硼掺杂的NiO修饰玻碳电极电催化氧化甲醇
孙 倩,杨 朵,高 丽,郁清涛,杨海棠,杨敬贺
(河南大学 化学化工学院,化工与清洁技术工程中心,河南 开封 475004)
硼掺杂的NiO修饰玻碳电极电催化氧化甲醇
孙 倩,杨 朵,高 丽*,郁清涛,杨海棠,杨敬贺
(河南大学 化学化工学院,化工与清洁技术工程中心,河南 开封 475004)
采用计时电流法,线性扫描伏安法和安培曲线来研究B-NiO的电催化氧化甲醇过程. 研究结果表明,该B-NiO电极具有良好的电催化作用,活性高,稳定性好,在电极上对甲醇的氧化动力学过程为单扩散动力学控制过程. 与块状Ni(OH)2相比,硼掺杂的NiO纳米花在碱性介质中电催化氧化甲醇的电流密度提高了50倍. 由于B-NiO纳米花出色的电化学性能使其在电氧化甲醇上具有潜在的应用前景.
NiO;电氧化;甲醇;硼掺杂
Biography: SUN Qian(1991-), female, master, majoring in electrochemical catalysis.*Corresponding author, E-mail: gaoli@henu.edu.cn.
Direct methanol fuel cells (DMFCs) have attracted considerable interest, due to their low operating temperatures, high power density[1-6]. Anode electro-catalyst is the most important part of DMFCs. The most common catalyst in DMFCs is platinum and the activity of platinum catalyst is vigorously dependent on particle shape, size and structure[7]. Therefore, to improve the catalytic activity, much effort has been dedicated to prepare various morphology of Pt catalysts, such as Pt nanowire[8-10], Pt nanotubes[11-12], Pt nanocubes[13-15], Pt nanoparticles[16-18]. However, as to the Pt-based catalyst, the high cost and the poisoning resulting from adsorbed intermediate such as COads-like poisoning species leading to significant over-potential and loss in DMFC efficiency[19-20]. Therefore, many researches have been paid attention to search for alternative low-cost transition metal oxides/hydroxides (such as NiO, Ni(OH)2, CoO, Co(OH)2) catalysts for methanol oxidation[21-25]. Particularly, NiO is potential electrode material applied as electro-catalyst of methanol, because of its lower toxicity, low lost, ease synthesis and electro-catalytic activity[21-22,26-28]. By the way, the kinetic feature would also affect the electrochemical properties of transition metal oxides/hydroxides. The nanostructure materials can promote the electrochemical redox reactions and alleviate diffusion resistance. The properties of NiO can be improved by providing special nanostructure. The doping other atoms modification is another important way to improve its properties.
In this study, boron-doped porous nickel oxide (B-NiO) was fabricated through coprecipitation and thermal decomposition of nickel hydroxide. The activity of B-NiO was higher than the commercial bulk NiO.
1 Experimental section
1.1 Reagents and materials
Nafion (5% ethanol solution, mass fraction) was purchased from Alfa Aesar, and diluted to 0.1% with doubly distilled water in use. The surfactant copolymer poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide), commercially known as Pluronic (P123 EO20-PO70-EO20) was purchased from Sigma-Aldrich. Sodium borohydride was obtained from Sinopharm Chemical Reagent Co. Ltd (Shanghai, China). Methanol was purchased from Sinopharm Chemical Reagent Co. Ltd. The commercial Ni(OH)2and NiO were from Aladdin. All of these reagents were of analytical grade.
1.2 Synthesis of boron-doped nickle oxide
We got boron-doped NiO nanoflowers by calcination the Ni(OH)2at 873 K for 4 h with the heating rate 10 K·min-1in air. Ni(OH)2was based on a self-assembly between a triblock copolymer template P123 and two precursors (sodium borohydride and nickel species) in a flask as describe in reference we have reported[29]. Briefly, Ni(NO3)2·6H2O (8.6 g) solution was put into the 440 mL solution of P123(4 g), and then stirring at 313 K for more than 3 h. Then dropped sodium borohydride (1.5 g) solution into the mixture. After 3 h, the same quantity of sodium borohydride solution was added into the mixture too. After 12 h, light green sediments were formed. The mixture was filtered and washed with water and ethanol alternately until no foam of surfactant P123 in the filtrate, then we got Ni(OH)2with boron. The control material bulk-Ni(OH)2-773 K was prepared by calcination the commercial Ni(OH)2at 873 K for 4 h with the heating rate 10 K·min-1in air.
