基于四元羧酸钙配位聚合物的合成、结构及其荧光性能
陈 一,杜 毅,蔡 婷,杨乐乐,韩宇阳,陆云霞,邵彩云,杨立荣*
(1.河南大学 化学化工学院,河南省多酸化学重点实验室,河南 开封 475004; 2.河南大学 图书馆, 河南 开封 475001)
基于四元羧酸钙配位聚合物的合成、结构及其荧光性能
陈 一1,杜 毅1,蔡 婷1,杨乐乐1,韩宇阳1,陆云霞2,邵彩云1,杨立荣1*
(1.河南大学 化学化工学院,河南省多酸化学重点实验室,河南 开封 475004; 2.河南大学 图书馆, 河南 开封 475001)
通过水热法合成了一种结构稳定的金属有机框架化合物{[Ca(atba)4·2H2O]·H2O}n(H4atba = 偶氮苯-3,3′,5,5′-四羧酸). 运用X射线单晶衍射、红外谱图及X射线粉末衍射对其进行了结构表征. 晶体结构测试表明该配合物通过{Ca2(CO2)2}结构单元形成了一维链状结构, 继而通过配体之间的连接形成二维层状结构, 进一步通过共价键构筑成三维结构. 荧光光谱分析表明, 标题化合物对L-组氨酸具有选择性识别作用.
金属-有机框架物;水热合成;结构表征;荧光识别
Biography: CHEN Yi (1996-), male, majoring in coordination chemistry.*Corresponding author, E-mail: lirongyang@henu.edu.cn.
1 Experimental section
1.1 Materials and physical measurements
All chemicals were commercially purchased and used without further purification. IR spectra in the range of 400-4 000 cm-1were obtained with an AVATAR 360 FT-IR spectrometer (KBr pellets were used). The crystal structure was determined with a Bruker Smart CCD X-ray single-crystal diffractometer. Excitation and emission spectra were recorded with an F-7000 FL spectrofluorometer at room temperature. Powder X-ray diffraction (PXRD) patterns were recorded on a Bruker D8.
1.2 Synthesis of the coordination
A mixture of calcium chloride (11.1 mg, 0.2 mmol), azobenzene-3,3,5,5-tetracarboxylic acid (37.4 mg, 0.1 mmol), 4′4 bipyridyl (13.6 mg, 0.1 mmol) and water (10 mL) was homogenized by stirring for 30 min, afterwards, the pH value of the mixture was tuned to 6, then transferred into 25 mL Teflon-linepd stainless steel autoclave under autogenous pressure at 125 ℃ for 3 days. After cooling the reaction system to room temperature at a rate of 5 ℃/h, and transparent block crystals suitable for X-ray diffraction analysis were obtained. IR data (KBr pellet, cm-1): 3 337(m), 3 117(w), 1 607(s), 1 563(s), 1 481(m), 1 439(s), 1 380(s), 1 208(m), 1 103(w), 1 006(w), 783(m), 670(w), 542(w), 484(w).
1.3 Crystallographic data collection and refinement
Single-crystal diffraction data were collected suitable single crystals of the coordination polymers on a Bruker Smart CCD X-ray single-crystal diffractometer with graphite monochromated Mo Kα-radiation (λ= 0.071 073 nm). All independent reections were collected in a range of 3.69°-26.37° for the coordination polymer. Multi-scan empirical absorption corrections were applied to the data using the SADABS. The crystal structure was solved by direct methods and Fourier synthesis. Positional and thermal parameters were refined by the full-matrix least-squares method onF2using the SHELXTL software package. The final least-square cycle of refinement gaveR1= 0.032 7,WR2= 0.087 9 for the coordination polymer, the weighting schemeW= 1/[δ2(F20)+(0.041 0P)2+0.139 9P] for the coordination polymer, whereP= (F20+2Fc2)/3. The crystallographic data, selected bond lengths and bond angles for coordination polymer {[Ca(atba)4·2H2O]·H2O}nare listed in Table 1 and Table 2, respectively.
