光分析方法测定六价铬离子的研究进展
郑 阳,吴一微
(湖北师范大学化学化工学院,湖北 黄石 435002)
光分析方法测定六价铬离子的研究进展
郑 阳,吴一微
(湖北师范大学化学化工学院,湖北 黄石 435002)
主要从光分析的角度综述了自2015年以来六价铬离子的八种测试方法,详细比较了这些检测方法的优缺点,并简要展望了六价铬离子测试的发展趋势。
六价铬离子;光分析方法;综述
1 六价铬离子的概述
地壳中的铬元素和各种铬盐作为重要的工业原料,广泛用于采矿、冶金、制革、纺织印染、电镀以及木材防腐等领域,导致铬的环境污染,进而通过生物链进入生物体内,随着铬离子的毒性、迁移和生物积累研究的深入,人们逐渐意识到铬的生物毒性与其总量和形态分布均有关[1]。无机铬主要是以三价和六价的形式存在,一般说来铬(VI)的毒性是铬(III)的几百倍[2,3],其中,六价铬会引起机体DNA变性,且在生物体内以CrO42-和HCrO4-的形式穿过细胞膜,在谷胱甘肽作用下还原为铬(III),产生铬(V)中间体及活性氧自由基(或羟基自由基),对生物体造成严重而持久的危害,是公认的有毒环境污染物[4]和致癌物[5,6]。因此,建立简单、灵敏高效检测实际样品中六价铬的方法受到越来越多分析化学家们的重视,本文主要从光分析的角度综述比较了自2015年以来六价铬离子的八种测试方法的优缺点。
2 铬离子的测定方法
自2015年来,用于检测铬(VI)的光分析方法主要包括原子吸收光谱法[7-18]、电感耦合等离子体发射光谱法[19,20]、电感耦合等离子质谱法[21-23]、分子荧光光谱法[24-29]、X射线荧光光谱法[30-32]、拉曼散射光谱分析法[33]、紫外-可见分光光度法[34]和比色分析法[35]等,综述如下。
从表1可知,尽管AAS方法具有选择性好、仪器廉价、快速简便的优点,但实际样品中六价铬的含量较低,且存在复杂基体效应,AAS不能直接用于检测实际样品中痕量六价铬,因此,检测前,作者都对样品进行了预分离富集[7,8,10,12,13]。例如:在酸性介质中,Cr6+以HCrO4-阴离子形式存在,而Cr3+以阳离子形式存在,Rossi[7]等将N-甲基-D-葡糖胺固定在交换柱上,利用带正电的氮原子选择性吸附HCrO4-从而达到与Cr3+分离的目的;氧化石墨烯(GO),由于具有单层sp2杂化碳原子的二维蜂巢平面结构,大的比表面积,优良的理化性能,基面和板边众多的吸氧官能团等优势,被用作新型固相萃取材料[14,15]分离富集铬; 引入亲水基团的GO用于铬的分离富集时具有高吸附容量、 好的选择性以及快速吸附动力学性质[16,17]; 此外, 功能化纳米磁性氧化石墨烯在分离富集铬时可提高其分散性[15,18]。
2.1原子吸收光谱法
原子吸收光谱法(Atomic Absorption Spectrometry, AAS)是一种常规的单元素分析方法,常常被广泛用于铬的定量分析,近三年,用于铬离子的分析检测的原子吸收光谱法情况如表1.
