基于 3,5-二(3-吡啶)-4-氨基-1,2,4-三唑配体的Co髤和Cu髤配合物的合成与晶体结构
余 沁 王大鹏 马建平 俞 飞 曹子恒 胡天皓 王 鹏 王海英*,
基于 3,5-二(3-吡啶)-4-氨基-1,2,4-三唑配体的Co髤和Cu髤配合物的合成与晶体结构
余 沁1王大鹏1马建平2俞 飞1曹子恒1胡天皓1王 鹏*,3王海英*,1
(1南京大学化学化工学院,配位化学国家重点实验室,人工微结构科学与技术协同创新中心,南京 210023)
(2山东师范大学化学与化工学院,济南 250014)
(3山东科技大学化学与环境工程学院,青岛 266590)
利用 3,5-二(3-吡啶)-4-氨基-1,2,4-三唑(L)配体与 Co髤/Cu髤盐室温下反应得到了一维的配位聚合物{[CoL(H2O)4]SO4·H2O}n(1)和单核配合物[Cu(hfac)2L2](2,hfac=hexafluoroacetylacetonate)。通过红外、元素分析及X射线单晶衍射等检测手段对所合成的配合物进行了表征。结构研究表明,配合物1中,配体L呈顺式构型,采取双齿配位方式桥联Co髤离子形成一维正弦链状结构,一维链通过多种氢键相互作用连接进一步形成三维网状结构;溶剂水分子和硫酸根阴离子通过氢键连接在框架上。配合物2中,配体L则采取单齿配位方式,与Cu髤离子形成离散型的单核结构,通过多重氢键作用进而连接成三维网状结构。
三唑;配合物;氢键;晶体结构
0 Introduction
Coordination polymers(CPs)are intriguing metalorganic hybrid materials and have attracted enormous attention owing to their potential applications in a plethora of areas,including sensing,solar energy harvesting,energy storage,etc[1-3].In CPs,metal ions or metal-containing clusters act as nodes,and organic ligands act as spacers,both of which are linked via coordination bonds to form one-,two-or three-dimensional extended solids[4].Meanwhile,the exquisite control that can be achieved over the design of coordination complexes via rational structural modifications to the component metal ions and ligands provides unique prospects for designing structures and elucidating fundamental structure-function relationship[5-7].A wide variety of coordinational groups such as carboxyl,pyridyl,cyan,amino,and N-oxidized pyridine etc.,have been used to synthesize multifunctionalCPsunderdifferentsynthetic conditions,giving rise to unprecedented structural diversity which has been tailored for specific applications[8-13].Ligands containing pyridyl functionalities are well-known to coordinate with metallic centers and produce interesting multifunctional materials[14-16].The important characteristics of triazole-containing linkers are rigidity,the number and orientation of binding sites(coordination numbers and coordination geometries),and the relative distance(s)between the coordinating functionalities.
In this context,the triazole-containing bispyridine rigid crooked ligand L(Scheme 1)was chosen to construct CPs.Initially,the two pyridyl groups of the L ligand can partially or completely coordinate with the metal ions,giving rise to a range of coordination structures.Furthermore,multiple configurations exist when the L ligand coordinates with the metal ions.Additionally,the N atoms of 1,2,4-triazole can serve as potential binding sites and H-bond acceptors.Meanwhile,the-NH2group of 4-amino-1,2,4-triazole rings can serve as H-bond donors.
In this paper,the new one-dimensional complex{[CoL(H2O)4]SO4·H2O}n(1) and discrete complex[Cu(hfac)2L2](2,hfac=hexafluoroacetylacetonate)based on 3,5-bis(3-pyridyl)-4-amino-1,2,4-triazole (L)have been synthesized and characterized.

Scheme 1 Three Typical Possible Conformations of L
1 Experimental
1.1 Materials and instruments
All reagents and solvents were obtained from commercial sources and used without further purification. Triazole-containing bispyridine rigid crooked ligand L was prepared according to literature method[17].IR spectra were recorded in range of 400~4000 cm-1on a Vector27 Bruker Spectrophotometer with KBr pellets.C,H,N and S analyses were carried out on a Perkin-Elmer 240C analyzer.
1.2 Synthesis of 1
A solution of L(4.76 mg,0.020 mmol)in MeOH(10 mL)was layered onto a solution of CoSO4·H2O(6.92 mg,0.040 mmol)in H2O(10 mL).The resulting solution were left for about 4 days at room temperature,and pale pink crystals were obtained.Yield:71%(based on L).Anal.Calcd.for C12H20CoSN6O9(%):C,29.82;H,4.17;N,17.39;S,6.63.Found(%):C,29.87;H,4.21;N,17.46;S,6.70.IR (KBr,cm-1):3 476(w),3 250(w),1 620(m),1 580(m),1 474(m),1 423(w),1 130(s),983(s),845(w),700(w).