1.3 Characterization
The samples crystalline structure were examined by X-ray diffraction (XRD), which was carried out on X-ray D8 Advance (Bruker, Germany) instrument with Cu Kαradiation(λ= 0.154 18 nm). Samples microstructure was determined using a scanning electron microscope (SEM). The morphology and dispersion of the samples were observed using TEM, which was carried out with a FEI Tecnai G2T20. Fourier transform infrared (FT-IR) spectra were measured by transmission on a Bruker Vertex 80 FT-IR Spectrometer on KBr pellets with 2 cm-1resolution.
1.4 Electrochemical measurements and preparation of modified GCE
Electrochemical experiments were tested on a CHI660D electrochemical workstation (Shanghai, China) using three electrode system. The working electrode was glassy carbon electrode (GCE) (3 mm in diameter). B-NiO, commercial bulk-Ni(OH)2-773 K and commercial bulk-NiO were used as the working electrode. A Pt wire and Ag/AgCl electrode were used as the counter and reference electrodes, respectively. Cyclic Voltammetry (CV), linear sweep voltammetry were condusted at a rate of 50 mV·s-1in alkaline aqueous solution. The chronoamperometry was recorded in alkaline aqueous solution at 0.5 V for 1 000 s. The Amperometrici-tCurve was measured in alkaline aqueous solution at 0.5 V for 600 s. Electrochemical impedance spectroscopy (EIS) was measured in the same system at open circuit potential over a frequency range from 106 Hz to 0.01 Hz at the ampliture of the sinusoidal voltage of 0.005 V.
The bare GCE was polished with 1.0, 0.3, and 0.05 μm alumina slurry, rinsed thoroughly with ethanol and deionized water, and dried by N2atmosphere. B-NiO, bulk-Ni(OH)2-773 K and bulk-NiO of 2.5 mg was added into 1 mL 0.1%(mass fraction) nafion solution to form different suspending mixtures. The mixture was then ultrasonicated to forge a homogeneous solution. Next, the various suspensions mixtures of 10 μL were drop in the surface of GCE. Finally, the as-prepared catalyst film was dried under the infrared lamp. For comparision, a bare GCE that had been polished and cleaned was also dried for electrochemical measurement.
2 Results and discussion
The structures and composition of the obtained B-NiO and NiO nano-catalyst were studied by XRD and FTIR spectrum. As shown in Fig.1 (A), all of the diffraction peaks of B-NiO could be perfectly showed to the JCPDS 4-0835 of the cubic phase. The peaks of B-NiO centered at 36.9° (111), 43.8° (200), 62.8° (220), 75.3° (311) and 78.5° (222) belong to the cubic structure of B-NiO. No other obvious diffraction peaks were monitored, demonstating the high quality of the sample. The XRD spectrum of bulk-NiO is similar to B-NiO.
