 3H, CH3), 1.57(s, 3H, CH3);13C NMRδ: 173.4, 170.9, 159.6, 138.9, 137.4, 135.3, 129.6, 128.9, 128.8, 128.6, 128.2, 125.9, 125.6, 125.0, 119.3, 77.4, 77.2, 72.9, 63.8, 47.9, 32.1, 21.1, 17.1; IRν: 3 020, 1 713, 1 594, 1 500, 1 455, 1 368, 1 327, 1 246, 1 187, 1 095, 1 016, 857, 816, 753 cm-1; HR-MS(ESI)m/z: Calcd for C28H27N4O2{[M+H]+}451.212 9, found 451.213 4。
3c: 白色固体,产率68%, m.p.184~186 ℃;1H NMRδ: 7.81~7.79(m, 2H, ArH), 7.41(t,J=8.0 Hz, 2H, ArH), 7.23(t,J=7.2 Hz, 1H, ArH), 7.13~7.05(m, 6H, ArH), 7.02~7.00(m, 2H, ArH), 5.70(s, 1H, CH), 4.37(s, 1H, CH), 3.93~3.87(m, 1H, CH), 3.22~3.15(m, 1H, CH), 3.08~3.02(m, 1H, CH), 2.95~2.88(m, 1H, CH), 2.28(s, 3H, CH3), 2.27(s, 3H, CH3), 1.56(s, 3H, CH3);13C NMRδ: 172.9, 170.9, 159.7, 138.8, 137.9, 137.4, 132.2, 129.6, 129.5, 128.9, 128.7, 125.9, 125.6, 124.8, 119.2, 77.3, 72.9, 63.7, 48.2, 32.4, 21.1, 21.0, 17.2; IRν: 1 714, 1 596, 1 502, 1 453, 1 365, 1 322, 1 242, 1 184, 1 090, 1 029, 859, 821, 755 cm-1; HR-MS (ESI)m/z: Calcd for C29H29N4O2{[M+H]+}465.228 5, found 465.229 3。
3d: 白色固体,产率70%, m.p.178~180 ℃;1H NMRδ: 7.80(d,J=8.0 Hz, 2H, ArH), 7.41 (t,J=8.0 Hz, 2H, ArH), 7.23(t,J=7.2 Hz, 1H, ArH), 7.13~7.11(m, 2H, ArH), 7.07~7.00(m, 4H, ArH), 6.82~6.80(m, 2H, ArH), 5.69(s, 1H, CH), 4.37(s, 1H, CH), 3.93~3.87(m, 1H, CH), 3.76(s, 3H, OCH3), 3.22~3.15(m, 1H, CH), 3.08~3.01(m, 1H, CH), 2.95~2.89(m, 1H, CH), 2.27(s, 3H, CH3), 1.57(s, 3H, CH3);13C NMRδ: 172.9, 170.9, 159.8, 159.3, 138.8, 137.4, 129.6, 128.9, 128.7, 127.2, 126.2, 125.9, 125.6, 119.2, 114.2, 77.2, 73.0, 63.5, 55.2, 48.2, 32.3, 21.1, 17.2; IRν: 3 003, 2 834, 1 713, 1 599, 1 505, 1 455, 1 363, 1 318, 1 249, 1 184, 1 122, 1 087, 1 030, 923, 829, 758 cm-1; HR-MS(ESI)m/z: Calcd for C29H29N4O3{[M+H]+}481.223 4, found 481.224 0。
