Primary cilia as a novel horizon between neuron and environment
Gregory W. Kirschen, Qiaojie Xiong,
1 Medical Scientist Training Program, Stony Brook University, Stony Brook, NY, USA
2 Department of Neurobiology & Behavior, Stony Brook University, Stony Brook, NY, USA
Primary cilia as a novel horizon between neuron and environment
Gregory W. Kirschen1,2, Qiaojie Xiong2,*
1 Medical Scientist Training Program, Stony Brook University, Stony Brook, NY, USA
2 Department of Neurobiology & Behavior, Stony Brook University, Stony Brook, NY, USA
How to cite this article:Kirschen GW, Xiong Q (2017) Primary cilia as a novel horizon between neuron and environment. Neural Regen Res 12(8):1225-1230.
Funding: GWK currently receives support from the National Institutes of Health (NIH 1 F30 MH110103).
G protein-coupled receptor; sonic hedgehog; seizure; stroke; stem cell; neurogenesis; plasticity
While few would still go so far as to disparage primary cilia as inert, vestigial appendages inherited from our single-celled ancestors, the fact remains that these hair-like sensory organelles continue to receive less attention than they deserve. First discovered in protozoans in the late 1600s by the Dutch scientist Antony Van Leeuwenhoek while examining pond water samples under the microscope, cilia and flagella, microtubule-based organelles found in most eukaryotic cells, have been a topic of curiosity and controversy (Haimo and Rosenbaum, 1981; Satir, 1995). Although we now appreciate at least some of the various motor and sensory functions of these organelles, thanks largely to pioneering work by the Czech biologist Jan Purkyne and his student G.G. Valentin, the field of cilia biology is still relatively uncharted (Teich, 1970). We have since discovered that most cells of the human body express primary cilia, which are immotile and sensory in nature, while a handful of specialized cells express the beating, motile variety (Afzelius, 1976; Wheatley et al., 1996). After many years of speculation, poo pooing, and neglect of primary cilia, we are fi nally beginning to develop tools and approaches to more fully understand their importance to normal cellular physiology as well as their relevance to human disease. Even more recently, we have learned that primary cilia exist and signal throughout the CNS.
The first clue that primary cilia have not become irrelevant to mammalian biology is, of course, the host of problems that arise when primary cilia fail to assemble or signal properly, particularly during development of the organism. Indeed, the wide range of primary ciliopathies, including retinal degeneration, brain and spinal cord malformations, and Bardet-Biedl syndrome, a genetically-related obesity syndrome, has focused the scientific community’s attention on the critical role of primary cilia during embryonic development (Gerdes et al., 2009; Lee and Gleeson, 2011). Although it has become widely acknowledged that primary ciliary signaling, for example through Hedgehog-mediated control of cell cycle progressionviathe canonical Wnt pathway, is essential for proper proliferation, differentiation, and migration of cells throughout the developing embryo, much less is known regarding the potential homeostatic, stimulus-triggered, or disease relevant functions of primary cilia once developmental programs have become established (Clement et al., 2009; Wong et al., 2009; Schneider et al., 2010; Gilliam et al., 2012). In particular, investigation of primary cilia within the CNS has increased in recent years, as it has been discovered that they contribute to homeostatic mechanisms and may also be implicated in neuropathological states in the adult organism.
In this review, we provide an overview of several recent technical and conceptual advances in the field of primary cilia biology related to their reparative potential in the CNS. We introduce several newly described non-canonical roles of primary cilia in neuroplasticity, and set forth a proposed research agenda for the study of the role of primarycilia in damage and repair in the context of neural injury. Novel methods to manipulate primary cilia structure or signaling will likely prove crucial not only to enhance our understanding of their roles in health and disease, but also to provide the foundation for novel therapeutics targeting these organelles.
Research Agenda: to Better Understand Neural Primary Cilia in Health and Disease
Until the past decade, it has been dif fi cult to probe the various functions of primary cilia due to a paucity of refined pharmacological or genetic tools targeting these organelles specifically. However, our understanding of cilia biology has benefited tremendously from new technologies such as transgenic mice conditionally deficient in intraflagellar transport (IFT) genes or Bardet-Biedl syndrome (BBS) genes, as well as virally-delivered DNA/RNA constructs that can downregulate or overexpress the various components of the ciliary protein machinery (Jonassen et al., 2008; Zaghloul and Katsanis, 2009; Boehlke et al., 2010; Kumamoto et al., 2012). Using these approaches, we are beginning to appreciate the many roles that cilia take on far beyond embryonic development and well into adulthood and senescence. With these considerations, we propose the following research agenda.