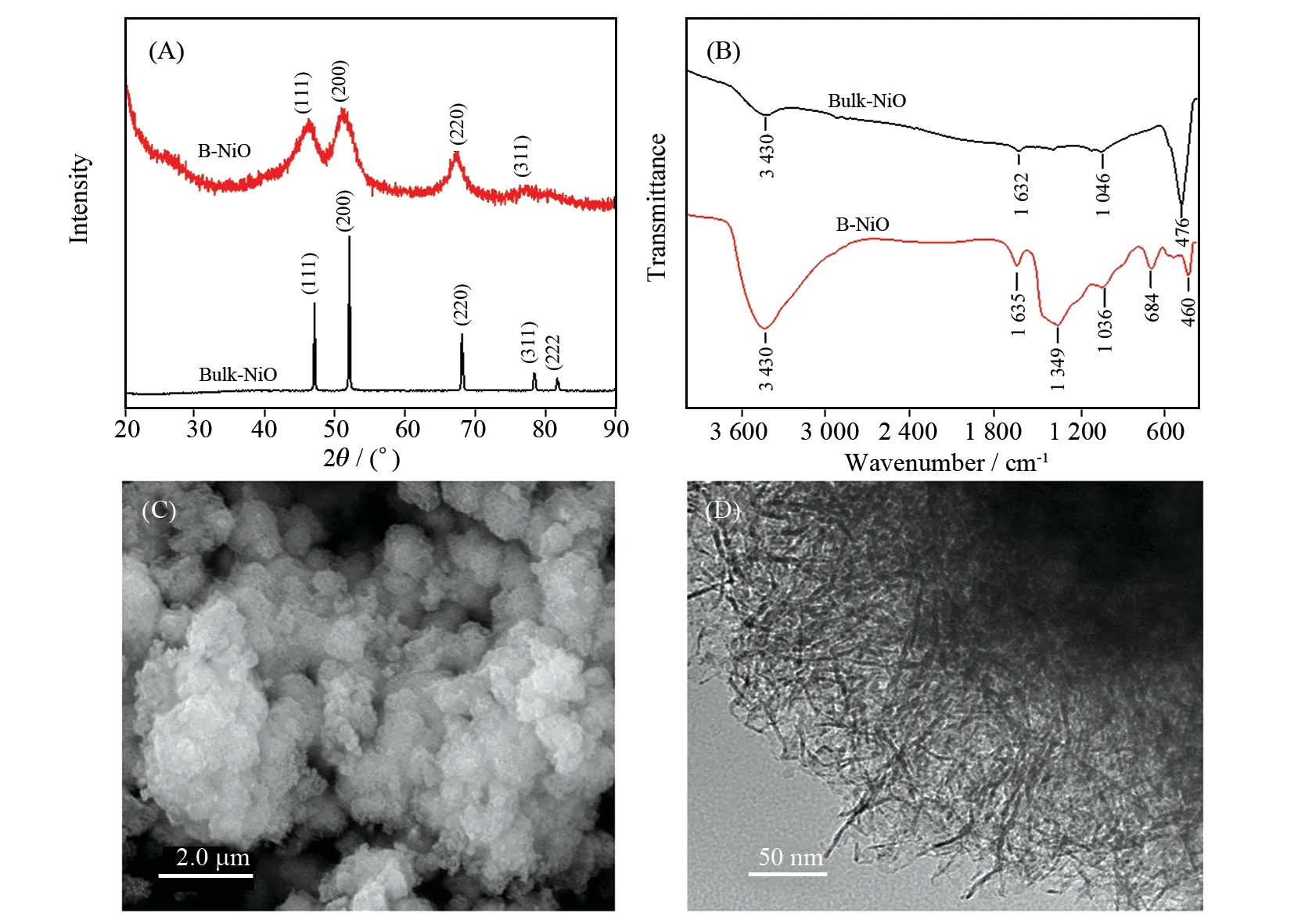
Fig.1 XRD patterns (A), FTIR patterns (B), SEM (C) and TEM (D) images of the synthesized B-NiO nanoparticle
The FTIR spectrum of B-NiO and bulk-NiO are shown in Fig.1 (B). As shown in the B-NiO FTIR spectrum, the strong and broad band around 3 430 cm-1is assigned to the stretching vibration of the adsorbed water, another one around 1 635 cm-1is assigned to the bending vibration of the adsorbed water. The peak of B-NiO at 450 cm-1is due to the Ni-O stretching vibration. The peak at 1 349 cm-1is assigned to the symmetric vibration of NO2groups. The FTIR spectrum of bulk-NiO is similar to B-NiO, too.
Fig.1 (C) showed the SEM micrograph of the B-NiO nanomaterial. It can be seen that the average particle size of the B-NiO spheres was 400 nm. As shown in the TEM images Fig.1(D) of the synthesized B-NiO nanomaterial, B-NiO was composed of approximately 30 nm nanoplates and the thickness of the nanosheets was approximately 3 nm.
The electro-oxidation properties of B-NiO, bulk-Ni(OH)2-773 K and bulk-NiO catalysts toward methanol oxidation were investigated. Fig.2(A) shows the linear sweep voltammetry (LSV) curves of methanol oxidation on the bulk-NiO, bulk-Ni(OH)2-773 K and B-NiO electrodes in 0.1 mol·L-1NaOH containing 0.5 mol·L-1methanol solution, at a scan rate of 50 mV· s-1. The magnitude of the peak current density in the forward scan from negative potential to positive (jpf) indicates the electro-catalytic activity for fresh methanol oxidation. The electrochemical performance of methanol oxidation on the bulk-NiO, bulk-Ni(OH)2-773 K and B-NiO electrodes is given in Table 1. The lower value of onset potential (Es) shows more easily electrochemically oxidized for methanol. TheEsof methanol oxidation is 0.45 V on the B-NiO electrode and it shifts positively on the two other electrodes. The reduction value ofEsshows an improvement in the kinetics. The cur-rent on B-NiO modified GCE is higher than that on the bulk-Ni(OH)2-773 K/GCE and bulk-NiO/GCE. The order forjpfon the electrodes is B-NiO > bulk-Ni(OH)2-773 K > bulk-NiO. The results show that the B-NiO electro-catalysts have higher activity than bulk-Ni(OH)2-773 K and bulk-NiO for methanol electro-oxidation.