3e: 白色固体,产率75%, m.p.190~192 ℃;1H NMRδ: 7.81~7.79(m, 2H, ArH), 7.44~7.40(m, 4H, ArH), 7.24(t,J=7.6 Hz, 1H, ArH), 7.11~7.01(m, 6H, ArH), 5.65(s, 1H, CH), 4.38(s, 1H, CH), 3.93~3.86(m, 1H, CH), 3.25~3.17(m, 1H, CH), 3.08~2.99(m, 1H, CH), 2.96~2.89(m, 1H, CH), 2.27(s, 3H, CH3), 1.57(s, 3H, CH3);13C NMRδ: 173.8, 170.6, 159.2, 139.0, 137.3, 134.6, 132.0, 129.6, 129.0, 128.4, 126.8, 125.9, 125.7, 122.1, 119.2, 77.3, 72.7, 63.4, 47.9, 31.9, 21.1, 17.3; IRν: 3 037, 2 936, 2 886, 1 709, 1 598, 1 497, 1 406, 1 369, 1 335, 1 290, 1 235, 1 181, 1 083, 1 008, 859, 819, 754 cm-1; HR-MS(ESI)m/z: Calcd for C28H26N4O2Br{[M+H]+}529.123 4, found 529.123 2。
3f: 白色固体,产率68%, m.p.154~156 ℃;1H NMRδ: 7.79~7.78(m, 2H, ArH), 7.43~7.39(m, 2H, ArH), 7.24~7.22(m, 1H, ArH), 7.19~7.11(m, 3H, ArH), 7.06~7.02(m, 1H, ArH), 6.98~6.87(m, 2H, ArH), 6.83~6.78(m, 2H, ArH), 5.69(s, 1H, CH), 4.35(s, 1H, CH), 3.92~3.86(m, 1H, CH), 3.74(s, 3H, OCH3), 3.22~3.15(m, 1H,CH), 3.08~3.00(m, 1H, CH), 2.96~2.89(m, 1H, CH), 2.27(s, 3H, CH3), 1.55(s, 3H, CH3);13C NMRδ: 170.9, 159.8, 159.6, 138.4, 137.4, 135.2, 128.9, 128.8, 128.7, 127.2, 125.6, 125.5, 123.4, 122.0, 119.3, 114.2, 72.8, 63.7, 55.1, 47.8, 32.0, 21.4, 17.2; IRν: 2 945, 2 836, 1 714, 1 601, 1 504, 1 455, 1 362, 1 299, 1 246, 1 176, 1 124, 1 086, 1 030, 847, 757 cm-1; HR-MS(ESI)m/z: Calcd for C29H28N4O3Na{[M+Na]+}503.205 9, found 503.205 7。
3g: 白色固体,产率64%, m.p.156~158 ℃;1H NMRδ: 7.78(d,J=7.6 Hz, 2H, ArH), 7.43~7.40(t,J=6.4 Hz, 2H, ArH), 7.23~7.22(m, 2H, ArH), 7.21~7.17(m, 2H, ArH), 7.16~7.13(m, 2H, ArH), 6.92~6.90(m, 1H, ArH), 6.80~6.78(m, 2H, ArH), 5.66(s, 1H, CH), 4.35(s, 1H, CH), 3.90~3.88(m, 1H, CH), 3.74(s, 3H, OCH3), 3.23~3.20(m, 1H, CH), 2.99~2.94(m, 2H, CH), 1.67~1.60(m, 1H, CH), 1.56(s, 3H, CH3);13C NMRδ: 174.4, 170.6, 159.9, 159.1, 137.7, 134.9, 130.2, 128.9, 128.3, 