土壤还原过程对氯代有机污染物还原脱氯的影响与机制
冯曦,朱敏,何艳,*
1. 浙江大学土水资源与环境研究所,杭州3100582. 浙江省农业资源与环境重点实验室,杭州310058
土壤还原过程对氯代有机污染物还原脱氯的影响与机制
冯曦1,2,朱敏1,2,何艳1,2,*
1. 浙江大学土水资源与环境研究所,杭州3100582. 浙江省农业资源与环境重点实验室,杭州310058
自然环境中,大多数氯代有机污染物厌氧还原脱氯反应是与土壤环境中一些生源要素的生物化学还原过程相伴生。有机污染物的种类、生物有效性以及毒性能够显著影响这些生源要素的转化,反过来,土壤中活跃的氧化还原反应也可以显著影响有机污染物的动力学转化过程。本文从氧化还原顺序上综述了反硝化过程、铁还原过程、硫酸盐还原过程和产甲烷过程对氯代有机污染物厌氧还原脱氯过程的影响与作用机制,旨在为氯代有机污染物在厌氧环境中还原脱氯的过程与机理的进一步研究、以及还原脱氯与微生物介导的生源要素氧化还原过程的耦合作用机制的揭示提供参考。
还原脱氯;反硝化;铁还原;硫还原;产甲烷
Received14 January 2017accepted30 April 2017
Abstract: Under anaerobic conditions, reductive dechlorination of chlorinated organic pollutants is thought to be coupled to biogeochemical processes of some biogenic elements in soil. The variety, bio-availability and toxicity of organic pollutants can significantly affect the transformation of the biogeochemical processes. Correspondingly, soil natural active redox processes can also significantly affect the dynamics of organic pollutant transformation. Based on the classical redox reactions sequence, the effects and mechanisms of denitrification process, dissimilatory iron reduction process, sulfate reduction process and methanogenic process on reductive dechlorination of chlorinated organic pollutants were reviewed and discussed in this paper. We also provided reference for the further study to disclose more in-depth mechanism regarding reductive dechlorination and their interaction with the co-occurring natural redox processes in soil under anaerobic environment.Keywords: reductive dechlorination; denitrification; dissimilatory iron reduction; sulfate reduction; methanogenesis
氯代有机物(chlorinated organic compounds,COCs)指的是氯原子取代了脂肪烃、芳香烃及其衍生物中的一个或几个氢原子后的产物,其种类繁多,主要包括氯代烃(chlorinated hydrocarbon)、有机氯农药(organochlorine pesticides,OCPs)、氯代苯(chlorinated biphenyls,CBs)、多氯联苯(polychlorinated biphenyls, PCBs)、氯代苯酚(chlorinated phenols,CPs)等,被广泛应用于化工、医药、农药、制革等行业。大多数COCs具有较低的水溶性和较高的辛醇-水分配系数,一旦进入环境,很难在短时间内自然降解,并能够进行长距离迁移和沉积,容易在生物体内聚集,可通过食物链积累,造成严重的环境污染和长期的生态破坏后果。
COCs与一般的芳香族化合物相比,其可生化性大大降低,这是因为氯原子引起了化合物分子结构特性的改变,所以脱氯是COCs生物降解的关键步骤。根据脱氯过程中的电子得失,其机理分为氧化脱氯和还原脱氯[1]。但在好氧条件下高氯代有机污染物很难被微生物利用[2],氯离子会阻碍双加氧酶对苯环的攻击,使得大部分好氧微生物降解高氯代污染物的能力和效率要低于低氯代有机污染物[3]。在缺氧条件下,一旦氯原子被脱去,其产物就对好氧的2,3-和3,4-双氧化酶等变得更为敏感[4],使氯代有机污染物形成容易进一步被好氧矿化的物质。例如氯代烯烃可以通过一系列还原脱氯作用,形成烯烃,进而转化为甲烷[5];而对于氯代芳烃,氯原子强烈的吸电子性使芳烃上电子云密度降低,在缺氧条件下,电子云密度较低的苯环在酶作用下很容易受到还原剂的亲核攻击,显示出较好的缺氧生物降解性[6]。因此,氯代有机污染物的缺氧脱氯越来越引起了研究者们的兴趣。

