吡唑-3-甲酸Cu髤/Mn髤配合物的合成、表征及常压下对二氧化碳催化转化
蒋秀燕 荣念新 邱田田 钱 瑞 王燕珍 赫庆鹏*, 黄现强*,
吡唑-3-甲酸Cu髤/Mn髤配合物的合成、表征及常压下对二氧化碳催化转化
蒋秀燕1荣念新2邱田田2钱 瑞2王燕珍2赫庆鹏*,2黄现强*,2
(1中国石油大学胜利学院化学工程学院,东营 257061)
(2聊城大学化学化工学院,山东省化学储能与新型电池技术重点实验室,聊城 252059)
合成和表征了2个吡唑-3-甲酸过渡金属配合物[Cu2(pca)2(H2O)6]·2H2O(1),[Mn(Hpca)2(phen)]·3H2O(2)(H2pca=吡唑-3-甲酸;phen=菲咯啉)。X射线单晶衍射分析结果表明,配合物1属于单斜晶系P21/n空间群,它是一个畸变八面体的双核铜配合物;配合物2是一个畸变八面体的单核锰配合物。配合物1和2分别通过分子间的O-H…O,N-H…O氢键形成了三维网状结构。配合物1在二氧化碳的环加成反应中显示出了良好的催化效率(转化率高达97.4%;选择性高达98.9%)。
吡唑-3-甲酸;配合物;晶体结构;二氧化碳转化
0 Introduction
In the last few decades,transition metal complexes have been highlighted by many researchers,owing to their outstanding abilities in gas storage,molecular recognition,structure analysis and catalysis[1-3].The combination of transition metal ions and organic ligands in many systems has not only led to more complicated and fascinating structural topologies,but also exerted synergetic effects and merged the merits of both aspects,which reveal potential applications in heterogeneous catalysis,selective absorption,and photonic,electronic,and magnetic functional materials[4-6].Among lots of organic ligands,pyrazole carboxylates play a key role in generating the excellent architectures because they not only possess potential coordination nodes and the strong coordination ability,but also can be acted as the multiple proton donors,acceptors and build high-dimensional supramolecular frameworks.However,to our best knowledge,transition metalcoordination complexes derived from pyrazole-3-carboxylic acid are very little[7-8],and the studies of catalytic properties of the transition metal coordination complexes are rather rare.
Herein,copper髤 and manganese髤 complexes 1 and 2 formed by pyrazole-3-carboxylic acid,namely,[Cu2(pca)2(H2O)6]·2H2O(1)and[Mn(Hpca)2(phen)]·3H2O(2),have been synthesized and structurally characterized.Additionally,two complexes were further investigated as heterogeneous catalysts for the synthesis of cyclic carbonates from epoxides at CO2atmospheric pressure.
1 Experimental
1.1 General methods and materials
All reagents and solvents for synthesis were purchased from commercial sources and used without further purification.The FT-IR spectra were recorded from KBr pellets in the range of 4 000~400 cm-1on Nicolet 170 SXFT/IR spectrometer.The GC analyses were performed on Shimadzu GC-2014C with a FID detector equipped with an Rtx-1701 Sil capillary column.The C,H and N elemental analyses were conducted on Perkin-Elmer 240C elemental analyzer.
1.2 Synthesis
Complex[Cu2(pca)2(H2O)6]·2H2O(1):To a solution of NaOH(0.004 g,0.1 mmol)and H2pca(0.044 8 g,0.4 mmol)in water(4 mL),Cu(NO3)2·3H2O(0.096 6 g,0.4 mmol)and H2O(5 mL)were sequentially added in a 25 mL glass beaker.The reaction mixture was stirred for 1 h at room temperature and filtrated.After that,the blue block crystals of 1 were obtained by slow evaporation of the filtrate over three weeks.The yield was ca.23%based on Cu.IR(KBr,cm-1):3 484(s),1 659(s),1 555(w),1 507(m),1 475(m),1 382(s),1 355(s),1 263(s),1 232(w),1 133(m),1 068(m),1 014(m),942(m),898(m),839(m),785(m),649(w),501(w).Anal.Calcd.for C8H20Cu2N4O12(%):C 19.55,H 4.10,N 11.40;Found(%):C 19.61,H 4.16,N 11.33.