127.2, 125.7, 125.3, 123.2, 123.2, 119.3, 114.3, 72.6, 63.2, 55.2, 47.3, 31.5, 17.2; IRν: 3 063, 2 995, 2 944, 2 830, 1 713, 1 601, 1 505, 1 460, 1 412, 1 364, 1 302, 1 246, 1 182, 1 089, 1 032, 907, 848, 758 cm-1; HR-MS(ESI)m/z: Calcd for C28H25N4O3ClNa{[M+Na]+} 523.151 3, found 523.151 8。
3h: 白色固体,产率65%, m.p.200~202 ℃;1H NMRδ: 7.81~7.79(m, 2H, ArH), 7.43~7.39(m, 4H, ArH), 7.25~7.22(m, 1H, ArH), 7.16~7.13(m, 2H, ArH), 7.03~7.01(m, 2H, ArH), 6.80~6.77(m, 2H, ArH), 5.64(s, 1H, CH), 4.36(s, 1H, CH), 3.93~3.86(m, 1H, CH), 3.74(s, 3H, OCH3), 3.24~3.17(m, 1H, CH), 3.06~3.00(m, 1H, CH), 2.96~2.89(m, 1H, CH), 1.58(s, 3H, CH3);13C NMRδ: 173.9, 170.6, 160.0, 159.2, 137.3, 134.6, 132.0, 129.0, 127.2, 126.8, 125.7, 123.2, 122.1, 119.1, 114.3, 77.3, 72.7, 63.3, 55.2, 47.8, 31.9, 17.3; IRν: 2 945, 1 707, 1 607, 1 503, 1 408, 1 370, 1 303, 1 248, 1 178, 1 086, 1 041, 826, 771 cm-1; HR-MS(ESI)m/z: Calcd for C28H26N4O3Br{[M+H]+}545.118 3, found 545.118 6。
3i: 白色固体,产率60%, m.p.168~170 ℃;1H NMRδ: 7.81~7.79(m, 2H, ArH), 7.44~7.40(m, 2H, ArH), 7.29~7.27(m, 4H, ArH), 7.25~7.23(m, 2H, ArH), 7.18~7.16(m, 2H, ArH), 7.14~7.12(m, 2H, ArH), 5.74(s, 1H, CH), 4.37(s, 1H, CH), 3.94~3.87(m, 1H, CH), 3.22~3.15(m, 1H, CH), 3.07~3.01(m, 1H, CH), 2.97~2.89(m, 1H, CH), 1.58(s, 3H, CH3);13C NMRδ: 173.5, 170.6, 159.2, 137.3, 135.2, 134.9, 130.4, 129.2, 129.0, 128.9, 128.3, 127.4, 125.8, 125.0, 119.2, 76.8, 72.8, 63.9, 47.9, 31.9, 17.1; IRν: 2 928, 2 843, 1 706, 1 598, 1 496, 1 366, 1 319, 1 245, 1 179, 1 096, 1 017, 847, 782, 708 cm-1; HR-MS(ESI)m/z: Calcd for C27H24N4O2Cl{[M+H]+}471.158 2, found 471.158 6。