2 Results and discussion
2.1 FT-IR spectroscopy and X-ray powder diffraction
The complex is stable at room temperature and insoluble in common solvents; such as CH3COCH3,CH3CH2OH and CH3CN, but they are slight soluble in H2O and soluble in CH3OH and DMF. The powder XRD patterns of the coordination polymer have been investigated. As shown in Fig.1, the experimental powder XRD patterns are consistent with the simulated ones on the basis of the single-crystal structure, which indicated that the corresponding samples are pure. Meanwhile, the structure of the coordination polymer was revealed by FT-IR (Fig.2). The strong and broad absorption bands within the scope of 3 496-3 342 cm-1are assigned to the water molecules in coordination and lattice forms. Some other strong absorption bands can be seen in the region of 1 610-1 606 cm-1and 1 383-1 375 cm-1, which may be ascribed to the asymmetric (COO-) and symmetric (COO-) stretching of carboxyl groups of atbt4-ligands in the coordination polymer. The values ofΔ[vas-vs] are about 223-235 cm-1, which indicate that the carboxyl groups are coordinated with the metal ions via bidentate-bridging mode. The absence of the characteristic bands around 1 700 cm-1demonstrate that the H4atba ligands are completely deprotonated in the form of atba4-anions upon reaction with the metal ions[24-27]. The same conclusions are also supported by the results obtained from X-ray diffraction measurements.

Table 1 Summary of crystallographic data for the complex

Table 2 Bond lengths and angles for the complex

Fig.1 Simulated and experimental powder XRD patterns

Fig.2 IR of coordination polymer
2.2 Structural description of the coordination polymer
The crystallographic data, selected bond lengths and bond angles for coordination polymer {[Ca(atba)4·2H2O]·H2O}nare listed in Table 1 and Table 2. The Single crystal X-ray diffraction analysis reveals that the complex crystallizes in the triclinic crystal system of theP-1 space group. The coordination geometry of center Ca (Ⅱ) is a contorted six-oxygen-coordinated octahedron (Fig.3d), and four of them are from four atba4-ligand, respectively, as well as another two oxygen atoms are from two water molecules (Fig.3a,b). The Ca-O bond distances range from 0.229 16(16) nm to 0.239 38(19) nm, and the angles of O-Ca-O are within the scopes of 7.753(6)°-17.405(6)°, which are consistent with the bond length data and angles in previous work covering the corresponding coordination polymers[28-29]. Two adjoining crystallographic equivalent Ca(Ⅱ) are bridged by two carboxyl groups from atba4-ligands to form the building unit of {Ca2(CO2)2}. Afterwards, based on such units, the complex is generated into a 1D infinite linear metallic chains (Fig.3c). The 1D chains are connected into 2D layers (Fig.3e) through atba4-ligands, which are further interlinked to a 3D porous framework (Fig.3f). Meanwhile, the stabilization are strengthen owing to the covalent bonding interactions between adjacent layers which contribute to the formation of 3D structure[30-31].
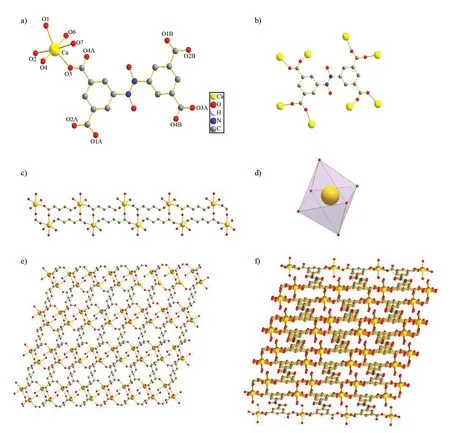
Fig.3 a) Coordination environment of Ca (Ⅱ) ion; b) Coordination mode of the ligand; c) the 1D chain in the complex; d) Diagram showing the coordination environment for Ca(Ⅱ) center; e) view of 2D layer in the complex ; f) The 3D structure of the complex
2.3 Luminescent properties
As mentioned above, metal ions or SBUs and organic ligands constitute the MOFs, in which, the part of organic ligands often contain aromatic or conjugated moieties that are subject to excitation, giving rise to optical emission or photoluminescence (PL) upon irradiation. Furthermore, the metal components can also contribute to photoluminescence, in which case lanthanides or various inorganic clusters are often involved.