To better understand normal neural primary cilia physiology and behavior in the adult CNS
While our knowledge of cilia signaling in orchestrating neuronal patterning and other developmental programs during embryogenesis has advanced significantly, the field of cilia biology is still relatively new with regard to mature/adult physiology, especially in the CNS. More basic research aimed at understanding the cilium’s role in neuronal excitability, plasticity and behavior, as well as its importance to glial cells, is warranted.
The presence of primary cilia on neurons was first reported in the late 1950s and early 1960s by several scientists working on different model organisms. Duncan and Dahl, each independently studying the rodent nervous system, noted the presence of cilia by ultrastructural analysis of notochord and cerebral cortex, respectively (Duncan, 1957; Dahl, 1963). Meanwhile, developmental geneticist Sydney Brenner and his colleagues also noticed the presence of cilia in the primitive nervous system of the nematodeCaenorhabditis elegans(Ward et al., 1975).us began the search into the structure and functions of neural primary cilia, work that until recently had largely focused on the cilium’s role in vertebrate neural tube development in the embryo through Sonic hedgehog signaling (Corbit et al., 2005; Caspary et al., 2007).
As a part of their normal functioning in the mature, intact nervous system, primary cilia contribute to neuroplasticity at the neural stem cell, electrophysiological, and behavioral levels. In both adult neurogenic regions of the brain, the lateral ventricle of the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampal dentate gyrus, neural progenitor cells harbor primary cilia, and so a logical question is whether these cilia contribute to the process of adult neurogenesis. Radial glia-like progenitors in the SGZ depleted of ciliary genes or whose ciliary Hedgehog signaling has become disrupted are unable to proliferate, thus leading to dramatic impairments in adult hippocampal neurogenesis (Han et al., 2008). Similarly, ciliary disruption in radial glia of the SVZviadeletion of IFT proteins (important in ciliogenesis and maintained cilia integrity) leads to suppression of neurogenesis in the ventral portion of the SVZ (Tong et al., 2014). Since adult neurogenesis is well acknowledged to provide an additional layer of plasticity to the adult brain above that provided by synaptic dynamics (Kirschen et al., 2017a; Sailor et al., 2017), primary cilia are likely crucial in promoting ongoing cellular reorganization that promotes brain circuit plasticity throughout adulthood.
In the mature neuron at the level of the synapse, primary cilia again demonstrate their role in shaping adult plasticity. Depleting primary cilia in mature dentate granule neurons of the hippocampus not only decreases evoked and spontaneous excitatory postsynaptic currents in these cells and impairs functional glutamatergic synapse formation, but further leads to defects in long term potentiation (LTP) in CA3 pyramidal neurons following high frequency stimulation at the mossy fi ber terminal (Kumamoto et al., 2012; Rhee et al., 2016). Such defects likely contribute to hippocampus-specif i c behavioral def i cits in contextual fear conditioning and spatial memory that others and we have observed upon primary cilia depletion (Berbari et al., 2014; Rhee et al., 2016). Still, the electrophysiological and behavioral relevance of primary cilia in other circuits and in other brain regions is yet to be determined. Given that virtually all neurons as well as some glial cells express primary cilia, their roles are likely to be numerous. A summary of known ciliary receptors, signaling pathways, and cellular processes directly related to primary cilia of various neural cell types is shown in Table 1.
To characterize the functional ramif i cations of loss of neuronal primary cilia structural integrity under disease conditions
We still do not know the full scope of neurological diseases to which neuronal cilia may contribute. In addition to determining the neurodegenerative or neuroinflammatory conditions in which cilia are implicated, strategies to target these organelles should be pursued.is could involve, for example, experimentally blocking ciliary disassembly or activating/inactivating of ciliary receptors during neurological insult and tracking functional outcomes both in terms of cellular physiology and behavioral correlates.