Fig.2 LSV (A) and CV (B) curves of 0.5 mol·L-1methanol in 0.1 mol·L-1 NaOH solution at different catalyst electrodes with a sweep rate of 50 mV· s-1. CVs of 0.5 mol·L-1 methanol in 0.1 mol·L-1 NaOH solution at B-NiO catalyst modified electrode at different scan rates (C). Line relation between peak current density and the square root of the scan rate (D)
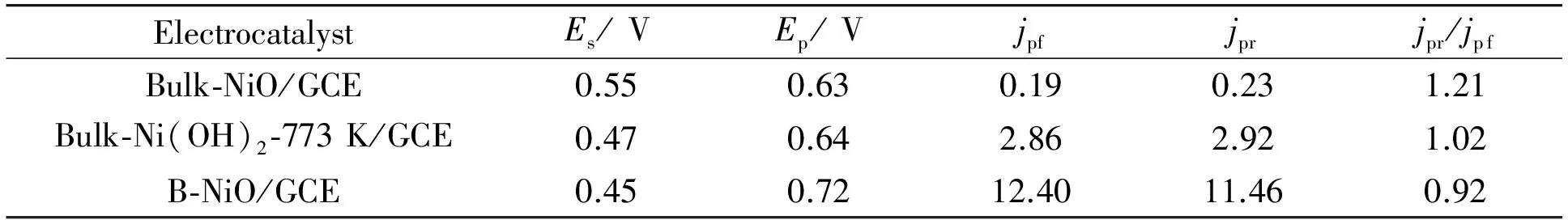
Table 1 Electrochemical performance of methanol oxidation on the bulk-NiO, bulk-Ni(OH)2-773 K and B-NiO electrodes
Fig.2(B) shows cyclic voltammograms (CVs) of methanol oxidation on the bulk-NiO, bulk-Ni(OH)2-773 K and B-NiO electrodes in 0.1 mol·L-1NaOH containing 0.5 mol·L-1methanol solution, at a scan rate of 50 mV· s-1, the potential was scanned positively from 0 to 1.0 V and then negatively to 0 V. The current at the potential range from 0.45 V to 0.9 V for methanol oxidation increases continuously in the forward sweep. This current is due to the electrochemical oxidation of fresh methanol. During this process, CO, CO2, HCOOH, HCOH and HCOOCH3form and CO molecules are absorbed on the surface of electrode, poisoning the electrocatalysts[30-31]. The current for methanol oxidation doesn’t drop from 0.7 V to 1.0 V in the forward sweep, indicating the poisoning resistance of these electro-catalysts is better[32]. The current of methanol oxidation on the catalysts didn’t drops from 0.7 V to 1.0 V in the forward sweep, indicating a poisoning-resistance happens on the catalysts. During the reverse sweep, the re-oxidation of CO and other adsorbed species occurs. The peak current density in the forward sweep (jpf) and the peak current density in the reverse sweep (jpr) are summarized in Table 1. The oxidation peak in the reverse sweep is according to the electrochemical oxidation CO and other adsorbed species. So the value ofjpr/jpfis smaller and the poisoning resistance of these electro-catalysts is better. The order for the value ofjpr/jpfon the Ni-oxide is bulk-NiO > bulk-Ni(OH)2-773 K > B-NiO. The value ofjPr/jpfon B-NiO is the smallest than that on the other catalysts, so the B-NiO will give the best stable performance of methanol oxidation.
As shown in Fig.2(C), the electro-catalytic properties of B-NiO was evaluated by cyclic voltammetry (CV). The CV behavior of the B-NiO at different scan rates (10-200 mV· s-1). It can be seen that oxidation peak current density (Ip) for methanol oxidation become larger with the increase of the scan rate. The line relation between peak current density (Ip) and square root of the scan rate (v1/2) is shown in Fig.2(D). The anodic peak currents increase linearly with the square root of scan rate indicating the electrochemical reaction controlled by the semi-infinite linear diffusion from the electrolyte to the electrode.