3j: 白色固体,产率78%, m.p.204~206 ℃;1H NMRδ: 7.81~7.79(m, 2H, ArH), 7.43(t,J=8.0 Hz, 2H, ArH), 7.28~7.27(m, 3H, ArH), 7.25~7.23(m, 2H, ArH), 7.18~7.16(m, 2H, ArH), 7.09~7.07(m, 2H, ArH), 5.68(s, 1H, CH), 4.36(s, 1H, CH), 3.93~3.86(m, 1H, CH), 3.23~3.16(m, 1H, CH), 3.08~3.02(m, 1H, CH), 2.97~2.90(m, 1H, CH), 1.55(s, 3H, CH3);13C NMRδ: 173.9, 170.3, 158.8, 137.2, 135.0, 134.2, 133.8, 130.1, 129.3, 129.1, 129.0, 127.3, 126.4, 125.9, 119.1, 72.7, 63.4, 47.8, 31.8, 17.2; IRν: 2 942, 2 885, 1 704, 1 494, 1 407, 1 370, 1 336, 1 285, 1 234, 1 184, 1 092, 1 009, 824, 762, 733, 701 cm-1; HR-MS(ESI)m/z: Calcd for C27H23N4O2Cl2{[M+H]+}505.119 3, found 505.119 8。
3k: 白色固体,产率75%, m.p.210~212 ℃;1H NMRδ: 7.79 (d,J=8.0 Hz, 2H, ArH), 7.44~7.40(m, 6H, ArH), 7.25~7.23(m, 1H, ArH), 7.10(d,J=8.0 Hz, 2H, ArH), 7.01(d,J=8.0 Hz, 2H, ArH), 5.65(s, 1H, CH), 4.34(s, 1H, CH), 3.93~3.86(m, 1H, CH), 3.22~3.15(m, 1H, CH), 3.09~2.99(m, 1H, CH), 2.96~2.89(m, 1H, CH), 1.55(s, 3H, CH3);13C NMRδ: 173.9, 170.3, 158.8, 137.1, 134.1, 132.2, 132.0, 130.7, 129.0, 127.6, 126.7, 125.9, 123.2, 122.3, 119.9, 72.5, 63.5, 47.8, 31.8, 17.2; IRν: 2 942, 1 740, 1 655, 1 600, 1 494, 1 406, 1 369, 1 336, 1 288, 1 238, 1 184, 1 082, 1 008, 823, 759 cm-1; HR-MS(ESI)m/z: Calcd for C27H23N4O2Br2{[M+H]+}593.018 2, found 593.018 1。
3l: 白色固体,产率78%, m.p.193~195 ℃;1H NMRδ: 8.19~8.14(m, 2H, ArH), 7.78(d,J=7.2 Hz, 2H, ArH), 7.48~7.42(m, 5H, ArH), 7.34~7.25(m, 2H, ArH), 7.12(d,J=7.6 Hz, 2H, ArH), 5.75(s, 1H, CH), 4.39(s, 1H, CH), 3.99~3.92(m, 1H, CH), 3.28~3.21(m, 1H, CH), 3.07~2.93(m, 2H, CH), 1.51(s, 3H, CH3);13C NMRδ: 175.3, 170.1, 158.2, 148.4, 137.9, 136.9, 132.2, 131.1, 130.4, 130.1, 129.0, 127.6, 126.1, 123.3, 120.3, 119.3, 72.3, 63.4, 47.2, 31.1, 17.2; IRν: 3 072, 2 930, 1 712, 1 596, 1 534, 1 494, 1 403, 1 354, 1 240, 1 180, 1 088, 1 006, 822, 761, 718 cm-1; HR-MS(ESI)m/z: Calcd for C27H22N5O4BrNa{[M+Na]+}582.075 3, found 582.074 7。