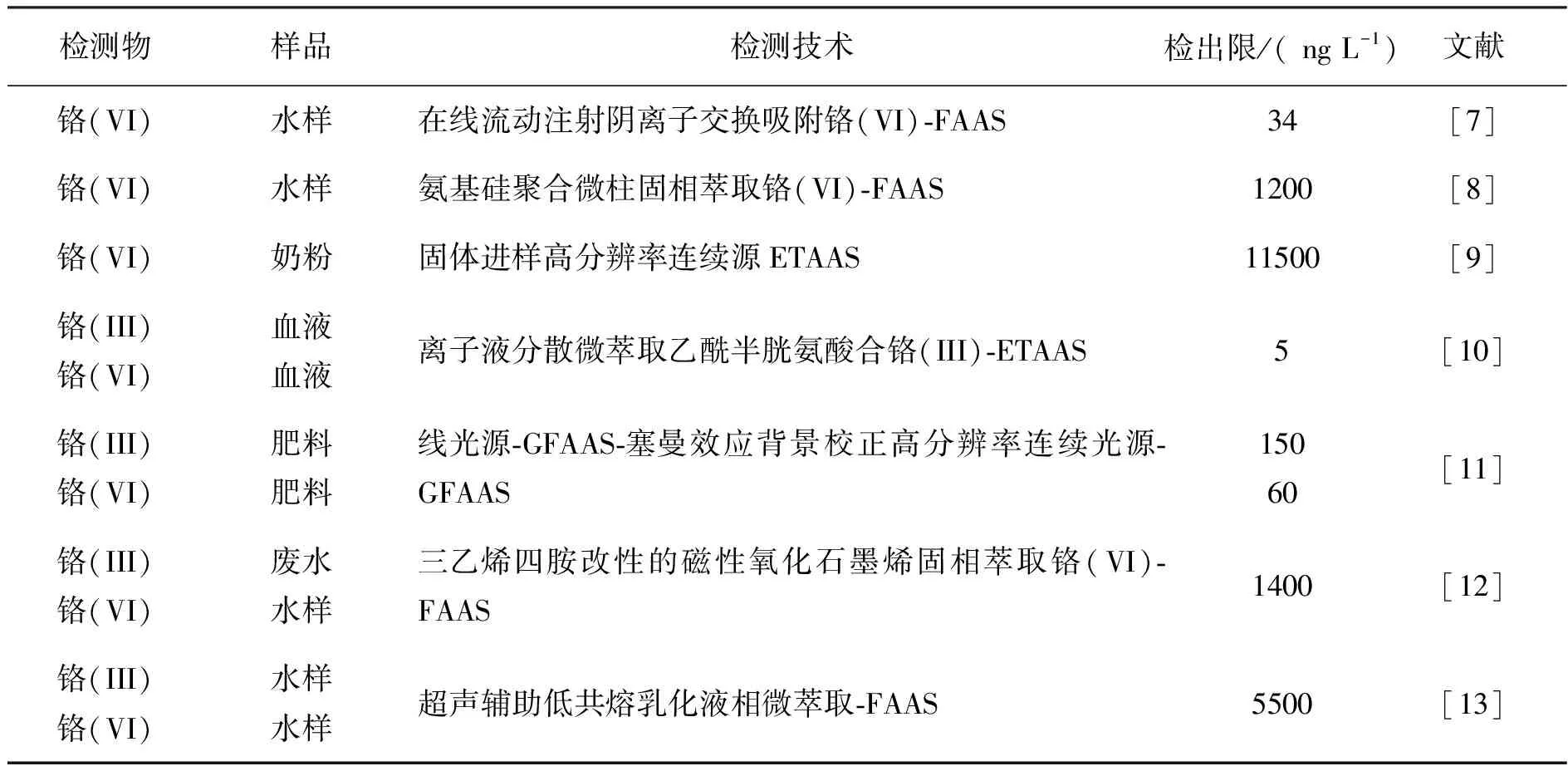
表1 原子吸收光谱法检测铬离子的情况
2.2电感耦合等离子体原子发射光谱法
电感耦合等离子体原子发射光谱法(Inductively Coupled Plasma-Optical Emission Spectrometer, ICP-OES)是一种多元素同时测定的方法,也常被用于六价铬的测定。Vu等[19]利用GO有效吸附重金属离子的优越性能,设计合成了一种外包海藻酸钠分子的磁性氧化石墨烯纳米粒子,采用这种高性能的绿色化学吸附剂,成功检测了废水中的铬(VI),该方法具有快速吸附磁分离的优点,且适用于高盐含量的工业废水中无机铬的分析。Weibel等[20]探讨了不同样品基体对富铬土壤、水泥、煤灰、粉尘中萃取铬(VI)可能产生的影响。电感耦合等离子体原子发射光谱法虽然精密度好,线性动态范围宽,基体干扰少,但运行费用较高。
2.3电感耦合等离子体质谱法
电感耦合等离子体质谱法(Inductively Coupled Plasma-Mass Spectrometry,ICP-MS)是一种多元素同时测定的质谱型分析技术之一,多被用作同位素检测和痕量元素形态分析。将其与高效液相色谱(HPLC)联用,分离铬(VI)和铬(III)后检出,是目前分析检测痕量铬(VI)的灵敏方法。Jia等[21]利用有机共聚物在线固相萃取、液相色谱分离铬(VI)和铬(III),ICP-MS检测奶制品、面粉、贝类、果汁冲剂中的铬(VI),方法检出限6.8 ngL-1;ICP-MS测定前利用离子交换色谱法分离铬(VI)和铬(III)[22];Leese等[23]建立了ICP-MS测定工厂工人的呼吸冷凝液和饮用水中铬(VI)含量的新方法。
与传统无机分析技术相比,ICP-MS具有高灵敏度、低检测限,进样少、分析速度快、分析精密度和准确度高的优点,常用于超痕量重金属离子的检测,但仪器昂贵,运行成本高。
2.4分子荧光光谱法
分子荧光光谱法(Molecular fluorescence spectrometry, MFS)也叫荧光分光光度法,是借助荧光染料或发荧光的物质产生反映该物质特性的一种荧光光谱分析技术。同为分子光谱法,相比于紫外可见分光光度法(UV-vis),MFS最大的优点就是灵敏度高和选择性好,对微量物质的检测可达到10-10数量级,比UV-vis灵敏了2~3个数量级。近三年,运用分子荧光光谱法检测铬离子的方法如表2.
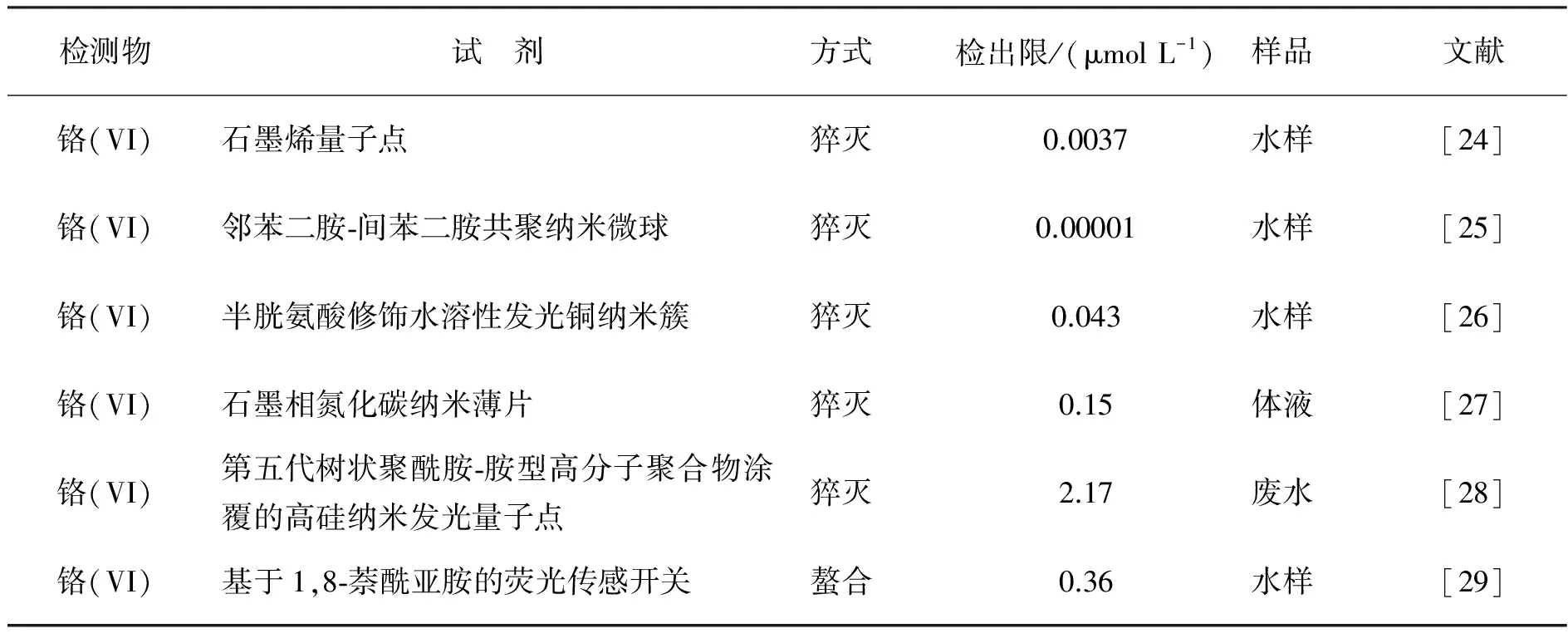