To evaluate the stability of nano-catalysts, amperometrici-tcurves of B-NiO, bulk-Ni(OH)2-773 K, bulk-NiO and bare GCE were measured in 0.1 mol·L-1NaOH containing 0.5 mol·L-1methanol solution (Fig.3(A)). The measurement under a constant potential of 0.5 V (vs. Ag/AgCl) is 600 s. As shown in Fig.3(A), the decay was slow and the current density finally reached a stable current density. Among the three nano-catalysts, B-NiO showed the higher activity and higher stability for methanol oxidation. This implies reasonable good mechanical and electro-catalytic stability of the above electrode towards MeOH at the prevailed experimental conditions.
To evaluate the rate of surface poisoning and the stability of nanocatalysts, chronoamperometry (CA) curves of B-NiO, bulk-Ni(OH)2-773 K, bulk-NiO and bare GCE were measured in 0.1 mol·L-1NaOH containing 0.5 mol·L-1methanol solution (Fig.3(B)). CA curves (performed at 0.5 V for 1 000 s) exhibits that the B-NiO nanoflower has higher reaction current density and a slower current degradation over time compared with the other electrocatalyst for the entire time course, which further verified that the B-NiO nanoflower exhibited better electrocatalytic performance, the ability to tolerate CO ads-like species formed in the methanol oxidation process[33-34]and the best catalytic stability.
Electrochemical impedance spectroscopy (EIS) is an effective method to investigate the parameters affecting the performance of an electrode, including its charge-transfer and diffusion properties. EIS spectra contain two portions, i.e. semicircle at higher frequencies correspond to the electron transfer limited process and linear line relatively at lower frequencies correspond to the diffusion process. Fig.3(C) shows the Nyquist plots of the B-NiO, bulk-Ni(OH)2-773 K and bulk-NiO measured at 0.049 43 V (vs. Ag/AgCl) in the frequency ranging from 100 kHz to 0.01Hz in 0.1 mol·L-1NaOH containing 0.5 mol·L-1CH3OH solution. From Figure 3(C), it is seen that there are no well-defined semicircle in the desired frequency range which indicates that they possess good electron transfer kinetics. The impedance of B-NiO is lower than that of the bulk-Ni(OH)2-773 K and bulk-NiO catalysts in the case of methanol oxidation indicating the layer of