自然环境中,大多数氯代有机污染物厌氧还原脱氯反应在土壤环境中是与反硝化反应、铁还原反应、硫酸盐还原反应、产甲烷反应等重要的生物化学过程相伴生。有机污染物的种类、生物有效性以及毒性能够显著影响这些生源要素的转化,反过来,土壤中活跃的氧化还原对也可以显著影响氯代有机污染物的动力学转化过程[10]。随着土壤环境中的生物地球化学循环过程等热点问题的提出,土壤生源要素氧化还原循环参与条件下的有机污染物消减的调控过程及作用机制引起了国内外广大学者的关注。本文介绍了反硝化过程、铁还原过程、硫酸盐还原过程和产甲烷过程对氯代有机污染物厌氧还原脱氯过程的影响与作用机制,旨在为氯代有机污染物在厌氧环境中还原脱氯的过程与机理的进一步研究、以及还原脱氯与微生物介导的生源要素氧化还原过程的耦合作用机制的揭示提供参考。
1 反硝化过程与还原脱氯过程(Denitrification process and reductive dechlorination process)
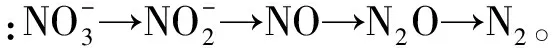


2 异化铁还原与还原脱氯过程(Dissimilatory iron reduction process and reductive dechlorination process)
铁,作为自然环境中含量最为丰富的变价金属元素,其对地球生物化学循环过程有着深远的意义。自然界中,特别是在厌氧的土壤或沉积物环境下,铁物种通常以难溶性Fe(III)氧化物的形式存在,而这些含变价元素的铁氧化物在这些厌氧环境中也是天然存在的氧化剂[30]。大量的研究结果表明,土壤中Fe(III)的还原主要是由特定的异化铁还原微生物驱动,在异化型铁还原菌的异化作用中,Fe(III)被用作电子受体,还原产生的Fe(II)可以偶联多种有机物的氧化还原。所以说Fe(III)还原是某些土壤和沉积物中有机质分解中的一个重要过程,它们在氯代有机污染物的还原转化过程中扮演着重要的角色[31]。
当电子供体限制的条件下,土壤中可作为最终电子受体同时存在的Fe(III)和COCs为获取有限的电子而表现为竞争关系。Paul等[32-33]发现三价铁矿物的存在抑制了三氯乙烯(TCE)的厌氧还原脱氯,当然,这也与铁的存在形式、数量以及环境因子如pH等有关。另外,也有研究发现,Fe(III)还原产生的吸附态Fe(II)可以强化化学的还原脱氯反应过程。吸附于铁氧化物表面的Fe(II)由于中心周围的电子云密度增加,而且吸附态Fe(II)的氧化还原电位明显低于溶解态Fe(II),提高了脱氯转化的自发性[34-36]。同时,还原产生的二价铁也可以作为还原剂将COCs还原,反应式为:Fe2++ R-Cl → Fe3++ R+Cl-,即实现了铁氧化物还原溶解反应与化学脱氯反应的交互[37]。

表1 土壤中主要还原反应的热力学顺序(pH 7.0,25 ℃)aTable 1 Thermodynamic sequence of main reduction processes in soil (pH 7.0, 25 ℃)a
在微生物的参与下,铁还原过程与氯代有机污染物还原脱氯过程的关系显得更为复杂。厌氧土壤环境中,参与还原脱氯过程的微生物主要还是以氯代有机污染物为电子受体进行能量代谢的脱氯菌,而在铁还原条件下,铁还原菌作为主要优势微生物也会相对抑制脱氯菌活性以及还原脱氯过程,主要也是通过对电子供体的竞争而产生抑制作用[32, 38]。一些铁还原菌可以直接以有机氯作为电子受体氧化电子供体进行脱氯呼吸,为有机氯化合物污染的原位修复工作提供了一条新途径。例如,Anaeromyxobacter dehalogenans strain 2CP-C除了可以利用Fe(III)作为电子受体外,还可以通过邻位脱氯过程获得能量生长[39]。Desulfuromonas chlorethenica也可以同时利用Fe(III)还原和四氯乙烯以及三氯乙烯作为电子受体进行生长[40]。作为富铁土壤环境中的典型菌属,梭菌属微生物Clostridium sp.也常在氯代化合物污染区域活跃[36, 41-42]。同时具有脱氯呼吸作用的异化铁还原菌还包括Anaeromyxobacter dehalogenes、Desulfitobacterium frappieri、Desulfitobacterium hafniense、Desulfitobacterium metallireducens、Desulfuromonas michigansis、Trichlorobacter thiogenes等[43-46]。
由于异化铁还原反应可以利用的电子供受体种类多种多样,利用Fe(II)与Fe(III)之间的这种异化还原转化过程来进行多种有机污染环境的修复也是一直以来的研究热点。有研究表明,典型的异化铁还原菌——希瓦氏菌(Shewanella)能够通过还原Fe(III)过程来提高三氯、四氯、五氯等高氯代有机化合物的脱氯转化效果[47-48]。Fe(Ⅲ)微生物还原过程成为联系2个过程的“纽带”:一方面,它可以以有机氯化合物作为电子受体直接还原脱氯;另一方面,它可为化学还原脱氯过程提供生物源Fe(Ⅱ)。我们前期通过驯化不同的功能菌群发现,铁还原菌群可以通过耦合铁氧化物的还原显著提高五氯酚(PCP)的还原转化,而且对PCP还原转化能力的不同主要由菌群群落结构不同导致,其中,我们发现另一类典型的异化铁还原菌——地杆菌(Geobater)在该还原脱氯的过程中起重要作用[49]。此外,在铁氧化物和腐殖质充足的条件下,发酵型铁还原菌C. beijerinckiistrain Z可以将大量的Fe(III)还原为Fe(II),生成的吸附态Fe(II)可以显著促进PCP的脱氯过程。Wu等[50](2010)研究发现铁还原菌Comamonas koreensis CY01可以利用水铁矿、针铁矿、纤铁矿或者赤铁矿作为最终电子受体,同时促进了有机氯污染物2,4-二氯苯氧基乙酸(2,4-D)的还原。由此,铁氧化物界面发生了铁还原菌介导的异化还原溶解,并使得有机氯发生了铁物种介导的化学脱氯反应,这就形成了生物脱氯与化学脱氯的耦合反应过程(图1),进一步揭示了氯代有机污染物的生物与非生物脱氯的交互反应以及缺氧的污染区域中高氯代污染物的自然消解过程。