Complex [Mn(Hpca)2(phen)]·3H2O (2):H2pca(0.022 4 g,0.2 mmol),Mn(OAc)2·4H2O(0.024 5 g,0.1 mmol),1,10-Phenanthroline(0.019 8 g,0.1 mmol)and distilled water(10 mL)were sealed in a 23 mL Teflon-lined steel vessel and heated at 180℃for 72 h,and then cooled to room temperature at a rate of 0.1℃·min-1.The resulting colorless block crystals of 2 were obtained,washed with distilled water and then air-dried.The yield was ca.35%based on Mn.IR(KBr,cm-1):3 145(s),1 610(s),1 517(m),1 494(m),1 464(w),1 424(m),1 361(s),1 249(m),1 142(w),1 103(m),998(m),927(w),865(w),846(m),726(m),638(w),468(w).Anal.Calcd.for C20H20MnN6O7(%):C 46.97,H 3.94,N 16.43;Found(%):C 47.06,H 3.99,N 16.35.
1.3 Catalytic conversion of CO2to cyclic carbonate
The reactions were carried out in a 10 mL threenecked glass flask with reflux and CO2bubbling.Epoxides(10 mmol),catalyst(10%,n/n,the same below),Bu4NBr(4%),and biphenyl(1 mmol,internal standard for quantitative analysis by gas chromatography)were successively added.The reaction mixture was kept stirring at 333 K for 24 h.Upon completion,the reactant and catalyst were separated by centrifugation,washed with CH2Cl2,and air-dried.The supernatant was diluted by 25 mL acetonitrile and quantitatively analyzed by GC and GC-MS.
1.4 X-ray crystallography
Single-crystal X-ray diffraction data for complexes 1~2 were conducted on a Bruker-AXS CCD diffractometer equipped with a graphite-monochromated Mo Kα radiation (λ=0.071 073 nm)at 296 K.All absorption corrections were applied using multiscan technique.The structures were solved by the direct method and refined through full-matrix leastsquares techniques method on F2using the SHELXTL 97 crystallographic software package[9-10].The hydrogen atoms of the organic ligands were refined as rigid groups.Crystallographic data for complexes 1 and 2 are summarized in Table 1,and the selected bond distances and bond angles of complexes 1 and 2 are listed in Table 2.