1.3 Synthesis of 2
Blue crystals of 2 were obtained according to a similar procedure described for 1 with Cu(hfac)2·2H2O instead of CoSO4·H2O.Yield:68%(based on L).Anal.Calcd.for C34H22CuF12N12O4:C,42.80;H,2.32;N,17.62.Found(%):C,42.89;H,2.26;N,17.42.Selected IR data (KBr,cm-1):3 470(w),3 325(w),1 610(m),1 575(m),1 470(m),1 420(w),1 258(s),1 215(s),1 030(s),819(w),747(w).
1.4 Crystal structure determination
The crystal data was collected with Mo Kα radiation(λ=0.071 073 nm)on a CCD diffractometer.The cell parameters were retrieved and refined by using computer software (SMART and SAINT,respectively)[18].The SADABS[19]program was applied for absorption corrections.Structures were solved by direct methods using the program package SHELXL-97[20].All the non-hydrogen atoms were located in the Fourier maps and refined with anisotropic parameters.Crystallographic parameters are summarized in Table 1.Selected bond lengths(nm)and bond angles(°)are listed in Table 2.Hydrogen bond lengths and bond angles are in Table 3.
CCDC:1568579,1;1568580,2.
2 Results and discussion
2.1 Structure description of 1
Complex 1 crystallizes in the monoclinic space group P21/c.As shown in Fig.1,the asymmetric unit contains one Co髤center,one L ligand,one SO42-anion,and four coordinated and one free H2O molecules.The central cobalt髤ion are six-coordinated in anapproximately octahedral coordination environment,which is defined by two nitrogen donors from two L ligands and four oxygen donors from four coordinated water molecules.The pyridyl nitrogen atoms of the ligand occupy the axial positions with the shortest Co1-N6 bond length of 0.216 7(3)nm.The Co-O bonds range from 0.203 3(3)to 0.212 2(3)nm.L ligand adopts a cis-conformationⅡ (Scheme 1).The two terminal pyridyl groups and the bridging triazole heterocyclic ring do not lie in the same plane with two torsion angles of 4.7(4)°(C3-C4-C6-N2)and 29.6(5)°(C9-C8-C7-N3)).The L ligand acts as 2-connected node to link[Co(H2O)4]units into a 1D sinusoidal chain(Fig.1).The 1D chains link together to give a twodimensional (2D)network through hydrogen bonds between coordinated water molecules(O(4)-H(4A)…O(2),H…O 0.204 nm)(Fig.2).These networks stack in an ABAB sequence to form a 3D framework through hydrogen bonds between coordinated water molecules and the N atoms of the triazole rings(O(2)-H(2A)…N(3),H…O 0.202 nm,and O(3)-H(3A)…N(2),H…O 0.199 nm;Fig.3).Viewing the structure along the crystallographic c axis,rhombus-like channels are observed.The solvent H2O molecules and SO42-anion locate in the channels and are connected to the 3D framework through ten sets of hydrogen bonds(Fig.4).