With primary cilia so important for proliferation, migration, and patterning/organization of neural cells during normal development, it is natural to wonder whether they participate in similar processes under pathological conditions. Intriguingly, in the corneal epithelial cells of the developing eye, the precise timing of primary cilia assembly is crucial for the coordination of cell localization to form the uniquehexagonal monolayer of cells characteristic of the corneal epithelium (Blitzer et al., 2011). Following the completion of this developmental program, these cilia degenerate, but strikingly, they quickly regenerate following mechanical corneal injury, which disrupts the hexagonal cellular patterning. Could a similar phenomenon occur in the CNS following an insult that disrupts neuronal homeostasis? And by extension, could primary cilia play a central role in coordinating the response to this insult?
To address these questions, others and we have initiated a new line of investigations aimed at understanding primary cilia integrity during and following CNS injury. Firstly, unlike in the corneal epithelium, in the brain, primary cilia are constitutively expressed by neurons of both young and aged animals (with increasing length across the lifespan in certain brain regions) (Guadiana et al., 2016). Secondly, neocortical and hippocampal neuronal primary cilia in the adult brain display a characteristic radial alignment paralleling their cellular polarity, and also exhibit regional heterogeneity in lengths at baseline (Kirschen et al., 2017b) (Figure 1A). Upon seizure induction, however, cilia lengths and alignment become disrupted (Parker et al., 2016; Kirschen et al., 2017b). Similarly, cerebral hemispheric ischemia leads to changes in both length and alignment of neuronal cilia (Kirschen et al., 2017b). Intriguingly, the hippocampus and its innervating region the entorhinal cortex exhibit dif f erential responses to brain injury. While a pilocarpine-induced seizure disrupts ciliary positioning in both regions, cilia length is selectively disrupted in the entorhinal cortex but not in the hippocampus.
What these changes signify structurally and molecularly, however, remains to be determined. Given the regional heterogeneity of G protein-coupled receptors and other receptors/ef f ectors along the axoneme (the microtubule-composed cytoskeletal backbone of the cilium) and immediately surrounding area of the cell membrane, we hypothesize that these insults may induce preferential damage at some sites, with relative sparing of others, leading to differential activation or inactivation of downstream pathways involved in mounting an inf l ammatory or reparative response. Alternatively, changes in cilia length and positioning may represent distal appendage and/or ciliary disassembly, for exampleviaPitchfork (Pifo)-mediated Aurora A (AurA) activation and cilia retraction at the basal body.is could lead to changes in planar cell polarity (PCP) signaling and consequent basal body mis-localization (Sanchez and Dynlacht, 2016). An overview of known and potential physiological/pathophysiological functions of neural primary ciliais depicted in Figure 1B.
We also do not know whether these structural changes in cilia following brain injury represent an advantageous reaction that will lead to downstream repair pathways, or rather collateral damage or injury exacerbation. Regardless, it will be important to further characterize the temporal dynamics of ciliary morphological changes over the course of disruptions in CNS homeostasis, as well as to determine their functional consequences.e existing literature on primary cilia signaling in the CNS under physiological conditions is sparse; their role in CNS malfunctioning is even more sparse. Thus, we hope that these preliminary findings will draw more interest in cilia research in the pursuit of both basic neural cilia knowledge as well as their relevance to neuropathology.
Identify and characterize the normal and triggered signaling cascades within neuronal cilia
The aforementioned gap in our knowledge regarding how neural cilia work, from their housekeeping functions to their stimulus-evoked responses, will be important issues to address. Fortunately, given the well-mapped neuronal connectivity and the relatively well understood stimulus-response properties of neural circuits, the basic neuroscience foundation underlying these questions should be feasible to address. Moreover, we can take advantage of strides made in other areas of cilia biology to use as starting points for reference and guidance in the study of neural cilia. For instance, it will be important to determine the molecular makeup of the plasma membrane composing the neuronal cilium as well as the cilioplasm, both in healthy and disease states. Like cilia of other cell types, neuronal cilia throughout the brain cluster G protein-coupled receptors (GCPRs) including adenylyl cyclase III (ACIII) and somatostatin receptor type 3, which when triggered, signal through the second messenger cyclic AMP (cAMP) to regulate various metabolic and secretory functions of the cell (Berbari et al., 2007; Bishop et al., 2007; Ferone et al., 2009) (Table 1). Interestingly, primary cilia were hypothesized to sense and regulate calcium homeostasis, as they appeared to act as a unique calcium sequestering compartment (Delling et al., 2013). More recent evidence from the same group suggests, however, that this is not in fact the case (at least in renal cells), as transgenic mice co-expressing a fl uorescent cilium marker and a genetically-encoded calcium indicator exhibited no cilia-specif i c basal or stimulus-evoked changes in calcium fl ux (Delling et al., 2016).