CCDC:1055764,1;1055766,2.
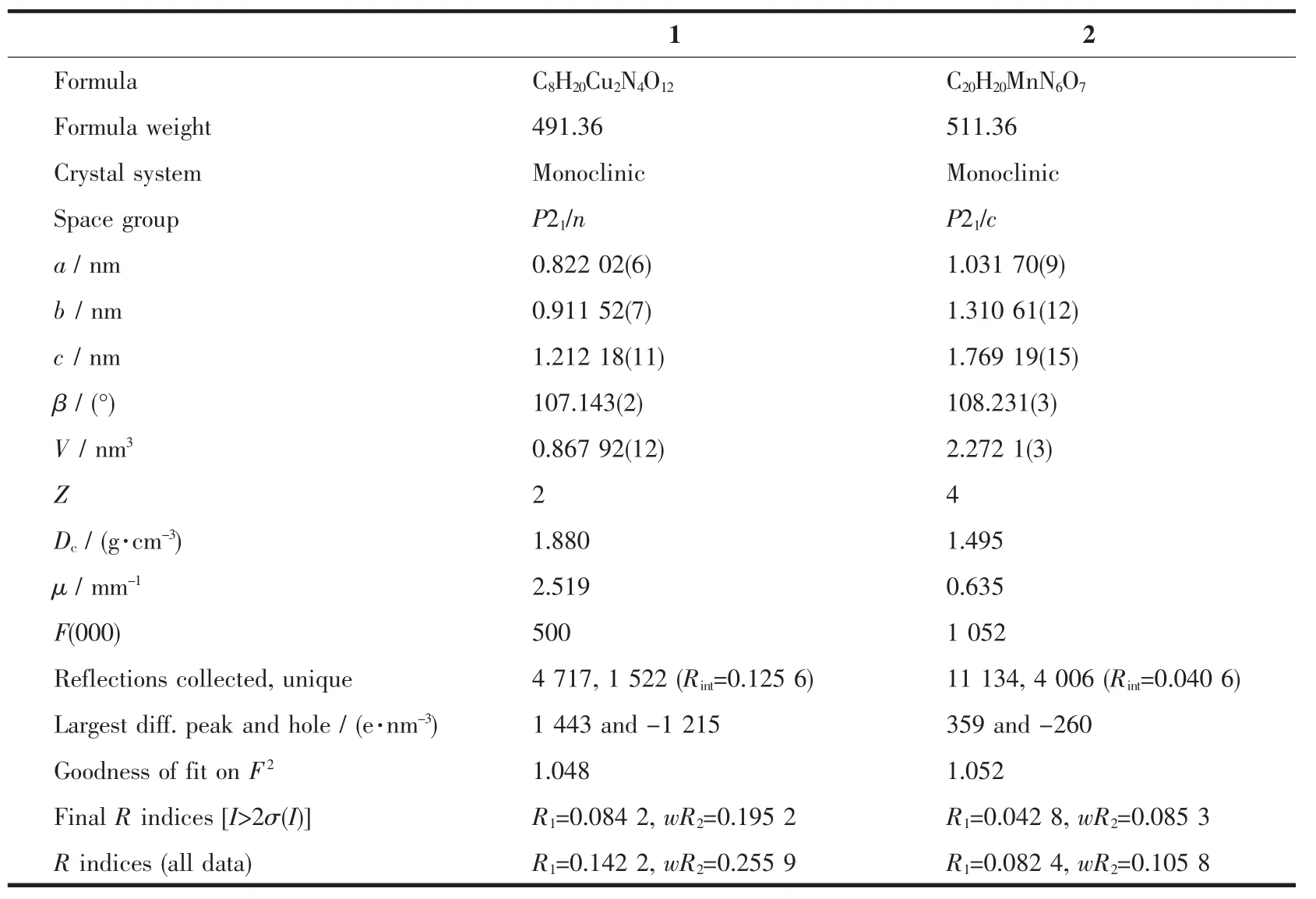
Table 1 Crystallographic data for complexes 1 and 2

Table 2 Selected bond distances(nm)and bond angles(°)for complexes 1 and 2
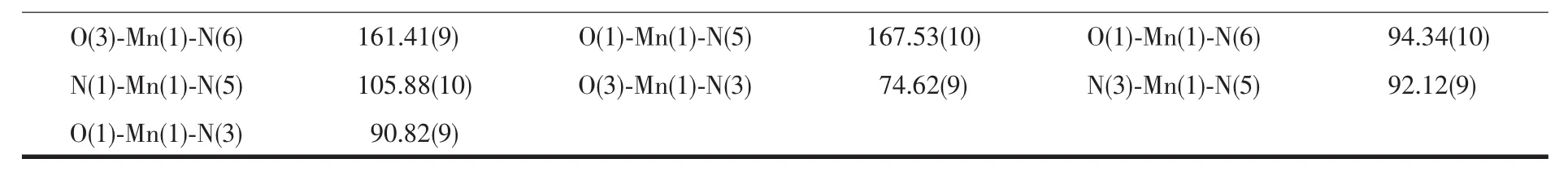
Continued Table 2
2 Results and discussion
2.1 Structure description

Fig.1 (a)Coordination environment of the Cu2+ions in 1 with thermal ellipsoids at 50%probability level;(b)Two dimensional supramolecular layer of 1 formed by hydrogen bonds in the bc plane;(c)Three dimensional supramolecular framework of 1 consisting of tetrahedral water clusters
The single-crystal X-ray diffraction reveals the asymmetric unit of complex 1 consists of one crystallographic independent Cu2+cation,one pca2-anion,three coordination water molecules and one free water molecule(Fig.1a).The Cu2+cation,which coordinated by oxygen atom (O1),nitrogen atom (N1)from pca2-anion,nitrogen atom (N2a)from the other pca2-anion and three oxygen atoms(O3,O4,O5)from three coordination watermolecules,displaying a distorted{CuO4N2}octahedral coordination geometry.The distances of the Cu-O and Cu-N are 0.206 6(6)~0.214 5(7)nm and 0.202 9(8)~0.204 2(8)nm,respectively,which are similar with those of reported complexes[11-12].The bond angles around Cu2+cation are in the range of 79.3(3)°~176.6(3)°.The six-membered ring of complex 1,which is formed by four pyrazole nitrogen atoms of two pca2-ligand and two Cu2+cations,is similar with that of the complex[Cu(NAA)2(bim)2]·H2O(HNAA=anaphthylacetic acid,bim=benzimidazole)[13].However,the distance of Cu…Cu (0.400 76 nm)in 1 is longer than that of the reported copper