B-NiO could form on the electrode surface and alower electrochemical polarization impedance. Fig.3(D) shows CVs of B-NiO nanoflower modified nickel foam electrode obtained after different numbers of cycles. With further cycling, the above oxidation/reduction waves decreased a little in amplitude and approached a stable value after 800 cycles suggesting the activation of B-NiO and the electro-active species on the nickel foam electrode is relatively stable.
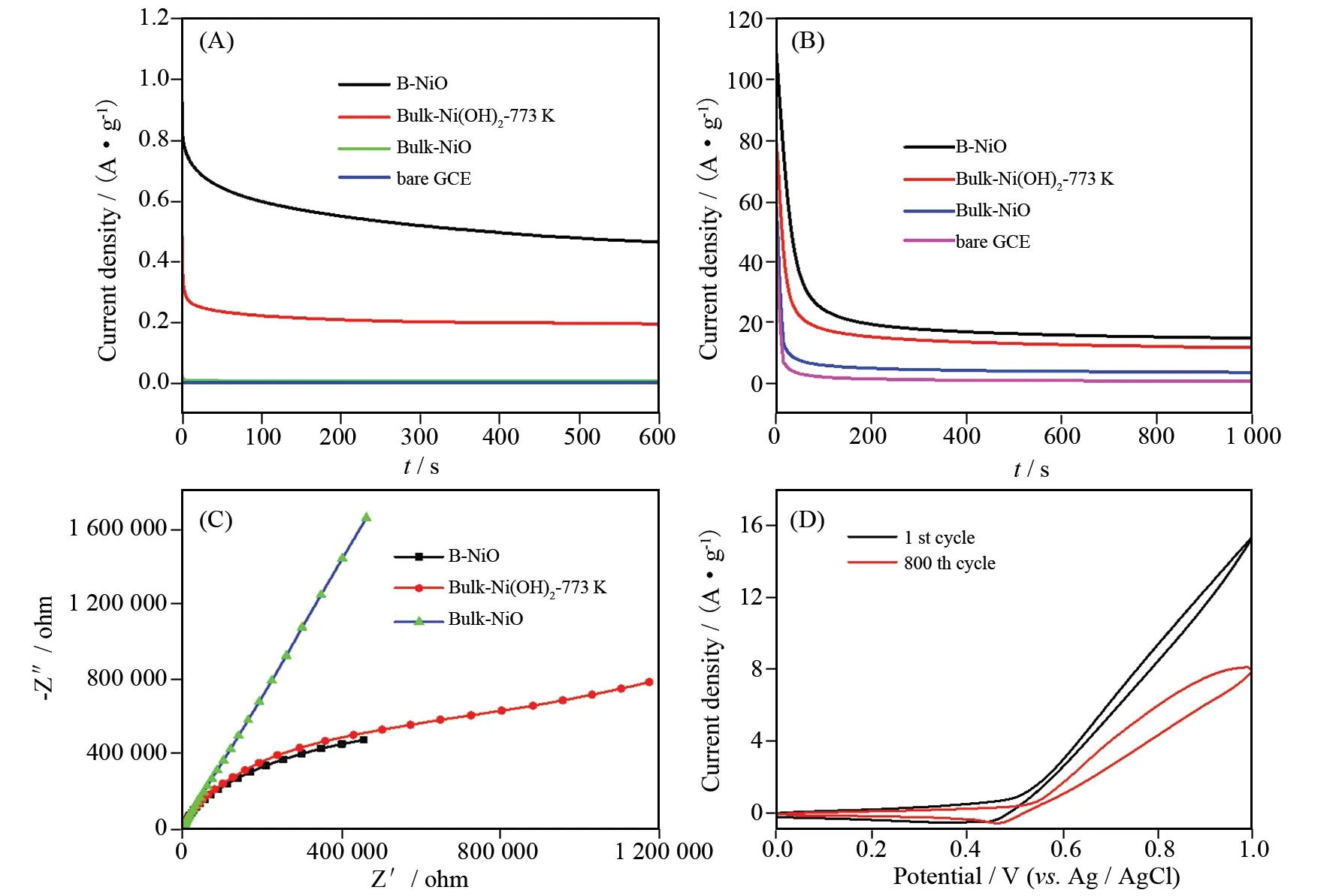
Fig.3 Amperometric i-t (A) and Chronoamperometry (B) curves of 0.5 mol·L-1 methanol in 0.1 mol·L-1 NaOH solution at different catalyst electrodes at 0.5 V. Electrochemical impedance spectra of 0.5 mol·L-1 methanol in 0.1 mol·L-1 NaOH solution at different catalyst electrodes at open circuit potential of 0.049 43 V (C). Stability CVs for methanol electro-oxidation of 0.5 mol·L-1 methanol in 0.1 mol·L-1 NaOH solution at B-NiO catalyst modified nickel foam electrode (D)
3 Conclusions
Using a simple one-step thermal decomposition method, Boron-doped nickle oxide (B-NiO) nanocatalysts was fabricated by thermal decomposition of nickel hydroxide. The porous B-NiO/GCE exhibited better electro-oxidation activity and stability than those of commercial Ni(OH)2after calcination and commercial bulk-NiO. The methanol oxidation of B-NiO was a di-ffusion controlled behavior in the range of scan rate from 10 mV· s-1to 200 mV· s-1. The results indicated that the B-NiO nanocatalysts modified GCE showed good electro-catalytic performance (including high electro-catalytic current density, good poison, and low onset potential) in comparison with commercial NiO nanocatalysts modified GCE in methanol oxidation reaction.
[1] LAMY C, BELGSIR E, LÉGER J M. Electrocatalytic oxidation of aliphatic alcohols: Application to the direct alcohol fuel cell (DAFC) [J]. Journal of Applied Electrochemistry, 2001, 31: 799-809.
[2] ACRES G J K. Recent advances in fuel cell technology and its applications [J]. Journal of Power Sources, 2001, 100(1/2): 60-66.
[3] JUSYS Z, BEHM R J. Methanol oxidation on a carbon-supported Pt fuel cell catalyst-a kinetic and mechanistic study by differential electrochemical mass spectrometry [J]. The Journal of Physical Chemistry B, 2001, 105(44): 10874-10883.