3m: 白色固体,产率74%, m.p.158~160 ℃;1H NMRδ: 8.18~8.15(m, 2H, ArH), 7.80~7.78(m, 2H, ArH), 7.42~7.32(m, 7H, ArH), 7.09(d,J=7.6 Hz, 2H, ArH), 5.74(s, 1H, CH), 4.37(s, 1H, CH), 3.94~3.89(m, 1H, CH), 3.25~3.19(m, 1H, CH), 3.01~2.95(m, 2H, CH), 1.25(s, 3H, CH3);13C NMRδ: 174.7, 169.9, 158.1, 147.7, 142.6, 137.0, 132.2, 130.3, 129.1, 127.6, 126.2, 126.0, 124.1, 123.3, 119.0, 72.4, 63.5, 47.5, 31.3, 29.6, 17.2; IRν: 3 072, 2 925, 2 854, 1 714, 1 599, 1 521, 1 495, 1 402, 1 344, 1 241, 1 181, 1 114, 1 011, 846, 757, 719 cm-1; HR-MS(ESI)m/z: Calcd for C27H22N5O4BrNa{[M+Na]+}582.075 3, found 582.075 6。
2 结果与讨论
2.1 合成
[27]方法合成3b,反应不完全,产率仅30%左右。延长反应时间,仍然有部分原料不能反应。加入酸(或碱)催化剂可以改善反应结果。当加入催化量的三乙胺时,反应可快速完成,顺利的合成3b,产率大幅提高(约60%)。在此反应条件下,可以中等至良好的产率合成预期产物。初步的构效分析表明,两种反应底物上的取代基对产率影响较小。
2.2 表征
(1)1H NMR
由于产物的吡唑环中存在3个手性碳原子,反应中可能形成多种非对映异构体。化合物的1H NMR谱图中仅显示一组特征吸收峰,这表明产物中仅存在一种非对映异构体。如3d在δ3.76处的特征峰为甲氧基的单峰,δ2.27和δ1.57处出现了两个甲基的单峰,δ5.89和δ4.33处单峰为吡咯环C—H的特征吸收峰,δ3.93~2.89处的多重峰为吡唑啉酮环中相邻的两个亚甲基由于非对映特性而出现的4个H的吸收峰。
(2) X-射线单晶衍射
图1~图4为3d,3h,3j和3l的单晶结构图。由图1~图4可见,4个分子具有相同的相对构型。两个芳基在新形成的吡唑环中均位于顺式位置。5-甲基-2-苯基吡唑-3-酮单元中的羰基位于两个芳基的反式位置,甲基伸向两个芳基的同一侧。结合1H NMR的分析,我们确定3a~3m均具有该相对构型,中间吡唑环中位于1,3-位上的两个芳基以顺式构型存在,这与文献[24]报道不同。值得注意的是,我们之前合成的螺[茚-2,2′-吡唑并[1,2-a]吡唑]和螺[二氢吲哚-3,2′-吡唑并[1,2-a]吡唑]也具有顺式1,3-二芳基的相对构型[27]。
综上可知,环状偶氮甲亚胺与环状亲1,3-偶极子的环环加成反应具有非常高的非对映选择性。

图1 3d(CCDC: 1538806)的单晶分子结构

图23h(CCDC: 1538807)的单晶分子结构
Figure2Single crystal structure of3h
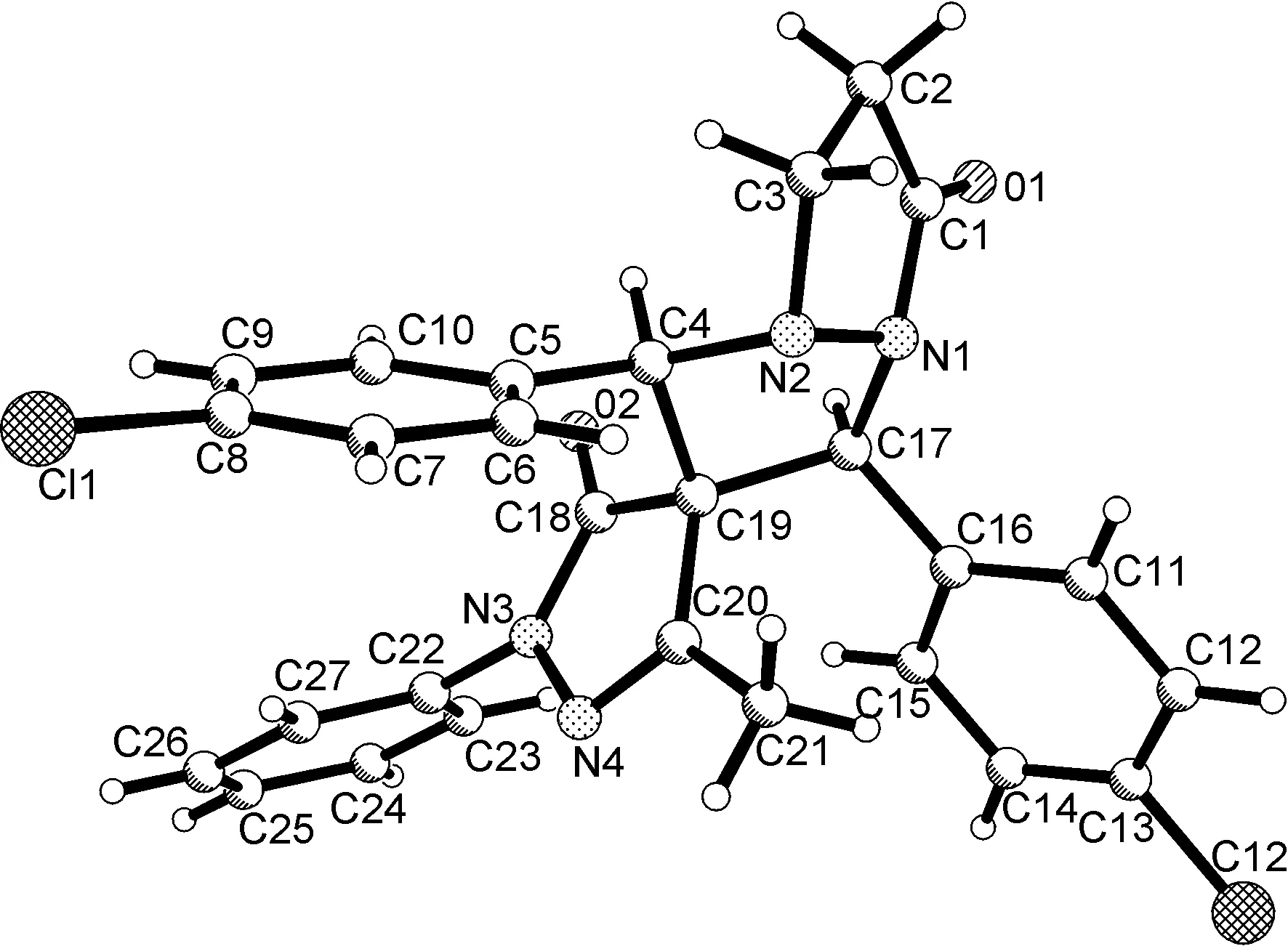
图3 3j(CCDC: 1538808)的单晶分子结构

图4 3l(CCDC: 1544352)的单晶分子结构
3 结论
通过环状偶氮甲亚胺与4-亚芳基-5-甲基-2-苯基吡唑-3-酮的1,3-偶极环加成反应合成了一系列顺-1,3-二芳基取代的螺[吡唑-4,2′-吡唑并[1,2-a]吡唑]。该多环体系由3个吡唑环分别以并环和螺环方式连接,结构新颖。该反应具有反应条件简单、底物普适性强、反应产率高,非对映选择性好等优点。合成的新化合物在药物研发中具有潜在的应用价值。
参考文献
[1] LALEU B, GAGGINI F, ORCHARD M,etal. First in class,potent,and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis[J].J Med Chem,2010,53:7715-7730.