complex[14].The oxygen atoms(O3,O4,O5)link the carboxylic oxygen atoms(O1,O2)to form the 2D supramolecular layer through O-H…O hydrogen bonding interactions(O3…O2 0.267 0(3)nm,O4…O2 0.276 9(4)nm,O5…O1 0.276 5(1)nm)(Fig.1b,Table 3).The free water molecules further connect the neighboring 2D supramolecular layers to generate 3D supramolecular framework via the hydrogen bonding interactions(Fig.1c)(O4…O6 0.275 3(6)nm,O5…O6 0.278 8(3)nm,O6…O2 0.285 9(7)nm,O6…O3 0.288 7(1)nm)(Table 3).
Single-crystalX-ray diffraction exhibits that complex 2 crystallizesin the monoclinic crystal system with P21/c space group.The crystal structure of 2 is composed of one Mn2+cation,one phen ligand,two Hpca-anions and three lattice water molecules(Fig.2a).The Mn2+center shows a distorted{MnO2N4}octahedral coordination geometry and coordinated with two nitrogen atoms(N1,N3)from two separate Hpcaanions,two chelate nitrogen atoms(N5,N6)from phen ligand and two carboxylic oxygen atoms(O1,O3).The bond distances of the Mn-O and Mn-N are 0.215 4(2)~0.217 3(2)nm and 0.223 2(3)~0.229 5(3)nm,respectively.The bond angles around Mn2+cation are in the range of 73.20(10)°~167.53(10)°.The Mn2+cation is coordinated with Hpca-anion and phen ligand to form three five-membered rings.Interestingly,the hexanuclear chair-like water clusters are formed by the intermolecular hydrogen bonding interaction of free water molecules in the complex 2(O6…O7 0.275 2(4)nm,O7…O5 0.288 0(2)nm,O7…O5 0.272 4(5)nm).Furthermore,a 3D supramolecular framework is formed by the oxygen atoms (O5,O6)of the hexanuclear water clusters and the carboxylic oxygen atoms(O1,O2,O4)and the pyrazole nitrogen atoms(N2)of the adjacent molecule(O5…O4 0.275 9(0)nm,O5…O1 0.273 8(9)nm,O6…O2 0.269 7(2)nm,N2…O6 0.265 4(9)nm)(Fig.2b,Table 3).