adapted to study primary cilia in the nervous system in response to electrical or chemical stimuli will be interesting to explore. Finally, novel technologies such as cilia-APEX, which selectively biotinylates cilia-specif i c proteins for subsequent proteomic analysis, may facilitate discovery of the unique protein makeup of cilia across different cell types and brain areas (Mick et al., 2015). It will be exciting to see our understanding of cilia structural/functional diversity blossom as these and other innovations allow us to answer a series of fundamental questions that currently remain unresolved.
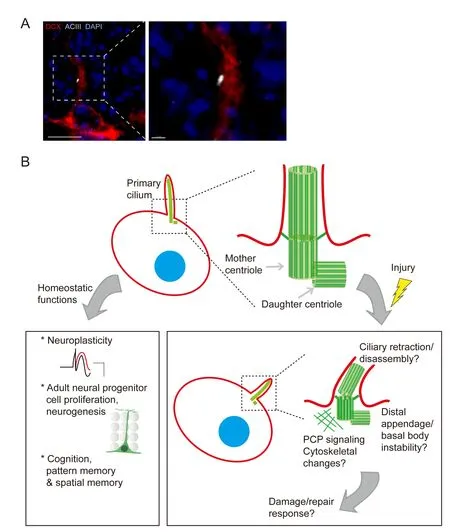
Figure 1 Functions of adult neuronal primary cilia, and potential disruptions following CNS injury.
Identify novel therapeutic agents to promote or restore cilia signaling under disease conditions
Aside from our incomplete understanding of how neural cilia signal under physiological conditions, we do not currently know whether structurally compromised primary cilia are still able to signal, and if so, how this signaling may dif f er from their homeostatic functions. As shown in Table 1, primary cilia of the CNS indeed express an array of receptors including GPCRs, which may suf fi ce as a starting point, however the precise downstream signaling cascades of these receptors, as well as those of as-of-yet unidentif i ed receptors, will require extensive characterization. As it stands, we now appreciate some of the important functions of adult neural primary cilia, and so the next step will be to elucidate the underlying mechanisms using pharmacological or genetic tools. If we can engineer such tools, we may be able to fi netune ciliary signaling, with the potential to either facilitate repair following neural injury, or identify candidate therapies for the treatment of primary ciliopathies. For example, a recent high-throughput screen of small molecules targeting ciliary signalingviaHedgehog identif i ed a number of small molecules that prevented aberrant Hedgehog signaling in a pancreatic ductal adenocarcinoma model, impeding the transition from G1 to S phase of the cell cycle and hence retarding cancer cell proliferation (Jung et al., 2016). More preclinical small molecule screening studies for neurological diseases (especially those involving aberrant cell proliferation or migration) will likely prove instrumental in such endeavors.
Acknowledgments:We thank Dr. Shaoyu Ge and Afrinash Ahamad for their critical feedback on this manuscript.
Author contributions:GWK wrote the original draft. GWK and QX edited the dra, and both of them approved the fi nal version.
Conf l icts of interest:None declared.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Abdel-Majid RM, Tremblay F, Baldridge WH (2002) Localization of adenylyl cyclase proteins in the rodent retina. Brain Res Mol Brain Res 101:62-70.
Afzelius BA (1976) A human syndrome caused by immotile cilia. Science 193:317-319.
Baudoin JP, Viou L, Launay PS, Luccardini C, Espeso Gil S, Kiyasova V, Irinopoulou T, Alvarez C, Rio JP, Boudier T, Lechaire JP, Kessaris N, Spassky N, Metin C (2012) Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron 76:1108-1122.
Berbari NF, Bishop GA, Askwith CC, Lewis JS, Mykytyn K (2007) Hippocampal neurons possess primary cilia in culture. J Neurosci Res 85:1095-1100.
Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K (2008) Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci U S A 105:4242-4246.
Berbari NF, Malarkey EB, Yazdi SM, McNair AD, Kippe JM, Croyle MJ, Kraft TW, Yoder BK (2014) Hippocampal and cortical primary cilia are required for aversive memory in mice. PLoS One 9:e106576.