图1 有机氯脱氯转化的铁还原菌-铁氧化物界面交互反应机制示意图[51]Fig. 1 The mechanism describing the dechlorination of organochlorine in the dissmilatory iron-reducing system of dissimilatory iron reduction bacteria (DIRB) and iron oxide[51]
此外,一些对铁还原过程产生影响的中间物质也会间接影响到有机氯还原脱氯的过程,在这一多过程耦合的过程中,提高铁还原速率是强化还原脱氯的关键。溶铁螯合剂的使用一方面可增加反应体系中生物可利用Fe(Ⅲ)的浓度,另一方面还可以提高Fe(Ⅲ)与铁还原菌直接接触的机率,进而提高铁还原的速率[52-53]。电子穿梭体如腐殖质、类腐殖质物质如anthraquinone-2,6-disulfonate (AQDS)等[54-56],以及现在新兴的污染修复材料如可导电的石墨烯、生物炭等[57-58],提高了电子的运转效率从而强化了COCs还原脱氯过程。
3 硫酸盐还原与还原脱氯过程(Sulfate reduction process and reductive dechlorination process)


目前,硫酸盐还原作用能否促进氯代有机物的还原消减仍然是一个有争议的话题,硫酸根作为电子受体对还原脱氯影响的两面性主要取决于硫酸盐形式和浓度、微生物群落结构的差异以及非生物还原脱氯对电子供体的竞争作用等众多因素[64]。Chang等[65](2008)和Yuan等[66](2011)研究发现添加硫酸盐可使氯代烃和多环芳烃的降解率分别提高25.5%和50%~77.8%。Yoshida等[67](2007)对稻田土壤中PCP的消减研究发现,外源添加的硫酸盐浓度在20 mmol·L-1的时候会抑制PCP还原,而10 mmol·L-1的时候却不产生抑制作用。而Ehlers等[68](2006)在分批补给反应器中研究硫还原条件下硫酸盐浓度对2,4,6-三氯酚(2,4,6-TCP)还原消减的影响时发现,当硫酸根浓度在110 mg·L-1时增强了2,4,6-TCP的还原转化,而在900mg·L-1时却抑制了其还原脱氯。我们前期研究也发现,外源添加硫酸盐对PCP还原脱氯过程的影响还与土壤类型有关,在黄斑田与黑土中表现为抑制作用,而对红壤和潮土中的PCP脱氯则起到了一定的促进作用,且前者的抑制作用随硫酸根浓度的增加而增强[17]。我们认为这种抑制作用可能由两方面造成,一是外源电子受体与PCP同作为电子受体的形成竞争,二是硫酸根的氧化性会提高环境氧化还原电位初始值并减缓了其下降速度。因此,在缺氧条件下,土壤本底条件、电子供体的种类和数量以及硫酸根浓度等均会影响氯代有机污染物的还原脱氯过程。

图2 硫酸盐还原菌的代谢过程[63]Fig. 2 The metabolic process of sulfate reducing bacteria[63]
Drzyzga等[69](2001)发现,在以乳酸盐为碳源且无硫酸盐作为电子受体的恒化器培养条件下,硫还原菌Desulfovibrio sp.与脱氯菌Desulfitobacterium frappieri TCE1仍可以共存,作者认为二者可以通过氢气和电子传递形成互营关系并降解四氯乙烯。也有研究发现,在以丁酸盐(H2释放基质)作为碳源的厌氧环境中,SRB会通过对H2的竞争来抑制四氯乙烯的还原脱氯,而且二者竞争的激烈程度取决于氢气的释放和消耗速率[70]。但也有研究表明,硫酸盐还原反应对H2的竞争并不是抑制脱氯的原因[71]。此外,有研究发现SRBs代谢还原产生的产物H2S反过来也会对微生物产生不利的影响,当H2S的浓度达到547 mg·L-1时会完全抑制SRBs的生长[72]。而硫酸盐还原过程虽然会与还原脱氯过程争夺电子,但当硫酸盐被SRB还原成H2S后,其也可能作为强还原性物质强化氯代化合物还原脱氯过程[73]。因此,碳源以及一些代谢中间产物如H2和H2S也可能成为影响硫酸盐还原过程和还原脱氯过程之间关系的因素之一。
另外,一些硫还原菌本身也可能具有还原脱氯功能,它们在氯代有机化合物消减过程中起着不可忽视的作用,这些微生物能够通过呼吸代谢PCBs、OCPs等氯代有机污染物,并从中获取自身生长所需能量[74]。已有大量研究通过筛选分离SRB或其富集物进行了还原脱氯研究,证实这些具有硫还原功能的微生物也能够直接参与脱氯[75-76]。微生物的生物脱氯代谢机制可以分为共代谢和脱氯呼吸,前者表现为微生物电子传递过程不以氯代有机物作为最终电子受体进行脱氯反应,而后者则需要以之作为最终电子受体[77],而具有脱氯功能的SRB究竟是以共代谢还是脱氯呼吸机制对进行脱氯这一关键问题目前尚不明确。
4 产甲烷过程与还原脱氯过程(Methanogenic process and reductive dechlorination process)
产甲烷(methanogensis)是一类可以将无机物或有机物在厌氧发酵条件下转化为甲烷和二氧化碳的过程。甲烷产生的过程主要可分为4步:(1)水解过程:在好氧、厌氧或兼性微生物的作用下,土壤或其他环境中沉积的高分子有机物水解为单分子(如糖苷、脂肪酸和氨基酸等);(2)酸化过程:在兼性或严格厌氧微生物的作用下,单分子化合物进一步酸解为酸性的小分子化合物(如甲酸、乙酸、丙酸等);(3)乙酸生成过程:厌氧细菌或同型乙酸细菌将酸性小分子化合物继续转化为乙酸和其他小分子H2和CO2;(4)甲烷形成过程:产甲烷菌利用少数几种C1、C2化合物(如CO2、HCOOH、CH3OH等)与H2还原生成甲烷[78]。产甲烷的发生要求体系中的氧化还原电位处于一个比较低的水平,而很多研究表明高氯代化合物还原脱氯过程的还原条件非常的严格,一般也只会发生在强还原条件下,因此,产甲烷环境也比较利于氯代有机物实现还原脱氯和进一步降解,产甲烷条件下氯代有机污染物的厌氧生物降解过程和机制也引起了诸多学者的关注。