[4] VERMA L K. Studies on methanol fuel cell [J]. Journal of Power Sources, 2000, 86(1/2): 464-468.
[5] LIU H S, SONG C J, ZHANG L, et al. A review of anode catalysis in the direct methanol fuel cell [J]. Journal of Power Sources, 2006, 155(2): 95-110.
[6] ZAINOODIM A M, KAMARUDIN S K, DAUD W R W. Electrode in direct methanol fuel cells [J]. International Journal of Hydrogen Energy, 2010, 35(10): 4606-4621.
[7] TIWARI J N, TIWARI R N, SINGH G, et al. Recent progress in the development of anode and cathode catalysts for direct methanol fuel cells [J]. Nano Energy, 2013, 2(5): 553-578.
[8] WANG S Y, JIANG S P, WANG X, et al. Enhanced electrochemical activity of Pt nanowire network electrocatalysts for methanol oxidation reaction of fuel cells [J]. Electrochimica Acta, 2011, 56 (3): 1563-1569.
[9] RUAN D J, GAO F, GU Z Y. Enhanced electrochemical properties of surface roughed Pt nanowire electrocatalyst for methanol oxidation [J]. Electrochimica Acta, 2014, 147 (20): 225-231.
[10] LI B, YAN Z Y, HIGGINS D C, et al. Carbon-supported Pt nanowire as novel cathode catalysts for proton exchange membrane fuel cells [J]. Journal of Power Sources, 2014, 262(15): 488-493.
[11] ALIA S M, ZHANG G, KISAILUS D, et al. Porous platinum nanotubes for oxygen reduction and methanol oxidation reactions [J]. Advanced Functional Materials, 2010, 20: 3742-3746.
[12] BI Y P, LU G X. Control growth of uniform platinum nanotubes and their catalytic properties for methanol electrooxidation [J]. Electrochemistry Communications, 2009, 11(1): 45-49.
[13] HAN S B, SONG Y J, LEE J M, et al. Platinum nanocube catalysts for methanol and ethanol electrooxidation [J]. Electrochemistry Communications, 2008, 10(7): 1044-1047.
[14] LEE Y W, HAN S B, KIM D Y, et al. Monodispersed platinum nanocubes for enhanced electrocatalytic properties in alcohol electrooxidation [J]. Chemical Communications, 2011,47: 6296-6298.
[15] NOGAMI M, KOIKE R, JALEM R, et al. Synthesis of porous single-crystalline platinum nanocubes composed of nanoparticles [J]. The Journal of Physical Chemistry Letters, 2010, 1(2): 568-571.
[16] ENSAFI A A, JAFARI-ASL M, REZAEI B. A new strategy for the synthesis of 3-D Pt nanoparticles on reduced graphene oxide through surface functionalization, Application for methanol oxidation and oxygen reduction [J]. Electrochimica Acta, 2014, 130: 397-405.
[17] RAUBER M, ALBER I, MULLER S, et al. Highly-ordered supportless three-dimensional nanowire networks with tunable complexity and interwire connectivity for device integration [J]. Nano Letters, 2011, 11(6): 2304-2310.
[18] SUN S H, YANG D Q, VILLERS D, et al. Template-and surfactant-free room temperature synthesis of self-assembled 3d Pt nanoflowers from single-crystal nanowires [J]. Advanced Materials, 2008, 20(3): 571-574.
[19] CARAM J A, GUTIERREZ C. Cyclic voltammetric and potential-modulated reflectance spectroscopic study of the electroadsorption of methanol and ethanol on a platinum electrode in acid and alkaline media [J]. Journal of Electroanalytical Chemistry, 1992, 323(1/2): 213-230.
[20] KIM Y, SOUNDARARAJAN D, PARK C,et al. Electrocatalytic properties of carbon nanofiber web-supported nanocrystalline pt catalyst as applied to direct methanol fuel cell [J]. International Journal of Electrochemical Science, 2009, 4: 1548-1559.
[21] SPINNER N, MUSTAIN W E. Effect of nickel oxide synthesis conditions on its physical properties and electrocatalytic oxidation of methanol [J]. Electrochimica Acta, 2011, 56 (16): 5656-5666.
[22] GU C D, HUANG M L, GE X, et al. NiO electrode for methanol electro-oxidation: Mesoporous vs. nanoparticulate [J]. International Journal of Hydrogen Energy, 2014, 39(21): 10892-10901.