Bishop GA, Berbari NF, Lewis J, Mykytyn K (2007) Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol 505:562-571.
Blitzer AL, Panagis L, Gusella GL, Danias J, Mlodzik M, Iomini C (2011) Primary cilia dynamics instruct tissue patterning and repair of corneal endothelium. Proc Natl Acad Sci U S A 108:2819-2824.
Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Godel M, Muller K, Herbst M, Hornung M, Doerken M, Kottgen M, Nitschke R, Igarashi P, Walz G, Kuehn EW (2010) Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 12:1115-1122.
Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D (2000) Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res 872:271-275.
Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T (2008) Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A 105:13127-13132.
Caspary T, Larkins CE, Anderson KV (2007)e graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell 12:767-778.
Christensen ST, Clement CA, Satir P, Pedersen LB (2012) Primary cilia and coordination of receptor tyrosine kinase (RTK) signalling. J Pathol 226:172-184.
Clement CA, Kristensen SG, Mollgard K, Pazour GJ, Yoder BK, Larsen LA, Christensen ST (2009)e primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte dif f erentiation. J Cell Sci 122:3070-3082.
Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF (2005) Vertebrate smoothened functions at the primary cilium. Nature 437:1018-1021.
Dahl HA (1963) Fine structure of cilia in rat cerebral cortex. Z Zellforsch Mikrosk Anat 60:369-386.
de Quidt ME, Emson PC (1986) Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system--II. Immunohistochemical analysis. Neuroscience 18:545-618.
Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE (2013) Primary cilia are specialized calcium signalling organelles. Nature 504:311-314.
Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE (2016) Primary cilia are not calcium-responsive mechanosensors. Nature 531:656-660.
Duncan D (1957) Electron microscope study of the embryonic neural tube and notochord. Tex Rep Biol Med 15:367-377.
Ferone D, Gatto F, Arvigo M, Resmini E, Boschetti M, Teti C, Esposito D, Minuto F (2009)e clinical-molecular interface of somatostatin, dopamine and their receptors in pituitary pathophysiology. J Mol Endocrinol 42:361-370.
Gerdes JM, Davis EE, Katsanis N (2009)e vertebrate primary cilium in development, homeostasis, and disease. Cell 137:32-45.
Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151:1029-1041.
Guadiana SM, Parker AK, Filho GF, Sequeira A, Semple-Rowland S, Shaw G, Mandel RJ, Foster TC, Kumar A, Sarkisian MR (2016) Type 3 adenylyl cyclase and somatostatin receptor 3 expression persists in aged rat neocortical and hippocampal neuronal cilia. Front Aging Neurosci 8:127.
Haimo LT, Rosenbaum JL (1981) Cilia, fl agella, and microtubules. J Cell Biol 91:125s-130s.
Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A (2008) Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11:277-284.
Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES (2012) Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell 23:925-938.
Jalalvand E, Robertson B, Wallen P, Grillner S (2016) Ciliated neurons lining the central canal sense both fl uid movement and pH through ASIC3. Nat Commun 7:10002.
Jonassen JA, San Agustin J, Follit JA, Pazour GJ (2008) Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 183:377-384.
Jung B, Messias AC, Schorpp K, Geerlof A, Schneider G, Saur D, Hadian K, Sattler M, Wanker EE, Hasenoder S, Lickert H (2016) Novel small molecules targeting ciliary transport of Smoothened and oncogenic Hedgehog pathway activation. Sci Rep 6:22540.
Kirschen GW, Sailor KA, Ge S (2017a) Structural plasticity induced by adult neurogenesis: Elsevier Science.
Kirschen GW, Liu H, Lang T, Liang X, Ge S, Xiong Q (2017b)e radial organization of neuronal primary cilia is acutely disrupted by seizure and ischemic brain injury. Front Biol (Beijing) 12:124-138.
Kondo S, Sato-Yoshitake R, Noda Y, Aizawa H, Nakata T, Matsuura Y, Hirokawa N (1994) KIF3A is a new microtubule-based anterograde motor in the nerve axon. J Cell Biol 125:1095-1107.
Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru K, Levine J, Ge S (2012) A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci 15:399-405, S391.
Lee JE, Gleeson JG (2011) Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol 24:98-105.
Loktev AV, Jackson PK (2013) Neuropeptide Y family receptors traf fi c via the Bardet-Biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep 5:1316-1329.