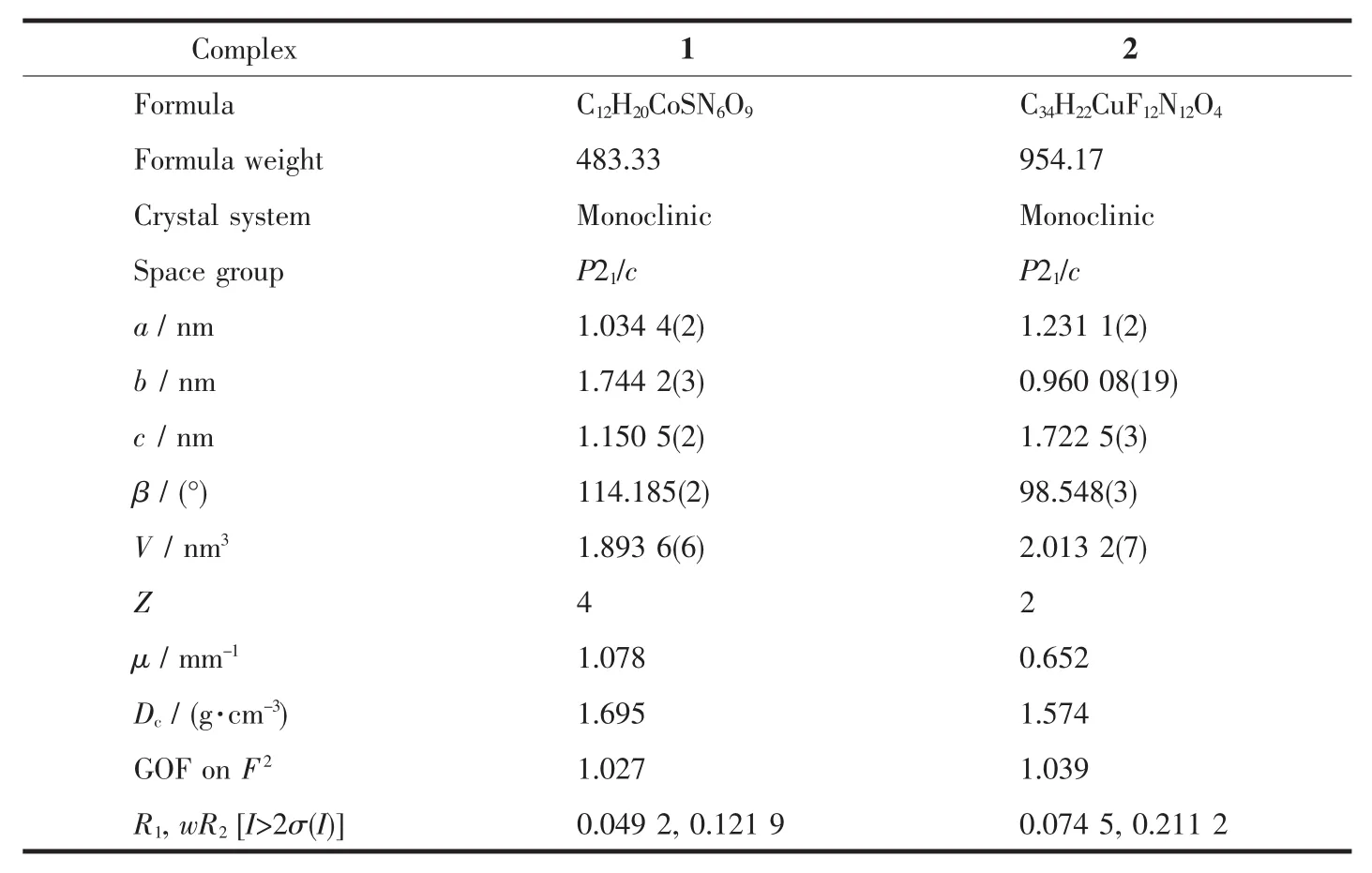
Table 1 Crystallographic parameters for 1 and 2
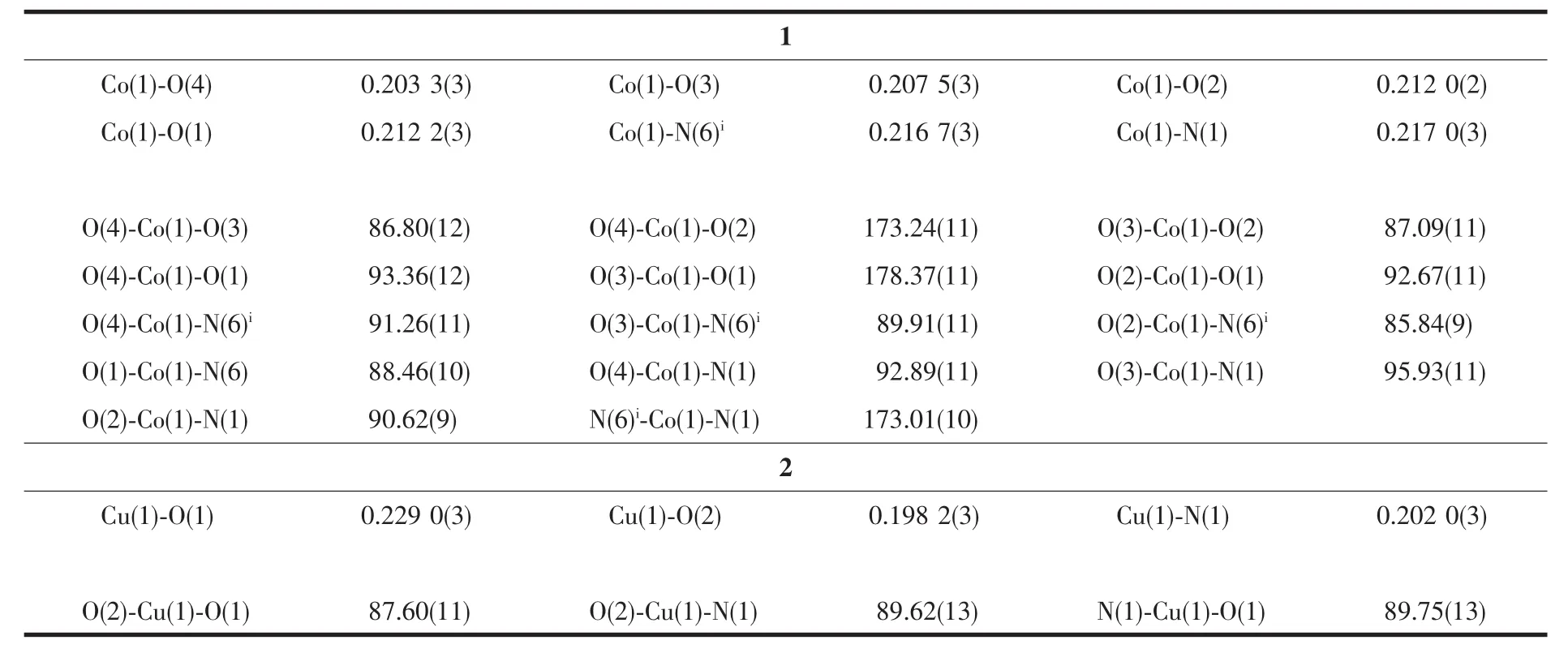
Table 2 Selected bond lengths(nm)and bond angles(°)of the complexes 1 and 2
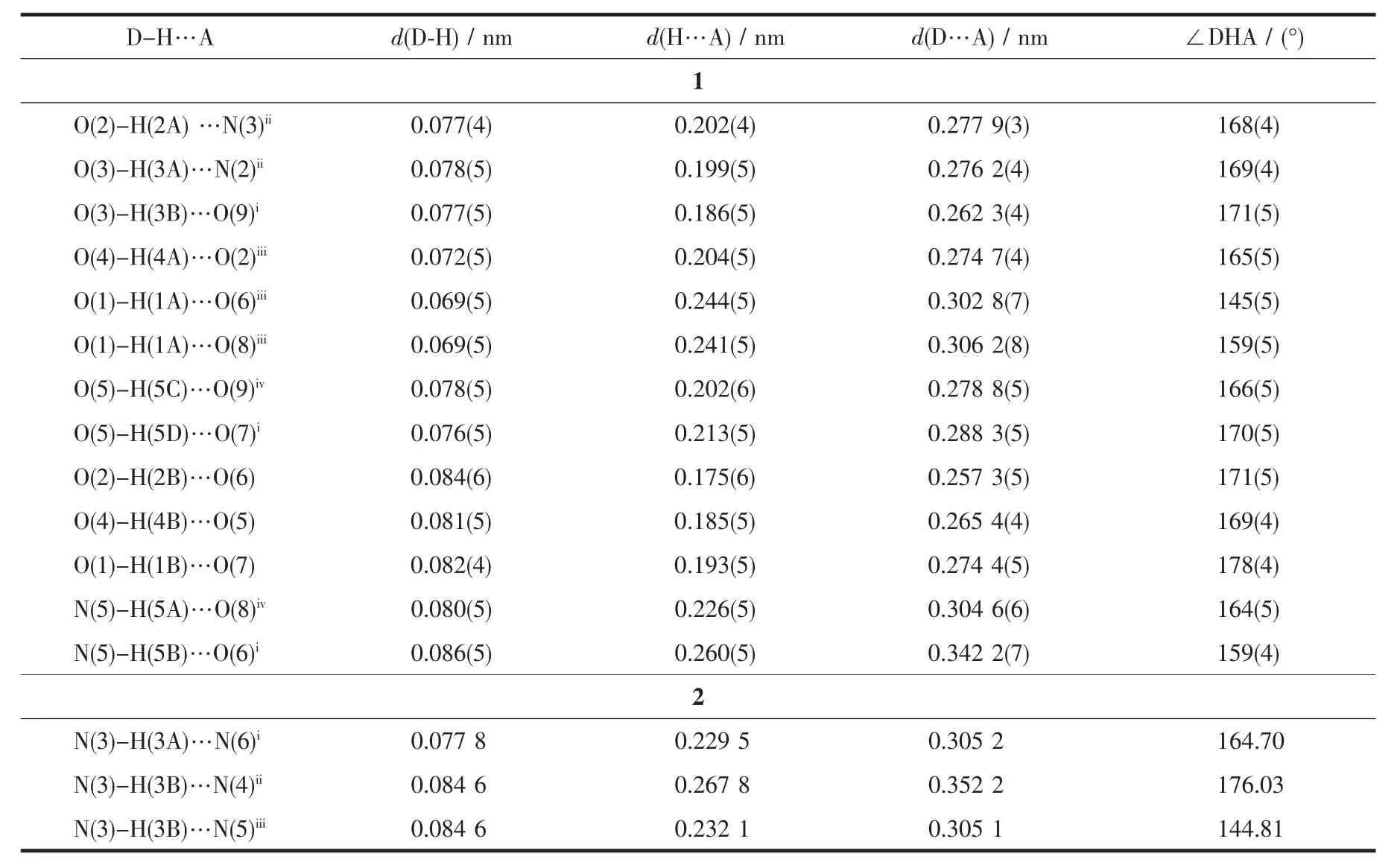
Table 3 Structural parameters of hydrogen bonds for complexes 1 and 2
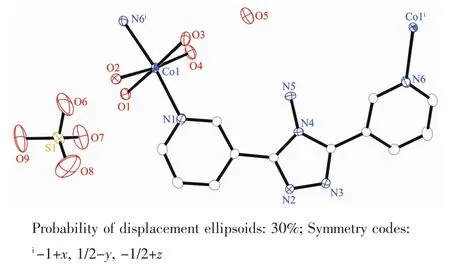
Fig.1 Coordination environment of the Co髤ion in 1
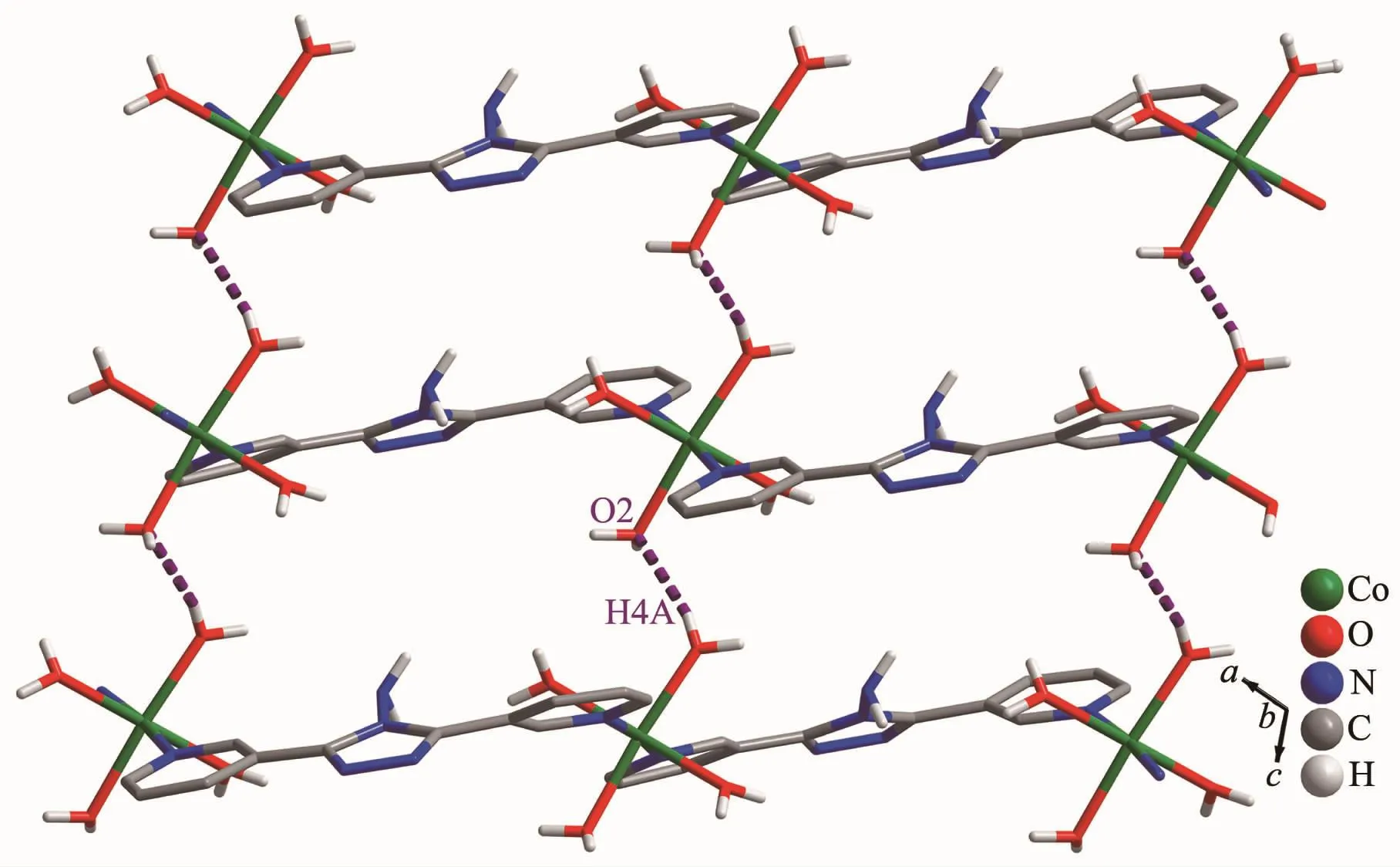
Fig.2 Hydrogen-bonded 2D network in 1
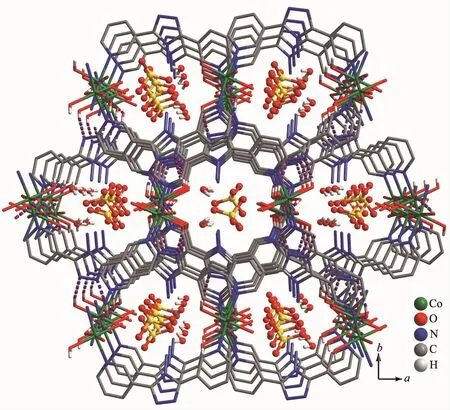