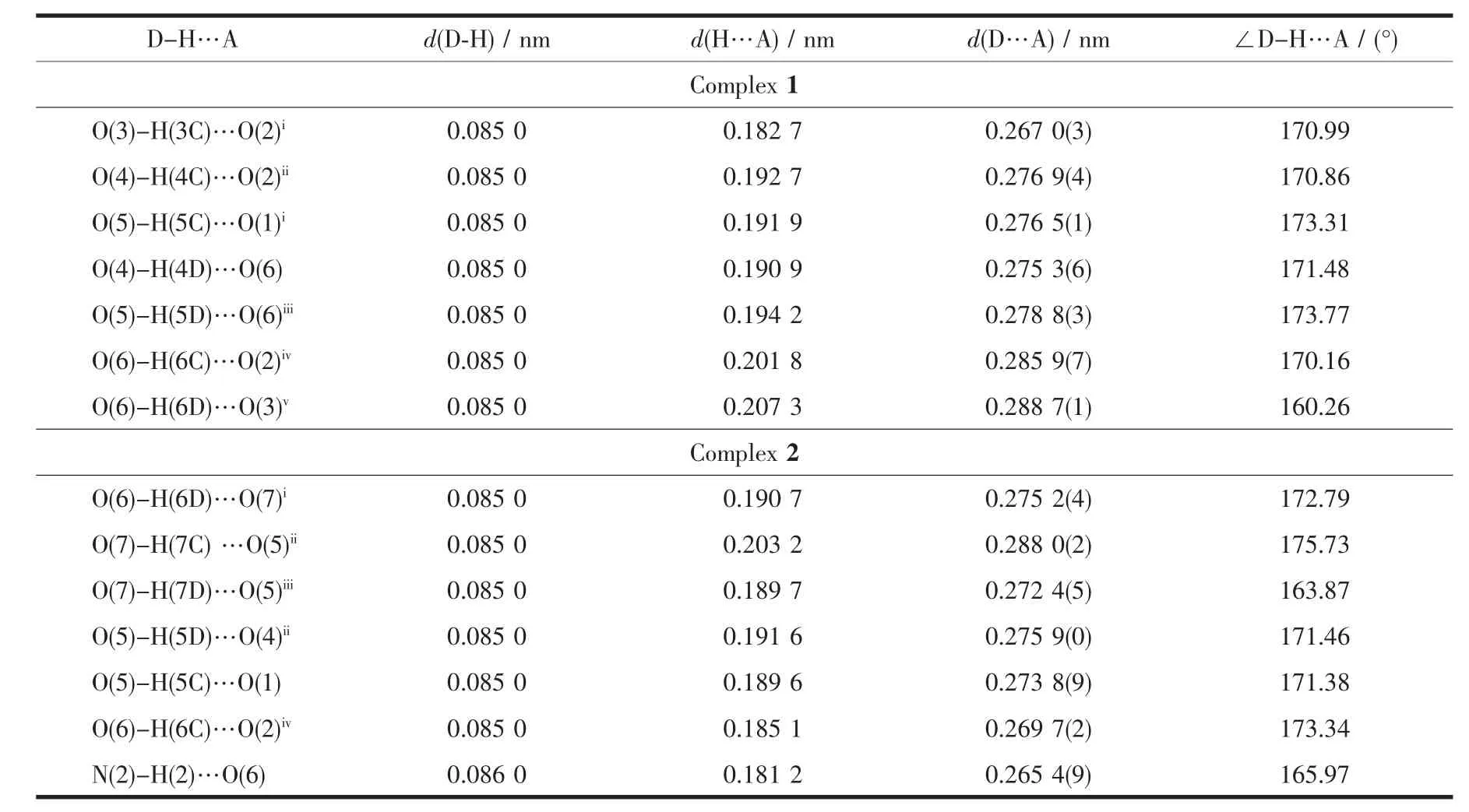
Table 3 Hydrogen-bonding geometry for complexes 1 and 2
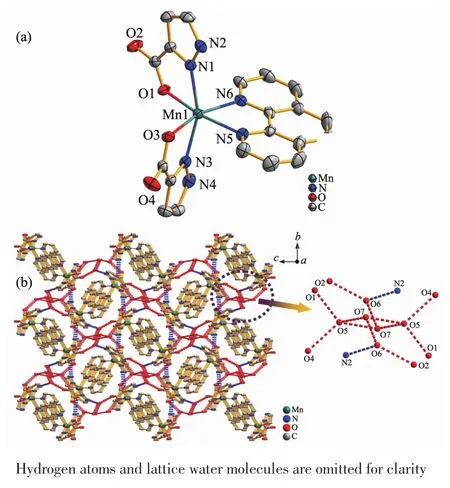
Fig.2 (a)Coordination environment of the Mn2+ions in 2 with thermal ellipsoids at 50%probability level;(b)Three dimensional supramolecular framework of 2 consisting of(H2O)6clusters
2.2 Catalytic conversion CO2to cyclic carbonate catalyzed by complexes 1 and 2
Carbon dioxide is not only the main greenhouse gas,but also is an attractive C1 building block in organic synthesis as it is highly functional,abundant,inexpensive,nontoxic,and nonflammable.Chemical fixation of CO2to obtain the valuable chemical materials is of both scientific and practical significance[15].One of the most promising pathways is the synthesis of cyclic carbonate from CO2and epoxide.Thusobtained five-membered cyclic carbonates can serve as valuable monomers for polycarbonates and electrolytes in secondary batteries and chemical sources[16-17].Our cycloaddition studies focused on the case of cycloaddition of CO2,with the aim to understand the catalytic property of pyrazole-3-carboxylic acid complexes for developing a new cycloaddition system in organic chemistry.The reactions were performed in a 10 mL flask in the presence of Bu4NBr.To optimize the product conversion and selectivity,the cycloaddition of CO2to styrene oxide to styrene carbonate was selected as a model reaction to evaluate the catalytic activities of the different catalysts in the presence of Bu4NBr(Scheme 1).The influences of different reaction