Ma X, Peterson R, Turnbull J (2011) Adenylyl cyclase type 3, a marker of primary cilia, is reduced in primary cell culture and in lumbar spinal cord in situ in G93A SOD1 mice. BMC Neurosci 12:71.
Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV (2015) Proteomics of Primary Cilia by Proximity Labeling. Dev Cell 35:497-512.
Moser JJ, Fritzler MJ, Rattner JB (2009) Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer 9:448.
Moser JJ, Fritzler MJ, Rattner JB (2014) Ultrastructural characterization of primary cilia in pathologically characterized human glioblastoma multiforme (GBM) tumors. BMC Clin Pathol 14:40.
Muresan V, Lyass A, Schnapp BJ (1999)e kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J Neurosci 19:1027-1037.
Parker AK, Le MM, Smith TS, Hoang-Minh LB, Atkinson EW, Ugartemendia G, Semple-Rowland S, Coleman JE, Sarkisian MR (2016) Neonatal seizures induced by pentylenetetrazol or kainic acid disrupt primary cilia growth on developing mouse cortical neurons. Exp Neurol 282:119-127.
Rhee S, Kirschen GW, Gu Y, Ge S (2016) Depletion of primary cilia from mature dentate granule cells impairs hippocampus-dependent contextual memory. Sci Rep 6:34370.
Sailor KA, Schinder AF, Lledo PM (2017) Adult neurogenesis beyond the niche: its potential for driving brain plasticity. Curr Opin Neurobiol 42:111-117.
Sanchez I, Dynlacht BD (2016) Cilium assembly and disassembly. Nat Cell Biol 18:711-717.
Satir P (1995) Landmarks in cilia research from Leeuwenhoek to us. Cell Motil Cytoskeleton 32:90-94.
Schmid A, Bai G, Schmid N, Zaccolo M, Ostrowski LE, Conner GE, Fregien N, Salathe M (2006) Real-time analysis of cAMP-mediated regulation of ciliary motility in single primary human airway epithelial cells. J Cell Sci 119:4176-4186.
Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hof f mann EK, Yoder BK, Schwab A, Satir P, Christensen ST (2010) Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem 25:279-292.
Teich M (1970) Purkyne and Valentin on ciliary motion: an early investigation in morphological physiology. Br J Hist Sci 5:168-177.
Tong CK, Han YG, Shah JK, Obernier K, Guinto CD, Alvarez-Buylla A (2014) Primary cilia are required in a unique subpopulation of neural progenitors. Proc Natl Acad Sci U S A 111:12438-12443.
Wheatley DN, Wang AM, Strugnell GE (1996) Expression of primary cilia in mammalian cells. Cell Biol Int 20:73-81.
Willaredt MA, Hasenpusch-Theil K, Gardner HA, Kitanovic I, Hirschfeld-Warneken VC, Gojak CP, Gorgas K, Bradford CL, Spatz J, Wolf l S,eil T, Tucker KL (2008) A crucial role for primary cilia in cortical morphogenesis. J Neurosci 28:12887-12900.
Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr., Dlugosz AA, Reiter JF (2009) Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 15:1055-1061.
Yoshimura K, Kawate T, Takeda S (2011) Signaling through the primary cilium af f ects glial cell survival under a stressed environment. Glia 59:333-344.
Zaghloul NA, Katsanis N (2009) Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest 119:428-437.
*< class="emphasis_italic">Correspondence to: Qiaojie Xiong, Ph.D., Qiaojie.xiong@stonybrook.edu.
Qiaojie Xiong, Ph.D., Qiaojie.xiong@stonybrook.edu.
orcid: 0000-0003-1371-8137 (Gregory W. Kirschen) 0000-0002-9221-860X (Qiaojie Xiong)
10.4103/1673-5374.213535
Accepted: 2017-07-11
- 中国神经再生研究(英文版)的其它文章
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats
- Optical coherence tomography and T cell gene expression analysis in patients with benign multiple sclerosis
- Impact of Pitx3 gene knockdown on glial cell linederived neurotrophic factor transcriptional activity in dopaminergic neurons
- Dried Rehmannia root protects against glutamateinduced cytotoxity to PC12 cells through energy metabolism-related pathways
- Dexmedetomidine mitigates isof l urane-induced neurodegeneration in fetal rats during the second trimester of pregnancy