产甲烷菌是一种严格厌氧菌,在生物发酵池、稻田、湿地,甚至一些温泉、海底热水喷口等与氧气隔绝的极端环境中,几乎都有产甲烷菌的存在。由于产甲烷菌处于有机物厌氧降解末端,但如果没有产甲烷菌分解有机酸产生甲烷的平衡作用,必然导致有机酸的积累使得发酵环境酸化,其可能最主要的作用就是产物抑制作用的消除,使还原脱氯反应能够连续进行。再者,产甲烷菌所能利用的基质有限,需要不产甲烷菌先将复杂有机物分解为简单化合物,因此,前面几步间接参与产甲烷过程的其他微生物也可能影响还原脱氯过程[79-80]。

而在以发酵型碳源乳酸作为电子供体时,Wen等[84]发现,产甲烷过程产生的中间产物H2以及产甲烷菌产生的辅酶等促进了脱氯菌Dehalococcoides的生长,从而促进了TCE的生物降解。Chen等[85]也是发现,SRB和产甲烷菌是湿地中氯仿生物降解过程中的关键微生物,通过共代谢的作用促进了氯仿的生物脱氯过程。当然,也有研究发现产甲烷过程对还原脱氯过程并无影响[86-88],因此,产甲烷过程对氯代有机污染物厌氧还原脱氯过程的作用与前面提到的氮/铁/硫还原过程类似,是多种因素影响下的综合表现。
5 结论与展望(Conclusion and prospect)
综上所述,厌氧条件下,在自然界中,有多种还原环境存在,如典型的反硝化环境、铁还原环境、硫酸盐还原环境以及产甲烷环境,很多研究发现一些难降解的三氯、四氯、五氯等高氯代有机化合物均能在这些还原环境中发生。土壤中天然存在的不同形态的氮、铁、硫等活跃的氧化还原能够显著影响这些难降解有机污染物的还原脱氯过程,反过来,有机污染物的种类、生物有效性以及毒性能够显著影响这些生源要素的转化。二者的关系可能表现为抑制、促进或无关,土壤本底条件、电子供/受/穿梭体的种类和数量、以及参与代谢的微生物及中间产物等均会影响氯代有机污染物的还原脱氯过程。

此外,在厌氧环境中,氯代有机污染物的厌氧还原转化与碳、氮、铁、硫等生源要素生物化学循环过程本质上均是微生物呼吸代谢介导的电子传递过程,这种过程受到电子供、受体之间协调性和竞争性的影响。但目前对于这种过程如何协调厌氧环境中微生物与土壤环境之间的交互效应、诱导微生物种群和关键功能菌的定向变化、并通过改变电子传递路径最终影响氯代有机污染物在厌氧环境中的还原转化过程和机制还缺乏系统认识。特别是如何基于改变电子供/受/穿梭体的数量(浓度)、种类等途径调控电子传递流向,协调污染物快速削减与铁硫等还原性毒害物质产生、以及与温室气体排放的关系,实现多赢的修复目标,在未来的土壤污染控制与修复研究中显得尤为重要。
[1] 王菊思, 赵丽辉. 合成有机化合物的生物降解性研究[J]. 环境化学, 1993, 12(3): 161-172
Wang J S, Zhao L H. A survey on the microbial degradation of synthetic organic compounds [J]. Environmental Chemistry, 1993, 12(3): 161-172 (in Chinese)
[2] Atuanya E I, Purohit H J, Chakrabarti T. Anaerobic and aerobic biodegradation of chlorophenols using UASB and ASG bioreactors [J]. World Journal of Microbiology & Biotechnology, 2000, 16(1): 95-98
[3] Bergauer P, Fonteyne P, Nolard N, et al. Biodegradation of phenol and phenol-related compounds by psychrophilic and cold-tolerant alpine yeasts [J]. Chemosphere, 2005, 59(7): 909-918
[4] 孟紫强. 环境毒理学[M]. 北京: 中国环境科学出版社, 2000: 202-207
[5] 傅旭庆, 吕德伟, 汪叔雄. 生物处理含氯代脂肪烃废水的研究进展[J]. 环境科学,1994, 15(3): 84-87
Fu X Q, Lu D W, Wang S X. Progress in research on the biological treatment of wastewater containing chlorinated aliphatics [J]. Chinese Journal of Environmental Science, 1994, 15(3): 84-87 (in Chinese)
[6] 瞿福平, 张晓健, 吕昕, 等. 氯代芳香化合物的生物降解性研究进展[J]. 环境科学, 1997, 18(2): 74-78
Qu F P, Zhang X J, Lv X, et al. Advances on research of biodegradability for the chlorinated aromatic compounds [J]. Chinese Journal of Environmental Science, 1997, 18(2): 74-78 (in Chinese).