conditions about the cycloaddition of CO2to styrene carbonate have been investigated.The conversion and selectivity of each reaction are summarized and illustrated in Fig.3.After the preliminary optimization,the best conversion of styrene oxide(95.1%)was obtained during the cycloaddition of CO2to styrene carbonate(10 mmol),with catalyst 1(10%)and 4%Bu4NBr at 333 K for 24 h.
Scheme 1 Conversion of styrene oxide to styrene carbonate
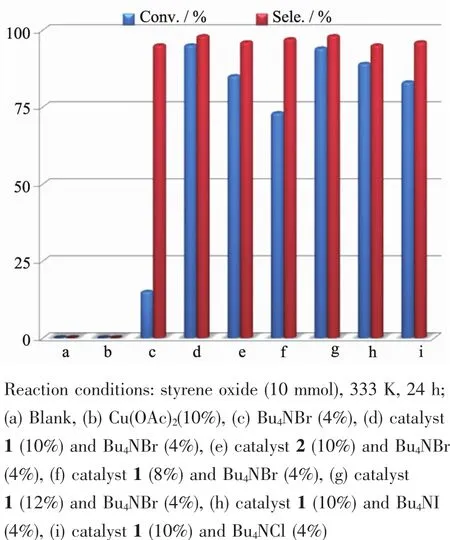
Fig.3 Conversion and selectivity of styrene oxide to styrene carbonate with different reaction conditions
Furthermore,control catalytic experiments of the cycloaddition of styrene oxide were performed under the same reaction conditions.No styrene carbonate was detected in the absence of Bu4NBr (Fig.3a,3b);The conversion was 16.7%in the absence of the catalyst,when the Bu4NBr was used as catalyst(Fig.3c),which is consistent with the previous reports[18].However,using the complex 1,the reaction can be finished in 24 h and the conversion reaches 95.1%,which overmatch the Cu(OAc)2and complex 2.Based on the above-mentioned facts,we conclude that the introduce of pyrazole-3-carboxylic acid ligand into the framework bring new coordination environment for the copper ions,which can enhance its catalytic capacity for the reaction material when combined with Bu4NBr.And indeed,catalytic activities of the complex 1 outperforms many effective catalysts,i.e.ionic liquid/Zn-PPh3catalysts[19],[Dmim]2ZnBr2Cl2[20]and pyridinium-based ionic liquids catalyst[21].