表2 分子荧光光谱法检测铬离子的情况
从表2中可见,对于本身不发荧光的铬(VI)离子而言,合适的荧光探针是测定铬(VI)的关键因素,检测机理主要基于以下两方面:利用铬(VI)对荧光探针的猝灭作用[24-28],或利用铬(VI)与有机试剂的螯合作用形成荧光物质[29]。
2.5X射线荧光光谱法
X射线荧光光谱法(X-ray fluorescence spectrometry, XRF)基于核内层电子跃迁建立起来的元素分析方法。Figueiredo等[30]利用XRF对药品和营养剂中铬(VI)的检测的可行性进行了研究,实验证明该方法符合欧洲药典规定的验证要求。其中全反射X射线荧光光谱法(Total reflection X-ray fluorescence spectrometry, TXRF)不仅具有背景低、信噪比高的特点,而且光线入射角和反射角可小到基体效应忽略不计,这也就为XRF超痕量分析开创了一个美好前景。Bahadir等[31]基于液液分散微萃取和TRXF,成功检测了水溶液中的铬(VI);Romero等[32]利用高弹性的石墨烯膜预浓缩富盐溶液中的铬(VI),结合TRXF检测,方法灵敏、选择性好,检出限为0.08 μg L-1.
2.6拉曼光谱分析法
拉曼光谱 (Raman spectra)是一种散射光谱分析方法,表面增强拉曼散射光谱(Surface Enhanced Raman Scattering, SERS)是对吸附在胶质金属微球表面的粒子进行分析的表面光谱技术。在SERS技术中,基底开发制备是关键,金属基底等离子体共振造成拉曼光谱信号可增强至3~6个数量级,灵敏度大大提高。Lv等[33]研究了涂覆有2~6 nm TiO2的Fe3O4@Au粒子光催化-SERS检测铬(VI),该方法为现场的快速分析铬(VI)提供了新的策略。拉曼光谱分析法具有灵敏度高、响应快及能分析待测物结构指纹信息等优点,吸引了越来越多的科研人员致力于这方面的研究。
2.7紫外-可见分光光度法
紫外-可见分光光度法(UV-vis Spectrophotometry)是基于物质分子对200-760 nm光谱区内光辐射吸收特性,建立的对物质定性、定量和结构分析的方法。Sereshti等[34]利用3-氨丙基三乙氧基硅修饰的热还原石墨烯萃取环境水中的铬(VI),与二苯碳酰二肼形成铬(VI)的络合物,最大吸收波长为540 nm,该方法成功用于检测自来水、河流、污水和地下水中的痕量铬(VI)。紫外-可见分光光度法仪器设备便宜、操作简便快速,但检测铬(VI)的灵敏度度不高,因此需要结合一些分离富集技术。