[2] INDELICATO J M, PASINI C E. The acylating potential ofγ-lactam antibacterials:Base hydrolysis of bicyclic pyrazolidinones[J].J Med Chem,1988,31:1227-1230.
[3] TERNANSKY R J, DRAHEIM S E. The chemistry of substituted pyrazolidinones:Applications to the synthesis of bicyclic derivatives[J].Tetrahedron,1992,48:777-796.
[4] MICHEL M, MANFRED B, FREDRIK C,etal. Aryldiones incorporating a [1,4,5]oxadiazepane ring. Part I:Discovery of the novel cereal herbicide pinoxaden[J].Bioorg Med Chem,2009,17:4241-4256.
[5] KAMATA M, YAMASHITA T, KINA A,etal. Symmetrical approach of spiro-pyrazolidinediones as acetyl-CoA carboxylase inhibitors[J].Bioorg Med Chem Lett,2012,22:4769-4772.
[6] SHENG Y, DANIEL G, KATHARINA W,etal. Synthesis and SERCA activities of structurally simplified cyclopiazonic acid analogues[J].Bioorg Med Chem,2011,19:4669-4678.
[7] BOYD D B. Application of the hypersurface iterative projection method to bicyclic pyrazolidinone antibacterial agents[J].J Med Chem,1993,36:1443-1449.
[8] COLDHAM I, HUFTON R. Intramolecular dipolar cycloaddition reactions of azomethine ylides[J].Chem Rev,2005,105:2765-2810.
[9] XU X F, DOYLE M P. The [3+3]-cycloaddition alternative for heterocycle syntheses:Catalytically generated metalloenolcarbenes as dipolar adducts[J].Acc Chem Res,2014,47:1396-1405.
[10] NA R S, JING C F, XU Q H,etal. Phosphine-catalyzed annulations of azomethine imines:Allene-dependent [3+2],[3+3],[4+3],and [3+2+3] pathways[J].J Am Chem Soc,2011,133:13337-13348.
[11] Imaizumi T, Yamashita Y, Kobayashi S. Group 11 metal amide-catalyzed asymmetric cycloaddition reactions of azomethine imines with terminal alkynes[J].J Am Chem Soc,2012,134:20049-20052.
[12] ZHU R Y, WANG C S, ZHENG J.etal. Organocatalytic asymmetric inverse-electron-demand 1,3-dipolar cycloaddition ofN,N′-cyclic azomethine imines[J].J Org Chem,2014,79:9305-9312.
[13] PEZDIRC L, BEVK D, GROSELJ U.etal. Combinatorial solution-phase synthesis of alkyl (1S*,2S*,3R*,5R*,6R*)-1-alkyl-3-aryl-6-benzoylamino-1-hydroxy-7-oxo-5-phenylhexahydropyrazolo[1,2-a]- pyrazole-2-carboxylates[J].J Comb Chem,2007,9:717-723.
[14] NA R S, LIU H L, LI Z,etal. Thermal [3+2] cycloaddition reaction of azomethine imines with allenoates for dinitrogen-fused heterocycles[J].Tetrahedron,2012,68:2349-2356.
[15] NOVAK A, TESTEN A, BEZENSEK J,etal. Synthesis of pyrazolo[1,2-a]pyrazole-based peptide mimetics[J].Tetrahedron,2013,69:6648-6665.
[16] CHEN W, DU W, DUAN Y Z,etal. Enantioselective 1,3-dipolar cycloaddition of cyclic enones catalyzed by multifunctional primary amines:Beneficial effects of hydrogen bonding[J].Angew Chem Int Ed,2007,46:7667-7670.
[17] HONG L, KAI M, WU C Y,etal. Enantioselective 1,3-dipolar cycloaddition of methyleneindolinones andN,N′-cyclic azomethine imines[J].Chem Commun,2013,49:6713-6715.
[18] LI Z, YU H, LIU L H,etal. Phosphine-catalyzed [3+2] cycloaddition reactions of azomethine imines with electron-deficient alkenes:A facile access to dinitrogen-fused heterocycles[J].Chem Eur J,2014,20:1731-1736.