[7] Nielsen L P, Risgaard-Petersen N, Fossing H, et al. Electric currents couple spatially separated biogeochemical processes in marine sediment [J]. Nature, 2010, 463(7284): 1071-1074
[8] Adriaens P, Chang P R, Barkovskii A L. Dechlorination of PCDD/F by organic and inorganic electron transfer molecules in reduced environments [J]. Chemosphere, 1996, 32(2): 433-441
[9] Paul G F, Tom F, Edward F D. The microbial engines that drive earth's biogeochemical cycles [J]. Science, 2008, 320(5879): 1034-1039
[10] Achtnich C, Bak F, Conrad R. Competition for electron donors among nitrate reducers, ferric iron reducers, sulfate reducers, and methanogens in anoxic paddy soil [J]. Biology and Fertility of Soils, 1995, 19(1): 65-72
[11] Klueglein N, Kappler A. Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe (II) oxidizer Acidovorax sp. BoFeN1-questioning the existence of enzymatic Fe (II) oxidation [J]. Geobiology, 2013, 11(2): 180-190
[12] Rosswall T. The internal nitrogen cycle between microorganisms, vegetation and soil [J]. Ecological Bulletins, 1976, 22: 157-167
[13] 郭建华, 彭永臻. 异养硝化, 厌氧氨氧化及古菌氨氧化与新的氮循环[J]. 环境科学学报, 2008, 28(8): 1489-1498
Guo J H, Peng Y Z. Heterotrophic nitrification,anaerobic ammonia oxidation and archaeal ammonia oxidation in a new nitrogen cycle [J]. Acta Science Circumstantiae, 2008, 28(8): 1489-1498 (in Chinese)
[14] Philippot L. Denitrifying genes in bacterial and Archaeal genomes [J]. Biochimca Biophysica Acta, 2002, 1577(3): 355-376
[15] Moura I, Moura J J. Structural aspects of denitrifying enzymes [J]. Current Opinion in Chemical Biology, 2001, 5(2): 168-175
[16] Postma D, Jakobsen R. Redox zonation: Equilibrium constraints on the Fe (III)/SO4-reduction interface [J]. Geochimica et Cosmochimica Acta, 1996, 60(17): 3169-3175
[17] Lin J, He Y, Xu J, et al. Verticalprofiles of pentachlorophenol and the microbial community in a paddy soil: Influence of electron donors and acceptors [J]. Journal of Agricultural and Food Chemistry, 2014, 62(41): 9974-9981
[18] Klüber H D, Conrad R. Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil [J]. FEMS Microbiology Ecology, 1998, 25(3): 301-318
[19] Lee T H, Yoshimi M, Ike M, et al. Characterization of an anaerobic soil enrichment capable of dechlorinating high concentrations of tetrachloroethylene [J]. Water Science and Technology, 1997, 36(6-7): 117-124
[20] Holliger C. The anaerobic microbiology and biotreatment of chlorinated ethenes [J]. Current Opinion in Biotechnology, 1995, 6(3): 347-351
[21] Yu H, Wang Y, Chen P, et al. Effect of nitrate addition on reductive transformation of pentachlorophenol in paddy soil in relation to iron(III) reduction [J]. Journal of Environmental Management, 2014, 132: 42-48
[22] Borch T, Ambus P, Laturnus F, et al. Biodegradation of chlorinated solvents in a water unsaturated topsoil [J]. Chemosphere, 2003, 51(2): 143-152
[23] Eisenbeis M, Bauer-Kreisel P, Scholz-Muramatsu H. Studies on the dechlorination of tetrachloroethene to cis-1,2-dichloroethene by Dehalospirillum multivorans in biofilms [J]. Water Science and Technology, 1997, 36(1): 191-198
[24] Gerritse J, Drzyzga O, Kloetstra G, et al. Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1 [J]. Applied and Environmental Microbiology, 1999, 65(12): 5212-5221
[25] Liu T, Li X, Zhang W, et al. Fe(III) oxides accelerate microbial nitrate reduction and electricity generation by Klebsiella pneumoniae L17 [J]. Journal of Colloid and Interface Science, 2014, 423: 25-32
[26] Kumaraswamy R, Ebert S, Gray M R, et al. Molecular- and cultivation-based analyses of microbial communities in oil field water and in microcosms amended with nitrate to control H2S production [J]. Applied Microbiology and Biotechnology, 2011, 89(6): 2027-2038
[27] Korte H L, Saini A, Trotter V V, et al. Independence of nitrate and nitrite inhibition of Desulfovibrio vulgaris Hildenborough and use of nitrite as a substrate for growth [J]. Environmental Science & Technology, 2015, 49(2): 924-931
[28] Zhang W, Li X, Liu T, et al. Competitive reduction of nitrate and iron oxides by Shewanella putrefaciens 200 under anoxic conditions [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 445: 97-104
[29] Stumm W, Morgan J J. Aquatic Chemistry [M]. New York: Wiley, 1981: 780