[23] EL-SHAFEI A A. Electrocatalytic oxidation of methanol at a nickel hydroxide/glassy carbon modified electrode in alkaline medium [J]. Journal of Electroanalytical Chemistry, 1999, 471(2): 89-95.
[24] XIA Y S, DAI H X, JIANG H Y, et al. Three-dimensional ordered mesoporous cobalt oxides: Highly active catalysts for the oxidation of toluene and methanol [J]. Catalysis Communications, 2010, 11(15): 1171-1175.
[25] ZAFEIRATOS S, DINTZER T, TESCHNER D, et al. Methanol oxidation over model cobalt catalysts: Influence of the cobalt oxidation state on the reactivity [J]. Journal of Catalysis, 2010, 269(2): 309-317.
[26] ASGARI M, MARAGHEH M G, DAVARKHAH R, et al. Methanol electrooxidation on the nickel oxide nanoparticles/multi-walled carbon nanotubes modified glassy carbon electrode prepared using pulsed electrode position [J]. Journal of the Electrochemical Society, 2011, 158(12): K225-K229.
[27] SHAMSIPUR M, NAJAFI M, MILANI HOSSEINI M R. Electrooxidation of alcohols at a nickel oxide/multi-walled carbon nanotube-modified glassy carbon electrode [J]. Journal of Applied Electrochemistry, 2013, 43(10): 1027-1033.
[28] LI S J, XIA N, LÜ X L, et al. A facile one-step electrochemical synthesis of graphene/NiO nanocomposites as efficient electrocatalyst for glucose and methanol [J]. Sensors and Actuators B: Chemical, 2014, 190: 809-817.
[29] YANG J H, YU Q T, LI Y M, et al. Batch fabrication of mesoporous boron-doped nickel oxide nanoflowers for electrochemical capacitors [J]. The Journal of Physical Chemistry Materials Research Bulletin, 2014, 59: 382-386.
[30] IWASITA T. Electrocatalysis of methanol oxidation [J]. Electrochimica Acta, 2002, 47(22/23): 3663-3674.
[31] LEUNG L W H, WEAVER M J. Real-time FTIR spectro-scopy as a quantitative kinetic probe of competing electrooxidation pathways of small organic molecules [J]. The Journal of Physical Chemistry, 1998, 92(14): 4019-4022.
[32] CORRIGAN D S, WEAVER M J. Mechanisms of formic acid, methanol, and carbon monoxide electrooxidation at platinum as examined by single potential alteration infrared spectroscopy [J]. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry, 1988, 241(1/2): 143-162.
[33] KATZ E, SHIPWAY A N, WILLNER I. Handbook of fuel cells-fundamentals, technology and applications [M]. John Wiley & Sons Ltd, 2003, 1: 1-27.
[34] LONG N V, OHTAKI M, NOGAMI M, et al. Effects of heat treatment and poly(vinylpyrrolidone) (PVP) polymer on electrocatalytic activity of polyhedral Pt nanoparticles towards their methanol oxidation [J]. Colloid and Polymer Science, 2011, 289(12): 1373-1386.
Electro-oxidationofmethanolonboron-dopednickleoxidemodifiedglassycarbonelectrode
SUN Qian, YANG Duo, GAO Li*, YU Qingtao, YANG Haitang, YANG Jinghe
(EngineeringCenterforCleanChemicalProcessandTechnology,CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China)
The boron-doped nickle oxide (B-NiO) nanoflowers were developed as an electro-catalyst for methanol electro-oxidation in alkaline media which is more than 50 times enhancement in current density compared to bulk nickel hydroxide powers. The positive correlation between the scan rates and the anodic currents implies a single diffusion-controlled kinetic process. The chronoamperometry, linear-sweep voltammetric, and amperometrici-tcurves were also employed for investigated the B-NiO and the results are also positive. This remarkable electrochemical performance will make B-NiO nanoflowers a promising electrode material for high performance electro-oxidation of methanol.
nickle oxide; electro-oxidation; methanol; boron-doped
O622.3DocumentcodeA
1008-1011(2017)05-0598-08
date: 2017-05-03.
Supported by the National Natural Science Foundation of China (21403053), the Joint Funds of the National Natural Science Foundation of China (U1404503) and Henan University Graduate Scientific Innovation Research Supporting Project (Y1427005).
[责任编辑:吴文鹏]