As a green catalyst,complex 1 was further used to explore the influence of catalyst recycles on the catalytic properties of cycloaddition of styrene oxide in a heterogeneous system.In the recycle experiments,the catalyst can easily be separated by filtration or centrifugation and washed using CH2Cl2.The IR spectra of the recovered complex 1 were identical to those of the freshly prepared complex 1 (Fig.4).The experiment results displayed that no obvious loss of activity was observed after three runs (Conv.95.1%for 1st,94.8%for 2nd,94.5%for 3rd)as shown in Fig.5.

Fig.4 FT-IR spectra of complex 1 after three runs of catalytic cycles
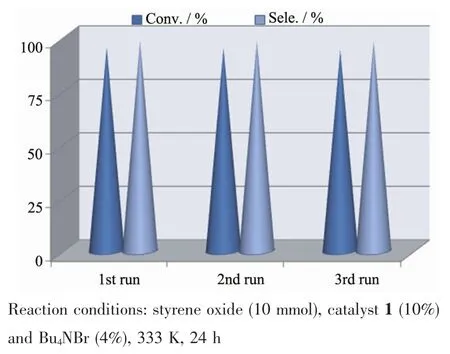
Fig.5 Recycle experiments of complex 1 on conversion of styrene oxide to styrene carbonate
Following the success of styrene epoxidecycloaddition and evaluating the scope and limitations of the current procedure,cycloaddition reactions with an array of epoxy compounds were examined using complex 1.A series of epoxide substrates were examined for the synthesis of corresponding cyclic carbonates(Table 4).Cyclic carbonates with alkyl side chain groups,aryl side chain groups were successfully synthesized from corresponding epoxidesin high conversion(up to 97.4%)and selectivity(up to 98.9%).The di-substituted epoxide,gave lower activity toward the production of the corresponding cyclic carbonates,which is presumably due to the effect of the high steric hindrance.These preliminary results exhibit that complex 1 and Bu4NBr can facilitate the cycloaddition of epoxides and serve as highly efficient and selective catalysts at CO2atmospheric pressure(101.5 kPa).

Table 4 Results of cycloaddition of epoxides catalyzed by complex 1a
3 Conclusions
In summary,Cu髤/Mn髤 complexes formed by pyrazole-3-carboxylic acid have been successfully synthesized and found that the pyrazole-3-carboxylic acid copper complexes can used as catalysts for highly efficient(Conv.90.5%~94.8%)and excellent selective(Sele.93.5%~98.3%)conversion of CO2.The other pyrazole-3-carboxylic acid transition metal complexes are in process,which will be reported timely.
[1]Han Y F,Jin G X.Acc.Chem.Res.,2014,47:3571-3579
[2]Lim D-W,Chyun S A,Suh M P.Angew.Chem.Int.Ed.,2014,53:7819-7822
[3]Wei T,Wang S,Lu X,et al.J.Am.Chem.Soc.,2016,138:207-214
[4](a)Zhang H,Lee J,Lammer A D,et al.J.Am.Chem.Soc.,2016,138:4573-4579(b)LI Zhi-Hua(李志华),LIU Hong(刘鸿),SONG Ling-Yong(宋凌勇),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33:237-242
[5]MAO Pan-Dong(毛盼东),ZHAO Xiao-Lei(赵晓雷),SHAO Zhi-Peng(邵志鹏),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33:890-896
[6]Lu Z,Abbina S,Sabin J R,et al.Inorg.Chem.,2013,52:1454-1465
[7]Liu G N,Zhu W J,Chu Y N,et al.Inorg.Chim.Acta,2015,425:28-35
[8]Hawes C S,Kruger P E.RSC Adv.,2014,4:15770-15775
[9]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of G觟ttingen,Germany,1997.