Fig.3 Perspective view of the hydrogen-bonded 3D network in 1
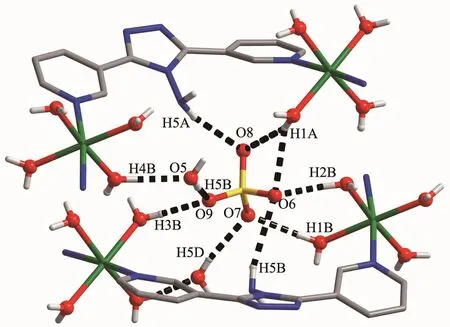
Fig.4 Hydrogen-bonds between free SO42-,H2O and framework of 1
2.2 Structure description of 2
For 2,the asymmetric unit consists of one Cu髤ion,two L ligands,and two hfac anions.As shown in Fig.5,Cu髤center also adopts a distorted octahedral coordination geometry,which consists of four O atoms from two bidentate hfac anions in the equatorial plane and two N atoms from two L ligands in apical positions.The Cu1-O1 and Cu1-O2 bond lengths are 0.229 0(3)and 0.198 2(3)nm,respectively.The Cu1-N1 bond length is 0.202 0(3)nm,which is shorter than that of 1.It is worth pointing out that the L ligand in 2 adopts the cis-conformationⅠ(Scheme 1)of the two terminal pyridyl rings to bind Cu髤atoms,versus the conformationⅡfor complex 1.The two terminal pyridyl groups and the bridging triazole heterocyclic ring are also not coplanar with two torsion angles of 53.5(5)°(C17-C13-C12-N5)and 22.7(6)°(C10-C9-C11-N4).The coordination behavior of L is different from that in 1.The ligand L herein acts as a mono-dentate spacer instead of a bidentate spacer to link[Cu(hfac)2]units into a discrete complex(Fig.1).As shown in Fig.6,an extensive H-bonding network exists,connecting the discrete complex into a 3D framework,in which the N atoms of amino serve as H-bond donors and the N atoms of the triazole rings as H-bond acceptors.The N…H distances are in the range of 0.229 5~0.267 8 nm,with angles in the range of 144.81°~176.03°.