2.8比色分析法
比色分析法是基于铬(VI)的颜色深浅与离子浓度存在的线性关系而建立起来的半定量分析方法。Guo等[35]开发出了一种灵敏检测铬(VI)的纸基比色传感技术,将牛血清白蛋白修饰的金纳米粒子截获在硅烷化二氧化钛修饰的滤纸上,纸基上的纳米金与铬(VI)反应逐渐浸出,出现明显颜色变化从而达到可视化分析检测铬(VI)的目的。虽然那些基于大型设备的方法(如ICP-MS、AAS、XRF等)通常具有优越的精度和稳定性,但它们不适合实时现场监测。基于金属纳米粒子的比色法不需要复杂的仪器和信号识别,仅通过肉眼识别颜色变化来半定量分析,引起了越来越多的关注。
3 结语与展望
近年来,随着电镀、制革、冶金等工业发展,铬(VI)对环境的污染和人体健康的危害越来越受到人们的广泛关注,铬(VI)的鉴定分离分析研究一直在进行。本综述针对“光分析方法用于铬(VI)检测分析”详细总结了火焰原子吸收光谱法、电感耦合等离子体发射法、电感耦合等离子体质谱法、分子荧光光谱法、X射线荧光光谱法、拉曼光谱分析法、紫外可见分光光度法、比色分析法这8种光分析方法近三年用于铬(VI)研究的发展近况。八种分析方法中仅比色分析实现了简便快速,低成本的实时现场检测铬(VI),但灵敏度低;其他大型仪器设备如ICP-MS虽然仪器设备昂贵,操作运行成本高,但检出限低、灵敏度高、线性范围宽,不仅适用于生物医药、生活用水、食品等实际样品中超痕量铬(VI)的定性定量检测,还适用于细胞、肿瘤组织和体液中超痕量的铬(VI)的检测;具有灵敏度高、选择性好的分子荧光光谱法也倍受关注,但必须找到特定的荧光探针或者是能与铬(VI)形成螯合物的有机试剂。这些都是铬(VI)测定的未来发展研究方向。
[1]Rosales R M, Faz A, Gómez-Garrido M, et al. Geochemical speciation of chromium related to sediments properties in the riverbed contaminated by tannery effluents [J]. J Soil Sediment, 2016: 1~12.
[2]Gill T, Singh S K, Kumar P A. Treatment of Heavy Metals Contaminated industrial waste water by Functionalized Polymers [J]. Int J Adv Research, 2015, (3): 178~181.
[3]Hokkanen S, Bhatnagar A, Repo E, et al. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution [J]. Chem Eng J, 2016, 283: 445~452.
[4]Najafabadi H H, Irani M, Rad L R, et al. Removal of Cu2+, Pb2+and Cr6+from aqueous solutions using a chitosan/graphene oxide composite nanofibrous adsorbent [J]. RSC Adv, 2015, (5): 16532~16539.
[5]Erdem E, Güng rm H, K ln arslan R. The investigation of some properties of cement and removal of water soluble toxic chromium (VI) ion in cement by means of different reducing agents [J]. Constr Build Mater, 2016, 124: 626~630.
[6]Markiewicz B, Komorowicz I, Bara kiewicz D. Accurate quantification of total chromium and its speciation form Cr (VI) in water by ICP-DRC-IDMS and HPLC/ICP-DRC-IDMS [J]. Talanta, 2016, 152: 489~497.
[7]Rossi E, Errea M I, de Cortalezzi M M F, et al. Selective determination of Cr(VI) by on-line solid phase extraction FI-SPE-FAAS using an ion exchanger resin as sorbent: An improvement treatment of the analytical signal [J]. Microchem J, 2017, 130: 88~92.