[19] PLESHCHEV M I, DAS GUPTA N V, KUZNETSOV V V,etal. An-mediated new,regioselective one-pot access to bicyclic cationic structures with 2,3-dihydro-1H-pyrazolo[1,2-a]pyrazol-4-ium core[J].Tetrahedron,2015,71:9012-9021.
[20] HAN Y, SHENG Y J, YAN C G. Convenient synthesis of triphenylphosphanylidene spiro[cyclopentane-1,3′-indolines] and spiro[cyclopent[2]ene-1,3′-indolines]viathree-component reactions[J].Org Lett,2014,16:2654-2657.
[21] GAO H, SUN J, YAN C G. Selective synthesis of functionalized spiro[indoline-3,2′-pyridines] and spiro[indoline-3,4′-pyridines] by Lewis acid catalyzed reactions of acetylenedicarboxylate,arylamines,and isatins[J].J Org Chem,2014,79:4131-4136.
[22] YANG F, SUN J, GAO H,etal. Unprecedented formation of spiro[indoline-3,7′-pyrrolo[1,2-a]azepine] from multicomponent reaction of L-proline,isatin and but-2-ynedioate[J].RSC Adv,2015,5:32786-32794.
[23] WU P, GAO H, SUN J,etal. 1,3-Dipolar cycloaddition reaction for diastereoselective synthesis of functionalized dihydrospiro[indoline-3,2′-pyrroles][J].Chin Chem Lett,2017,28:329-332.
[24] ZHANG D, ZHANG D M, XU G Y,etal. Copper-catalyzed 1,3-dipolar cycloaddition of methyleneindolinones andN,N′-cyclic azomethine imines[J].Chin Chem Lett,2015,26:301-303.
[25] KOPTELOV Y B, MOLCHANOV A P, KOSTIKOV R R. Regio- and diastereoselective cycloaddition of stable cyclic azomethine imines toN-arylitaconimides[J].Russ J Org Chem,2015,51:1134-1143.
[26] DUAN J D, CHENG J, CHENG Y Y,etal. Synthesis of dinitrogen-fused spirocyclic heterocyclesviaorganocatalytic 1,3-dipolar cycloaddition of 2-arylidene-1,3-indandiones and an azomethine imine[J]Asian J Org Chem,2016,5:477-480.
[27] LU Y L, SUN J, JIANG Y H,etal. Diastereoselective synthesis of spiro[indene-2,2′-pyrazolo[1,2-a]pyrazoles] and spiro[indoline-3,2′-pyrazolo[1,2-a]pyrazoles]via1,3-dipolar cycloaddition[J].RSC adv,2016,6:50471-50748.
DiastereoselectiveSynthesisofNovelcis-1,3-Diarylspiro[pyrazole-4,2′-pyrazolo[1,2-a]pyrazoles]
ZHU Mei-jun1, ZHANG Yu2, LU Yu-ling2, SUN Jing2, YAN Chao-guo2*
(1. Jianghai Polytechnic College, Yangzhou 225101, China; 2. College of Chemistry amp; Chemical Engineering, Yangzhou University, Yangzhou 225002, China)
Thirteen novel 1,3-diaryl-substituted spiro[pyrazole-4,2′-pyrazolo[1,2-a]pyrazoles] derivatives(3a~3m) were synthesized by triethylamine catalyzed 1,3-dipolar cycloaddition of cyclic azomethine imines with 4-arylidene-5-methyl-2-phenylpyrazol-3-ones in refluxing acetonitrile. The structures were characterized by1H NMR,13C NMR, IR and HR-MS(ESI). The single crystal structures of3d,3h,3jand3lwere investigated by X-ray single diffraction. The results indicated that3have unusualcis-1,3-diaryl-configuration.
azomethine imine; prazol-3-one; spiro compound; 1,3-dipolar cycloaddition; diastereoselective synthesis
2017-09-07;
2017-11-07
国家自然科学基金资助项目(21572196)
朱美军(1975-),女,汉族,江苏扬州人,硕士,主要从事有机合成的研究。 E-mail: happyfengjunyang@126.com
颜朝国,教授, E-mail: cgyan@yzu.edu.cn
O626.2
A
10.15952/j.cnki.cjsc.1005-1511.2017.12.17214