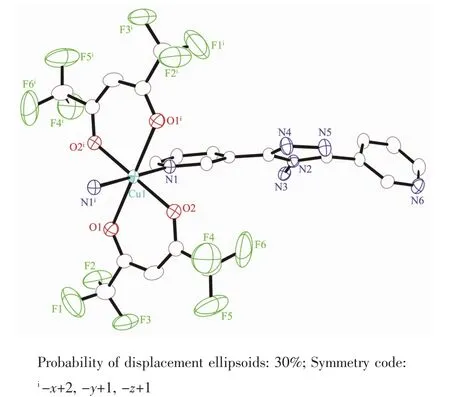
Fig.5 Coordination environment of the Cu髤ion in 2
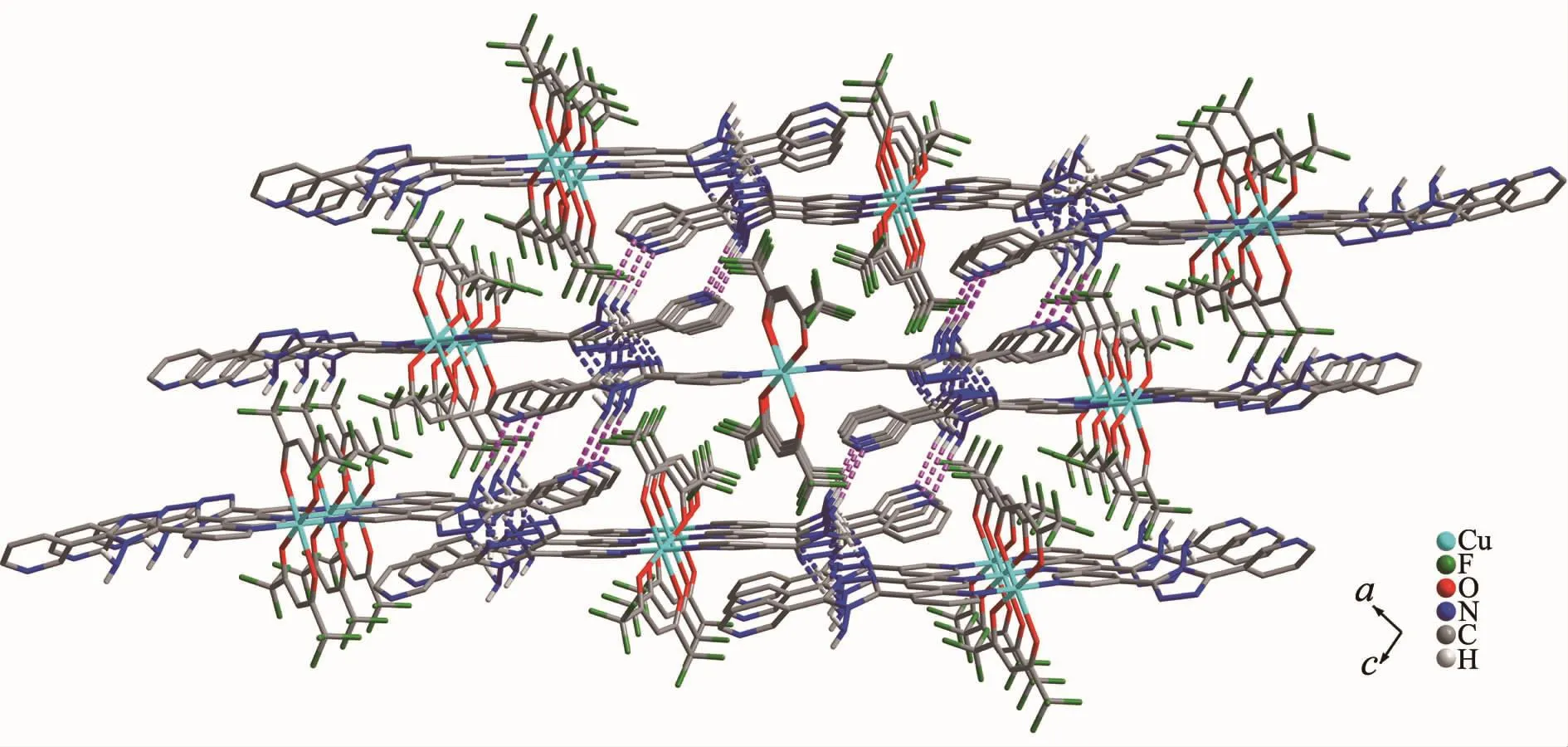
Fig.6 Perspective view of the hydrogen-bonded 3D network in 2
3 Conclusions
In this paper,one discrete Cu髤complex and one 1D Co髤complex based on the bent triazole-containing ligand were synthesized and structurally characterized.In complexes 1 and 2,the ligand L adopts cis-conformation,while,the relative orientation of the nitrogen donors on the pyridyl rings and the five-membered triazolespacing leadstodifferent structure motifs.The ligand L acts as a bidentate linker in 1 and a mono-dentate spacer in 2.Diverse hydrogen bonding interactions are observed,which contribute significantly to the alignmentofthe molecules of two complexes in the crystalline state.Further investigations on triazole-containing complexes with new structures and properties are ongoing in our laboratory.