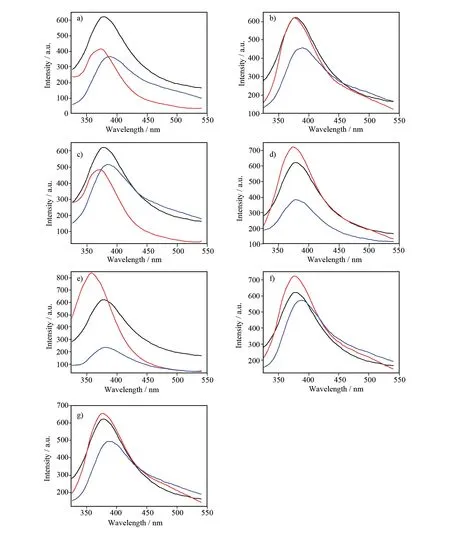
Fig.4 Emission spectra of the coordination polymer and amino acids at room temperature (Black, coordination polymer; Red, amino acids; Blue, mixture of coordination polymer and amino acids). a) L-Aspartic, b) L-Threonine, c) L-Glutamate, d) L-Arginin, e) L-Histidine, f) L-Glitamine, g) L-Phenylalanine
Luminescent properties of alkaline-earth metal coordination polymers are not well-studied up to now, although there are reports on the luminescent properties of alkaline earth metal-containing inorganic materials[32-34]. In this study, the original as-synthesized complex was used to sense amino acids. The investigation of luminescent properties for sensing amino acids was determined in the liquid state at room temperature. The crystalline samples were immersed in deionized water containing various amino acids to give 10-4mol/L solutions. Emission spectra of the complex in water containingL-Asp,L-Threonine,L-Glutamic acid,L-Arginine,L-Histidine,L-Glutamine,L-Hydrocinnamamide (10-4mol/L) are graphically shown in Fig.4 and Fig.5, respectively. The results indicate that most of the tested amino acids just gave slight effect on the fluorescence intensity of the initial coordination polymer exceptL-Histidine (as illustrated in Fig.4e). Furthermore, red shift occurs in the emission spectra. Detailedly, at the presence ofL-Histidine, the emission intensity is declined sharply. The high selectivity forL-Histidine sensing probably results from the electrostatic interaction between -COO-anions (deriving from the free carboxyl groups of side chains in the polymeric backbone) and -NH3+cations (belonging to amino acids), which affords signal amplification[35].

Fig.5 Luminescent intensities of the coordination polymer upon the addition of various amino acids at room temperature
3 Conclusion
In summary, we have successfully synthesized the complex based on alkaline earth metal under hydrothermal conditions, namely, {[Ca(atba)4·2H2O]·H2O}n. Structural analysis indicates that the complex presents a 1D uniform Ca-carboxylate chain based on the building unit of {Ca2(CO2)2}, thereafter, the 1D chains are connected to fabricate a 2D layered structure. Furthermore, these 2D layers are assembled into 3D network via covalent bonding. Luminescent properties of the complex have been studied at ambient temperature. Remarkably, the synthesized complex presents highly sensitive and selective towardL-Histidine and it may be acting as a promising sensor for its rapid detection.
[1] PERRY IV J J, PERMAN J A, ZAWOROTKO M J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks[J]. Chemical Society Reviews, 2009, 38(5): 1400-1417.
[2] O’KEEFFE M, YAGHI O M. Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets[J]. Chemical Reviews, 2011, 112(2): 675-702.
[3] HE Y, LI B, O’KEEFFE M, et al. Multifunctional metal-organic frameworks constructed from meta-benzenedicarbo-xylate units[J]. Chemical Society Reviews, 2014, 43(16): 5618-5656.
[4] KITAGAWA S. Metal-organic frameworks (MOFs)[J]. Chemical Society Reviews, 2014, 43(16): 5415-5418.
[5] HU Z, DEIBERT B J, LI J. Luminescent metal-organic frameworks for chemical sensing and explosive detection[J]. Chemical Society Reviews, 2014, 43(16): 5815-5840.
[6] TRANCHEMONTAGNE D J, MENDOZA-CORTÉS J L, O’KEEFFE M, et al. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1257-1283.
[7] O’KEEFFE M. Design of MOFs and intellectual content in reticular chemistry: a personal view[J]. Chemical Society Reviews, 2009, 38(5): 1215-1217.
[8] PERRY IV J J, PERMAN J A, ZAWOROTKO M J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks[J]. Chemical Society Reviews, 2009, 38(5): 1400-1417.
[9] BROZEK C K, DINCM. Cation exchange at the secondary building units of metal-organic frameworks[J]. Chemical Society Reviews, 2014, 43(16): 5456-5467.
[10] JASUJA H, PETERSON G W, DECOSTE J B, et al. Evaluation of MOFs for air purification and air quality control applications: Ammonia removal from air[J]. Chemical Engineering Science, 2015, 124: 118-124.
[11] HASEGAWA S, HORIKE S, MATSUDA R, et al. Three-dimensional porous coordination polymer functionalized with amide groups based on tridentate ligand: selective sorption and catalysis[J]. Journal of the American Chemical Society, 2007, 129(9): 2607-2614.
[12] ROSI N L, ECKERT J, EDDAOUDI M, et al. Hydrogen storage in microporous metal-organic frameworks[J]. Science, 2003, 300(5622): 1127-1129.