[30] Lovley D R. Dissimilatory Fe (III) and Mn(IV) reduction [J]. Microbiological Reviews, 1991, 55(2): 259-287
[31] 谭盈盈, 郑平, 姜辛. 微生物作用下铁(Ⅲ)对有机污染物的氧化[J]. 浙江大学学报: 农业与生命科学版, 2002, 28(3): 350-354
Tan Y Y, Zheng P, Jiang X. Organic contaminants bio-degradation with Fe(III) [J]. Journal of Zhejiang University: Agriculture & Life Sciences, 2002, 28(3): 350-354 (in Chinese)
[32] Paul L, Herrmann S, Bender Koch C, et al. Inhibition of microbial trichloroethylene dechorination by Fe (III) reduction depends on Fe mineralogy: A batch study using the bioaugmentation culture KB-1 [J]. Water Research, 2013, 47(7): 2543-2554
[33] Paul L, Smolders E. Inhibition of iron(III) minerals and acidification on the reductive dechlorination of trichloroethylene [J]. Chemosphere, 2014, 111: 471-477
[34] Stucki J W. A review of the effects of iron redox cycles on smectite properties [J]. Comptes Rendus Geoscience, 2011, 343(2-3): 199-209
[35] Neumann A, Petit S, Hofstetter T B. Evaluation of redox-active iron sites in smectites using middle and near infrared spectroscopy [J]. Geochimica et Cosmochimica Acta, 2011, 75(9): 2336-2355
[36] Li F, Wang X, Li Y, et al. Enhancement of the reductive transformation of pentachlorophenol by polycarboxylic acids at the iron oxide-water interface [J]. Journal of Colloid and Interface Science, 2008, 321(2): 332-341
[37] Chen M, Tao L, Li F, et al. Reductions of Fe(III) and pentachlorophenol linked with geochemical properties of soils from Pearl River Delta [J]. Geoderma, 2014, 217-218: 201-211
[38] Kouznetsova I, Mao X, Robinson C, et al. Biological reduction of chlorinated solvents: Batch-scale geochemical modeling [J]. Advances in Water Resources, 2010, 33(9): 969-986
[39] He Q, Sanford R A. Characterization of Fe(III) reduction by chlororespiring Anaeromxyobacter dehalogenans [J]. Applied and Environmental Microbiology, 2003, 69(5): 2712-2718
[40] Krumholz L R, Sharp R, Fishbain S S. A freshwater anaerobe coupling acetate oxidation to tetrachloroethylene dehalogenation [J]. Applied and Environmental Microbiology, 1996, 62(11): 4108-4113
[41] Li X, Li Y, Li F, et al. Interactively interfacial reaction of iron-reducing bacterium and goethite for reductive dechlorination of chlorinated organic compounds [J]. Science Bulletin, 2009, 54(16): 2800-2804
[42] Chen M, Cao F, Li F, et al. Anaerobic transformation of DDT related to iron(III) reduction and microbial community structure in paddy soils [J]. Journal of Agricultural and Food Chemistry, 2013, 61(9): 2224-2233
[43] Niggemyer A, Spring S, Stackebrandt E, et al. Isolation and characterization of a novel As (V)-reducing bacterium:Implications for arsenic mobilization and the genus Desulfitobacterium [J]. Applied and Environmental Microbiology, 2001, 67(12): 5568-5580
[44] De Wever H, Cole J R, Fettig M R, et al. Reductive dehalogenation of trichloroacetic acid by Trichlorobacter thiogenes gen. nov., sp. nov [J]. Applied and Environmental Microbiology, 2000, 66(6): 2297-2301
[45] Sung Y, Ritalahti K M, Sanford R A, et al. Characterization of two tetrachloroethene-reducing, acetate-oxidizing anaerobic bacteria and their description as Desulfuromonas michiganensis sp. nov [J]. Applied and Environmental Microbiology, 2003, 69(5): 2964-2974
[46] 王旭刚. 土壤胶体界面五氯酚的还原转化研究[D]. 杨凌: 西北农林科技大学, 2007: 9-11
Wang X G. Kinetic study of PCP reduction transformation on surface soil colloids [D]. Yangling: Northwest Sci-Tech University of Agriculture and Forestry, 2007: 9-11 (in Chinese)
[47] 陶亮, 周顺桂, 李芳柏. 土壤有机氯脱氯转化的界面交互反应[J]. 化学进展, 2009(4): 791-800
Tao L, Zhou S G, Li F B. Interactive reaction of polychlorinated compound dechlorinating transformation at the soil interface [J]. Progress in Chemistry, 2009(4): 791-800 (in Chinese)
[48] He Y T, Wilson J T, Wilkin R T. Transformation of reactive iron minerals in a permeable reactive barrier (biowall) used to treat TCE in groundwater [J]. Environmental Science & Technology, 2008, 42(17): 6690-6696
[49] 冯小莉. 水稻土混合菌群耦合铁氧化物对五氯酚还原转化的作用与机制[D]. 杭州: 浙江大学, 2014: 59-60
Feng X L. Reductive transformation and mechanism of pentachlorophenol by anaerobic microbial communities enriched from paddy soil coupled with iron oxides [D].Hangzhou: Zhejiang University, 2014: 59-60 (in Chinese)
[50] Wu C Y, Zhuang L, Zhou S G, et al. Fe(III)-enhanced anaerobic transformation of 2,4-dichlorophenoxyacetic acid by an iron-reducing bacterium Comamonas koreensis CY01 [J]. FEMS Microbiology Ecology, 2010, 71(1): 106-113