[1]Long J R,Yaghi O M.Chem.Soc.Rev.,2009,38(5):1213-1214
[2]Zhou H C,Long J R,Yaghi O M.Chem.Rev.,2012,112(2):673-674
[3]Zhou H C,Kitagawa S.Chem.Soc.Rev.,2014,43(16):5415-5418
[4]Batten S R,Champness N R,Chen X M,et al.CrystEngComm,2012,14(9):3001-3004
[5]Wang G,Xue Z,Pan J,et al.CrystEngComm,2016,18(43):8362-8365
[6]Ma J P,Zhao C W,Wang S Q,et al.Chem.Commun.,2015,51(78):14586-14589
[7]Ma J P,Wang S Q,Zhao C W,et al.Chem.Mater.,2015,27(11):3805-3808
[8]Cui L,Zhu F,Leong C F,et al.Sci.China Ser.B:Chem.,2015,58(4):650-657
[9]Chen B,Lü Z P,Hua C,et al.Inorg.Chem.,2016,55(9):4606-4615
[10]Li J H,Pan J,Xue Z Z,et al.J.Coord.Chem.,2017,70(1):84-92
[11]Dong Y B,Wang H Y,Ma J P,et al.Inorg.Chem.,2005,44(13):4679-4692
[12]Chen Y,Li C,Wang C,et al.Sci.China,Ser.B:Chem.,2009,52(10):1596-1601
[13]LI Xin-Wei(李 欣 玮),WANG Peng(王 鹏),ZHAO Ying(赵营),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(6):1361-1366
[14]Yao M,Zheng Q,Gao F,et al.Sci.China:Chem.,2012,55(6):1022-1030
[15]Dong Y B,Wang H Y,Ma J P,et al.Cryst.Growth Des.,2005,5(2):789-800
[16]WANG Gui-Xian(王桂仙),CAO Ke-Li(曹可利),XIA Yan(夏艳),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(12):2337-2342
[17]Bentiss F,Lagrenée M,Traisnel M,et al.J.Heterocycl.Chem.,1999,36(1):149-152
[18]SAINT-Plus,Ver.6.02,Bruker Analytical X-ray System,Madison,WI,1999.
[19]Sheldrick G M.SADABS:An Empirical Absorption Correction Program,Bruker Analytical X-ray Systems,Madison,WI,1996.
[20]Sheldrick G M.Acta Crystallogr.,Sect.A:Found.Crystallogr.,2008,A64:112-122
Syntheses and Crystal Structures of Co髤 and Cu髤 Complexes Based on 3,5-Bis(3-pyridyl)-4-amino-1,2,4-triazole Ligand
YU Qin1WANG Da-Peng1MA Jian-Ping2YU Fei1CAO Zi-Heng1HU Tian-Hao1WANG Peng*,3WANG Hai-Ying*,1
(1State Key Laboratory of Coordination Chemistry,School of Chemistry and Chemical Engineering,Collaborative Innovation Center of Advanced Microstructures,Nanjing 210023,China)
(2School of Chemistry,Chemical Engineering and Materials Science,Shandong Normal University,Jinan 250014,China)
(3College of Chemical and Environmental Engineering,Shandong University of Science and Technology,Qingdao,Shandong 266590,China)
Two new metal complexes of{[CoL(H2O)4]SO4·H2O}n(1)and[Cu(hfac)2L2](2,hfac=hexafluoroacetylacetonate)based on 3,5-bis(3-pyridyl)-4-amino-1,2,4-triazole(L)have been synthesized and characterized by infrared spectrum(IR),elemental analysis(EA),and X-ray single crystal diffraction.For 1,ligand L adopts a cisconformation and acts as a bidentate spacer to bind Co髤centers to form a one-dimensional(1D)sinusoidal chain.These sinusoidal chains are linked with each other and give a three-dimensional(3D)framework through O-H…O and O-H…N hydrogen bonds of neighboring chains.H2O guest molecules and SO42-anions are bound on the framework through hydrogen bonding system.For 2,while the ligand L also adopts a cis-conformation,it acts as a monodentate spacer to bind Cu髤centers to form a discrete mononuclear complex,which is also linked into a three-dimensional(3D)framework through hydrogen bonds.CCDC:1568579,1;1568580,2.
triazole;complex;hydrogen bond;crystal structure
O614.81+2;O614.121
A
1001-4861(2017)12-2345-06
10.11862/CJIC.2017.261
2017-08-16。收修改稿日期:2017-10-12。
国家自然科学基金(No.91433113,91422302)和江苏省自然科学基金(No.BK20130054)资助项目。
*通信联系人。 E-mail:haiyingqd@163.com