[10]Sheldrick G M.SHELXL-97,Program for Crystal Structure Refinement,University of G觟ttingen,Germany,1997.
[11]WU Hao(吴浩),CHEN Ze-Hua(陈泽华),YU Ya-Ping(于亚平),et al.Chinese J.Inorg.Chem.(无机化学学报),2017,33:699-704
[12]Ma P,Zhang Y,Li J.J.Coord.Chem.,2014,67:2238-2248
[13]Yin F J,Zhao H,Xu X Y,et al.Chin.J.Struct.Chem.,2013,32:786-791
[14]Thuery P.CrystEngComm,2013,15:6533-6545
[15]Leitner W.Angew.Chem.Int.Ed.,1995,34:2207-2221
[16]Benson E E,Kubiak C P,Sathrum A J,et al.Chem.Soc.Rev.,2009,38:89-99
[17]Appel A M,Bercaw J E,Bocarsly A B,et al.Chem.Rev.,2013,113:6621-6658
[18]Zou B,Hao L,Fan L Y,et al.J.Catal.,2015,329:119-129
[19]Wang W,Li C,Yan L,et al.ACS Catal.,2016,6:6091-6100[20]Palgunadi J,Kwon O-S,Lee H,et al.Catal.Today,2004,98:511-514
[21]Tharun J,Kathalikkattil A C,Roshan R,et al.Catal.Commun.,2014,54:31-34
Cu髤/Mn髤 Complexes Formed by Pyrazole-3-carboxylic Acid:Syntheses,Characterization and Highly Efficient Conversion of CO2at Atmospheric Pressure
JIANG Xiu-Yan1RONG Nian-Xin2QIU Tian-Tian2QIAN Rui2WANG Yan-Zhen2HE Qing-Peng*,2HUANG Xian-Qiang*,2
(1School of Chemical Engineering,Shengli College,China University of Petroleum,Dongying,Shandong 257061,China)
(2Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology,School of Chemistryamp;Chemical Engineering,Liaocheng University,Liaocheng,Shandong 252059,China)
Two transition metal complexes:Cu2(pca)2(H2O)6·2H2O(1)and Mn(Hpca)2(phen)·3H2O(2)(H2pca=pyrazole-3-carboxylic acid;phen=phenanthroline)have been prepared based on pyrazole-3-carboxylic acid and structurally characterized.The single crystal X-ray diffraction analysis reveals that complex 1 crystallizes in the monoclinic system P21/n space group and the binuclear copper center in 1 and mononuclear Mn髤center in 2 possess the distorted octahedral geometry.The three dimensional 3D framework of 1 and 2 are formed by the intermolecular O-H…O,N-H…O hydrogen bonding interactions.Importantly,complex 1 exhibits excellent heterogeneous catalytic performance (Conversion:up to 97.4%,Selectivity:up to 98.9%)in the catalytic conversion of CO2to cyclic carbonate.CCDC:1055764,1;1055766,2.
pyrazole-3-carboxylic acid;complex;crystal structure;conversion of CO2
O614.121;O614.7+11
A
1001-4861(2017)12-2303-08
10.11862/CJIC.2017.222
2017-05-23。收修改稿日期:2017-07-22。
国家自然科学基金(No.21401094)、山东省高等学校科技计划项目(No.J16LC53)和大学生创新创业基金(No.CXCY2028,CXCY2037)资助项目。
*通信联系人。 E-mail:heqingpeng@lcu.edu.cn,hxqqxh2008@163.com;会员登记号:S06N0131M1408。