[51] Li X M, Zhou S G, Li F B, et al. Fe(III) oxide reduction and carbon tetrachloride dechlorination by a newly isolated Klebsiella pneumoniae strain L17 [J]. Journal of Applied Microbiology, 2009, 106(1): 130-139
[52] Scott D T, McKnight D M, Blunt-Harris E L, et al. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms [J]. Environmental Science & Technology, 1998, 32(19): 2984-2989
[53] Doong R, Schink B. Cysteine-mediated reductive dissolution of poorly crystalline iron(III) oxides by Geobacter sulfurreducens [J]. Environmental Science & Technology, 2002, 36(13): 2939-2945
[54] Chen M, Shih K, Hu M, et al. Biostimulation of indigenous microbial communities for anaerobic transformation of pentachlorophenol in paddy soils of southern China [J]. Journal of Agricultural and Food Chemistry, 2012, 60(12): 2967-2975
[55] Xu Y, He Y, Feng X, et al. Enhanced abiotic and biotic contributions to dechlorination of pentachlorophenol during Fe(III) reduction by an iron-reducing bacterium Clostridium beijerinckii Z [J]. Science of The Total Environment, 2014, 473: 215-223
[56] Okutman Tas D, Pavlostathis S G. The influence of iron reduction on the reductive biotransformation of pentachloronitrobenzene [J]. European Journal of Soil Biology, 2007, 43(5-6): 264-275
[57] Yu L, Yuan Y, Tang J, et al. Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens [J]. Scientific Reports, 2015, 5: 16221
[58] Tong H, Hu M, Li F B, et al. Biochar enhances the microbial and chemical transformation of pentachlorophenol in paddy soil [J]. Soil Biology and Biochemistry, 2014, 70: 142-150
[59] 郭莹. 硫酸盐还原条件下三氯乙烯的降解研究[D]. 合肥: 合肥工业大学, 2014: 34-46
Guo Y. The degradation of trichlorethylene under sulfate-reducing conditions [D]. Hefei:Hefei University of Technology, 2014: 34-46 (in Chinese)
[60] Gibson G R. Physiology and ecology of the sulphate-reducing bacteria [J]. Journal of Applied Bacteriology, 1990, 69(6): 769-797
[61] Muyzer G, Stams A J M. The ecology and biotechnology of sulphate-reducing bacteria [J]. Nature Reviews Microbiology, 2008, 6(6): 441-454
[62] Häggblom M M, Young L Y. Chlorophenol degradation coupled to sulfate reduction [J]. Applied and Environmental Microbiology, 1990, 56(11): 3255-3260
[63] 李永峰, 李巧燕, 王兵,等. 环境生物技术:典型厌氧环境微生物过程[M]. 哈尔滨: 哈尔滨工业大学出版社, 2014: 403-404
[64] Stuart S L, Woods S L, Lemmon T L, et al. The effect of redox potential changes on reductive dechlorination of pentachlorophenol and the degradation of acetate by a mixed, methanogenic culture [J]. Biotechnology and Bioengineering, 1999, 63(1): 69-78
[65] Chang B, Chang I T, Yuan S Y. Anaerobic degradation of phenanthrene and pyrene in mangrove sediment [J]. Bulletin of Environmental Contamination and Toxicology, 2008, 80(2): 145-149
[66] Yuan S Y, Chen S J, Chang B V. Anaerobic degradation of tetrachlorobisphenol-A in river sediment [J]. International Biodeterioration & Biodegradation, 2011, 65(1): 185-190
[67] Yoshida N, Yoshida Y, Handa Y, et al. Polyphasic characterization of a PCP-to-phenol dechlorinating microbial community enriched from paddy soil [J]. Science of the Total Environment, 2007, 381(1-3): 233-242
[68] Ehlers G A, Rose P D. The potential for reductive dehalogenation of chlorinated phenol in a sulphidogenic environment in in situ enhanced biodegradation [J]. Water SA, 2006, 32(2): 243-248
[69] Drzyzga O, Gerritse J, Dijk J A, et al. Coexistence of a sulphate-reducing Desulfovibrio species and the dehalorespiring Desulfitobacterium frappieri TCE1 in defined chemostat cultures grown with various combinations of sulphate and tetrachloroethene [J]. Environmental Microbiology, 2001, 3(2): 92-99
[70] Aulenta F, Beccari M, Majone M, et al. Competition for H2between sulfate reduction and dechlorination in butyrate-fed anaerobic cultures [J]. Process Biochemistry, 2008, 43(2): 161-168
[71] Nelson D K, Hozalski R M, Clapp L W, et al. Effect ofnitrate and sulfate on dechlorination by a mixed hydrogen-fed culture [J]. Bioremediation Journal,2002, 6(3): 225-236
[72] Reis M A M, Almeida J S, Lemos P C, et al. Effect of hydrogen sulfide on growth of sulfate reducing bacteria [J]. Biotechnology and Bioengineering, 1992, 40(5): 593-600
[73] 左剑恶, 肖晶华, 陈莉莉. 氯代有机污染物在厌氧条件下还原脱氯的研究进展[J]. 环境污染治理技术与设备,2003, 4(6): 43-48