[8]Shirkhanloo H, Khaligh A, Golbabaei F, et al. On-line micro column preconcentration system based on amino bimodal mesoporous silica nanoparticles as a novel adsorbent for removal and speciation of chromium (III, VI) in environmental samples [J]. Iran J Environ Healt, 2015, 13: 1.
[9]Silva A S, Brandao G C, Matos G D, et al. Direct determination of chromium in infant formulas employing high-resolution continuum source electrothermal atomic absorption spectrometry and solid sample analysis [J]. Talanta, 2015, 144: 39~43.
[10]Shirkhanloo H, Ghazaghi M, Mousavi H Z. Chromium speciation in human blood samples based on acetyl cysteine by dispersive liquid-liquid biomicroextraction and in-vitro evaluation of acetyl cysteine/cysteine for decreasing of hexavalent chromium concentration [J]. J Pharmaceut Biomed, 2016, 118: 1~8
[11]Borges A R, Fran,cois L L, Becker E M, et al. Method development for the determination of chromium and thallium in fertilizer samples using graphite furnace atomic absorption spectrometry and direct solid sample analysis [J]. Microchem J, 2015, 119: 169~175.
[12]V Islam A, Ahmad H, Zaidi N, et al. A graphene oxide decorated with triethylenetetramine-modified magnetite for separation of chromium species prior to their sequential speciation and determination via FAAS [J]. Microchim Acta, 2016, 183: 289~296.
[13]Yilmaz E, Soylak M. Ultrasound assisted-deep eutectic solvent based on emulsification liquid phase microextraction combined with microsample injection flame atomic absorption spectrometry for valence speciation of chromium (III/VI) in environmental samples [J]. Talanta, 2016, 160: 680~685.
[14]Hu B, He M, Chen B. Nanometer-sized materials for solid-phase extraction of trace elements [J]. Anal Bioanal Chem, 2015, 407: 2685~2710.
[15]Sitko R, Zawisza B, Malicka E. Graphene as a new sorbent in analytical chemistry [J]. Trace-Trend Anal Chem, 2013, 51: 33~43.
[16]Islam A, Ahmad H, Zaidi N, et al. Graphene oxide sheets immobilized polystyrene for column preconcentration and sensitive determination of lead by flame atomic absorption spectrometry [J]. ACS Appl Mater Inter, 2014, (6): 13257~13265.
[17]Sayar O, Mehrani K, Hoseinzadeh F, et al. Comparison of the performance of different modified graphene oxide nanosheets for the extraction of Pb(II) and Cd(II) from natural samples [J]. Microchim Acta, 2014, 181: 313~320.
[18] Li J, Zhang S, Chen C, et al. Removal of Cu(II) and fulvic acid by graphene oxide nanosheets decorated with Fe3O4 nanoparticles [J]. ACS Appl Mater Inter, 2012, (4): 4991~5000.
[19] Vu H C, Dwivedi A D, Le T T, et al. Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr(VI) and As(V) from aqueous solutions: Role of crosslinking metal cations in pH control [J]. Chem Eng J, 2017, 307: 220~229.
[20]Weibel G, Waber H N, Eggenberger U, et al. Influence of sample matrix on the alkaline extraction of Cr(VI) in soils and industrial materials [J]. Environ Earth Sci, 2016, 75: 1~14.
[21]Jia X, Gong D, Xu B, et al. Development of a novel, fast, sensitive method for chromium speciation in wastewater based on an organic polymer as solid phase extraction material combined with HPLC-ICP-MS [J]. Talanta, 2016, 147: 155~161.
[22]Vacchina V, de la Calle I, Séby F. Cr(VI) speciation in foods by HPLC-ICP-MS: investigation of Cr(VI)/food interactions by size exclusion and Cr(VI) determination and stability by ion-exchange on-line separations [J]. Anal Bioanal Chem, 2015, 407: 3831~3839.
[23]Leese E, Morton J, Gardiner P H E, et al. Development of a method for the simultaneous detection of Cr(III) and Cr(VI) in exhaled breath condensate samples using μLC-ICP-MS [J]. J Anal Atom Spectrom, 2016, 31: 924~933.