[13] ZHAO X, XIAO B, FLETCHER A J, et al. Hysteretic adsorption and desorption of hydrogen by nanoporous metal-organic frameworks[J]. Science, 2004, 306(5698): 1012-1015.
[14] LEE J Y, FARHA O K, ROBERTS J, et al. Metal-organic framework materials as catalysts[J]. Chemical Society Reviews, 2009, 38(5): 1450-1459.
[15] STAVILA V, TALIN A A, ALLENDORF M D. MOF-based electronic and opto-electronic devices[J]. Chemical Society Reviews, 2014, 43(16): 5994-6010.
[16] LOERA-SERNA S, OLIVER-TOLENTINO M A, DE LOURDES LPEZ-NU′EZ M, et al. Electrochemical behavior of [Cu3(BTC)2] metal-organic framework: the effect of the method of synthesis[J]. Journal of Alloys and Compounds, 2012, 540: 113-120.
[17] KRENO L E, LEONG K, FARHA O K, et al. Metal-organic framework materials as chemical sensors[J]. Chemical Reviews, 2011, 112(2): 1105-1125.
[18] ACHMANN S, HAGEN G, KITA J, et al. Metal-organic frameworks for sensing applications in the gas phase[J]. Sensors, 2009, 9(3): 1574-1589.
[19] LIU J, CHEN L, CUI H, et al. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis[J]. Chemical Society Reviews, 2014, 43(16): 6011-6061.
[20] HU Z, DEIBERT B J, LI J. Luminescent metal-organic frameworks for chemical sensing and explosive detection[J]. Chemical Society Reviews, 2014, 43(16): 5815-5840.
[21] PERRIER M, KENOUCHE S, LONG J, et al. Investigation on NMR relaxivity of nano-sized cyano-bridged coordination polymers[J]. Inorganic Chemistry, 2013, 52(23): 13402-13414.
[22] ROESKY H W, ANDRUH M. The interplay of coordinative, hydrogen bonding and π-π stacking interactions in sustaining supramolecular solid-state architectures: A study case of bis (4-pyridyl)-and bis (4-pyridyl-N-oxide) tectons[J]. Coordination Chemistry Reviews, 2003, 236(1): 91-119.
[23] LI X F, HAN Z B, CHENG X N, et al. Studies on the radii dependent lanthanide self-assembly coordination behaviors of a flexible dicarboxylate ligand[J]. Inorganic Chemistry Communications, 2006, 9(11): 1091-1095.
[24] LI J R, YU Q, TAO Y, et al. Magnetic canting or not? two isomorphous 3D Co II and Ni II coordination polymers with the rare non-interpenetrated (10, 3)-d topological network, showing spin-canted antiferromagnetism only in the Co II system[J]. Chemical Communications, 2007(22): 2290-2292.
[25] DANESHFAR R, KLASSEN J S. Arrhenius activation parameters for the loss of neutral nucleobases from deprotonated oligonucleotide anions in the gas phase[J]. Journal of the American Society for Mass Spectrometry, 2004, 15(1): 55-64.
[26] TANCREZ N, FEUVRIE C, LEDOUX I, et al. Lanthanide complexes for second order nonlinear optics: evidence for the direct contribution of electrons to the quadratic hyperpolarizability 1[J]. Journal of the American Chemical Society, 2005, 127(39): 13474-13475.
[27] HUGHES C E, REDDY G N M, MASIERO S, et al. Determination of a complex crystal structure in the absence of single crystals: analysis of powder X-ray diffraction data, guided by solid-state NMR and periodic DFT calculations, reveals a new 2′-deoxyguanosine structural motif[J]. Chemical Science, 2017, 8(5): 3971-3979.
[28] ZHANG D, ZHANG R, LI J, et al. Two new 2D coordination polymers constructed from 2, 6-dimethylpyridine-3, 5-dicarboxylic acid ligands and alkaline earth metals (Sr and Ba)[J]. Inorganic Chemistry Communications, 2013, 35: 307-310.
[29] RUI-FEN D, FENG L, FU-WEI L, et al. Stabilization variation of organic conductor surfaces induced by π-π stacking interactions[J]. Chinese Physics B, 2012, 21(5): 056801.
[30] POATER J, SWART M, BICKELHAUPT F M, et al. B-DNA structure and stability: the role of hydrogen bonding, π-π stacking interactions, twist-angle, and solvation[J]. Organic & Biomolecular Chemistry, 2014, 12(26): 4691-4700.