Zuo J E, Xiao J H, Chen L L. A review on reductive dechlorination of chlorinated organic pollutants under anaerobic condition [J].Techniques and Equipment for Environmental Pollution Control, 2003, 4(6): 43-48 (in Chinese)
[74] Barbour J P, Smith J A, Chiou C T. Sorption of aromatic organic pollutants to grasses from water [J]. Environmental Science & Technology, 2005, 39(21): 8369-8373
[75] Guerrero-Barajas C, García-Pea E I. Evaluation of enrichments of sulfate reducing bacteria from pristine hydrothermal vents sediments as potential inoculum for reducing trichloroethylene [J]. World Journal of Microbiology and Biotechnology, 2010, 26(1): 21-32
[76] Bao P, Hu Z, Wang X, et al. Dechlorination of p,p′-DDTs coupled with sulfate reduction by novel sulfate-reducing bacterium Clostridium sp. BXM [J]. Environmental Pollution, 2012, 162: 303-310
[77] Hiraishi A. Biodiversity of dehalorespiring bacteria with special emphasis on polychlorinated biphenyl/dioxin dechlorinators [J]. Microbes and Environments, 2008, 23(1): 1-12
[78] 曾志华. 闽江河口区盐度梯度下潮汐沼泽湿地产甲烷菌群落结构及对环境因子的响应[D]. 福州: 福建师范大学, 2014: 5-8
Zeng Z H. The structure of methanogen comminities in marsh soil of salinity gradient in the main river estuary and response to environmental factors [D]. Fuzhou: Fujian Normal University, 2014: 5-8 (in Chinese)
[79] Heimann A C, Batstone D J, Jakobsen R. Methanosarcina spp. drive vinyl chloride dechlorination via interspecies hydrogen transfer [J]. Applied and Environmental Microbiology, 2006, 72(4): 2942-2949
[80] Ozuolmez D, Na H, Lever M A, et al. Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: Teamwork or coexistence? [J]. Frontiers in Microbiology, 2015, 6: 492
[81] Hu J, Hu Z, Cui X, et al. 2-bromoethanesulfonate (BES)enhances sulfate-reducing bacterial population and dichlorodiphenyltrichloroethane (DDT) dechlorination in an anaerobic paddy soil[J]. Soil and Sediment Contamination: An International Journal, 2012, 21(6): 732-738
[82] Men Y, Lee P K H, Harding K C, et al. Characterization of four TCE-dechlorinating microbial enrichments grown with different cobalamin stress and methanogenic conditions [J]. Applied Microbiology and Biotechnology, 2013, 97(14): 6439-6450
[83] Delgado A G, Parameswaran P, Fajardo-Williams D, et al. Role of bicarbonate as a pH buffer and electron sink in microbial dechlorination of chloroethenes [J]. MicrobialCell Factories, 2012, 11(1): 128
[84] Wen L, Zhang Y, Pan Y, et al. The roles of methanogens and acetogens in dechlorination of trichloroethene using different electron donors [J]. Environmental Science and Pollution Research, 2015, 22(23): 19039-19047
[85] Chen Y, Wen Y, Zhou J, et al. Transformation of chloroform in model treatment wetlands: From mass balance to microbial analysis [J]. Environmental Science & Technology, 2015, 49(10): 6198-6205
[86] Chiu P C, Lee M. 2-Bromoethanesulfonate affects bacteria in a trichloroethene-dechlorinating culture [J]. Applied and Environmental Microbiology, 2001, 67(5): 2371-2374
[87] Lv Y, Chen Y, Song W, et al. Enhanced selection of micro-aerobic pentachlorophenol degrading granular sludge [J]. Journal of Hazardous Materials, 2014, 280: 134-142
[88] Loffler F E, Ritalahti K M, Tiedje J M. Dechlorination of chloroethenes is inhibited by 2-bromoethanesulfonate in the absence of methanogens [J]. Applied and Environmental Microbiology, 1997, 63(12): 4982-4985
◆
EffectsandMechanismsofSoilRedoxProcessesonReductiveDechlorinationofChlorinatedOrganicPollutants
Feng Xi1,2, Zhu Min1,2, He Yan1,2,*
1. Institute of Soil and Water Resources and Environmental Science, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou 310058, China2. Zhejiang Provincial Key Laboratory of Agricultural Resources and Environment, Hangzhou 310058, China
10.7524/AJE.1673-5897.20170114004
2017-01-14录用日期2017-04-30
1673-5897(2017)3-151-11
X171.5
A
国家自然科学基金项目(41322006, 41771269); 国家重点研发计划项目(2016YFD0800207);中组部青年拔尖人才支持基金
冯曦(1992-),男,硕士研究生,研究方向为有机污染土壤修复,E-mail:13588221213@163.com
*通讯作者(Corresponding author), E-mail: yhe2006@zju.edu.cn
冯曦, 朱敏, 何艳. 土壤还原过程对氯代有机污染物还原脱氯的影响与机制[J]. 生态毒理学报,2017, 12(3): 151-161
Feng X, Zhu M, He Y. Effects and mechanisms of soil redox processes on reductive dechlorination of chlorinated organic pollutants [J]. Asian Journal of Ecotoxicology, 2017, 12(3): 151-161 (in Chinese)