[24]Huang S, Qiu H, Zhu F, et al. Graphene quantum dots as on-off-on fluorescent probes for chromium (VI) and ascorbic acid [J]. Microchim Acta, 2015, 182: 1723~1731.
[25]Song X, Sun H, Yang S, et al. Synthesis of photoluminescent o-phenylenediamine-m-phenylenediamine copolymer nanospheres: An effective fluorescent sensing platform for selective and sensitive detection of chromium (VI) ion [J]. J Lumin, 2016, 169: 186~190.
[26]Cui M, Song G, Wang C, et al. Synthesis of cysteine-functionalized water-soluble luminescent copper nanoclusters and their application to the determination of chromium (VI) [J]. Microchim Acta, 2015, 182: 1371~1377.
[27]Rong M, Lin L, Song X, et al. Fluorescence sensing of chromium (VI) and ascorbic acid using graphitic carbon nitride nanosheets as a fluorescent “switch” [J]. Biosens Bioelectron, 2015, 68: 210~217.
[28]Campos B B, Algarra M, Alonso B, et al. Fluorescent sensor for Cr(VI) based in functionalized silicon quantum dots with dendrimers [J]. Talanta, 2015, 144: 862~867.
[29]Zhang Z, Sha C, Liu A, et al. Highly selective detection of Cr(VI) in water matrix by a simple 1, 8-naphthalimide-based turn-on fluorescent sensor [J]. J Fluoresc, 2015, 25: 335~340.
[30]Figueiredo A, Fernandes T, Costa I M, et al. Feasibility of wavelength dispersive X-ray fluorescence spectrometry for the determination of metal impurities in pharmaceutical products and dietary supplements in view of regulatory guidelines [J]. J Pharmaceut Biomed, 2016, 122: 52~58.
[31]Bahadir Z, Bulut V N, Hidalgo M, et al. Cr speciation in water samples by dispersive liquid-liquid microextraction combined with total reflection X-ray fluorescence spectrometry [J]. Spectrochim Acta A B, 2016, 115: 46~51.
[32]Romero V, Costas-Mora I, Lavilla I, et al. Graphene membranes as novel preconcentration platforms for chromium speciation by total reflection X-ray fluorescence [J]. RSC Adv, 2016, (6): 669~676.
[33]Lv B, Sun Z, Zhang J, et al. Multifunctional satellite Fe3O4-Au@TiO2nano-structure for SERS detection and photo-reduction of Cr(VI) [J]. Colloid Surface A, 2016, 513: 234~240.
[34]Sereshti H, Farahani M V, Baghdadi M. Trace determination of chromium (VI) in environmental water samples using innovative thermally reduced graphene (TRG) modified SiO2 adsorbent for solid phase extraction and UV-vis spectrophotometry [J]. Talanta, 2016, 146: 662~669.
[35]Guo J, Huo D, Yang M, et al. Colorimetric detection of Cr(VI) based on the leaching of gold nanoparticles using a paper-based sensor [J]. Talanta, 2016, 161: 819~825.
Abstract: Eight kinds of optical analytical methods employed in detecting chromium (VI) ion were reviewed since 2015 years. The advantages and disadvantages of these methods were compared in detail, and the developing prospection of optical methods used to determine chromium ion is also proposed.
Keywords: chromium (VI) ion; optical analysis method; review
Progressofopticalanalyticalmethodsemployedindetectingchromium(VI)ion
ZHENG Yang, WU Yi-wei
(Department of Chemistry and Chemical Engineering, Hubei Normal University, Huangshi 435002)
O657.3
A
2096-3149(2017)03- 0047-06
10.3969/j.issn.2096-3149.2017.03.009
2017—07—03
湖北省教育厅重点基金(No.D20130501)
郑阳(1991— ),女,湖北荆门人,硕士生,研究方向为分离分析技术.
吴一微(1971— ),女,湖北武穴人,教授.