[31] HU Z, DEIBERT B J, LI J. Luminescent metal-organic frameworks for chemical sensing and explosive detection[J]. Chemical Society Reviews, 2014, 43(16): 5815-5840.
[32] SHUSTOVA N B, COZZOLINO A F, REINEKE S, et al. Selective turn-on ammonia sensing enabled by high-temperature fluorescence in metal-organic frameworks with open metal sites[J]. Journal of the American Chemical Society, 2013, 135(36): 13326-13329.
[33] JAYARAMULU K, KANOO P, GEORGE S J, et al. Tunable emission from a porous metal-organic framework by employing an excited-state intramolecular proton transfer responsive ligand[J]. Chemical Communications, 2010, 46(42): 7906-7908.
[34] DOUVALI A, PAPAEFSTATHIOU G S, GULLO M P, et al. Alkaline earth metal ion/dihydroxy-terephthalate MOFs: Structural diversity and unusual luminescent pro-perties[J]. Inorganic Chemistry, 2015, 54(12): 5813-5826.
[35] ZHOU Y, YOON J. Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids[J]. Chemical Society Reviews, 2012, 41(1): 52-67.
date: 2017-06-26.
Synthesis,structure,characterizationandfluorescencepropertiesofpolymersbasedoncalcium(Ⅱ)andazoxybenzene-3,3′,5,5′-tetracarboxyicacid
CHEN Yi1, DU Yi1, CAI Ting1, YANG Lele1, HAN Yuyang1, LU Yunxia2, SHAO Caiyun1, YANG Lirong1*
(1.HenanKeyLaboratoryofPolyoxometalateChemistry,CollegeofChemistryandChemicalEngineering,HenanUniversity,Kaifeng475004,Henan,China; 2.LibraryofHenanUniversity,Kaifeng475001,Henan,China)
A sable metal-organic framework of Ca (Ⅱ), namely, {[Ca(atba)4·2H2O]·H2O}n(H4atba=azoxybenzene-3,3′,5,5′-tetracarboxyic acid) has been synthesized under solvothermal conditions. The complex was characterized by single crystal X-ray analysis, IR spectroscopy and X-ray powder diffraction. Structural determination shows that the complex presents a 1D (one-dimensional) uniform Ca-carboxylate chain based on the building unit of {Ca2(CO2)2}, thereafter, the 1D chains are connected to form a 2D (two-dimensional) layered structure by ligands. These 2D layers are further linked into 3D (three-dimensional) network via covalent bonding. Luminescent properties of the complex have been studied at ambient temperature. The complex shows selective response toL-Histidine, suggesting that it may be promising luminescent selective recognition sensor forL-Histidine.
metal-organic framework; hydrothermal synthesis; structural characterization; luminescent property
O627.1DocumentcodeA
1008-1011(2017)05-0548-08
This research is financially supported by the Natural Science Foundation of Henan Province of China (Nos. 162300410010 and 13A150056).
Metal-organic frameworks (MOFs), also termed as porous coordination polymers (PCPs), are fascinating materials that are both fundamentally important and technologically relevant[1-5]. As indicated by the name, MOFs are consist of inorganic metal ions or metal-containing clusters (secondary building units or SBUs) and organic ligands via metal coordination bonds, and this mode formed the porous crystalline solid which possess the coordination mode of conjugate bridging ligands and the topological features the geometry of metal centers. Owing to the special structure, it’s significant to select suitable ligands with fixed geometry and variable bonding modes for designing and synthetizing coordination polymers with interesting geometric configurations[6-9]. Based on the intrinsic permanent porous interpenetration networks, various functionalities and potential applications in numerous areas can be accessed, such as gas storage and separation, heterogeneous catalysis, guest-exchange, molecular recognition, magnetic properties and selective luminescent probes[10-18]. Given the nearly limitless choices of metal and ligand combinations, MOFs can thrive on structural diversity, tunable chemical and physical properties[19-20]. Much effort has been focused on coordination polymers for decades, this filed has got enormous development, especially in lanthanide- and transition-metal-based coordination polymers. The assembly of the lanthanide ions and the transition metal ions in combination with organic linkers already could be systematically investigated. Nevertheless, the coordination polymers of the alkaline-earth metal ions still remain much less developed, and only several coordination polymers have been reported[21-23]. Following our longstanding research on the synthesis and separation of novel coordination polymers, in this work, we have successfully synthesized the complex {[Ca(atba)4·2H2O]·H2O}nbased on alkaline earth metal under hydrothermal conditions.
[责任编辑:刘红玲]

