Dried Rehmannia root protects against glutamateinduced cytotoxity to PC12 cells through energy metabolism-related pathways
Yong Liu, Lei Liu Xi-xiang Ying, Wen-juan Wei Chao Han Yang Liu Chun-hui Han, Ai-jing Leng, Jing-yun Ma Jing Liu
1 Regenerative Medicine Center, the First Af filiated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
2 Department of Traditional Chinese Medicine, the First Af filiated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
3 Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning Province, China
4 Traditional Chinese Medicine Pharmacy, the First Af filiated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
Dried Rehmannia root protects against glutamateinduced cytotoxity to PC12 cells through energy metabolism-related pathways
Yong Liu1,2, Lei Liu1, Xi-xiang Ying3, Wen-juan Wei1, Chao Han1, Yang Liu1, Chun-hui Han1,4, Ai-jing Leng4, Jing-yun Ma1, Jing Liu1,*
1 Regenerative Medicine Center, the First Af filiated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
2 Department of Traditional Chinese Medicine, the First Af filiated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
3 Liaoning University of Traditional Chinese Medicine, Shenyang, Liaoning Province, China
4 Traditional Chinese Medicine Pharmacy, the First Af filiated Hospital of Dalian Medical University, Dalian, Liaoning Province, China
How to cite this article:Liu Y, Liu L, Ying XX, Wei WJ, Han C, Liu Y, Han CH, Leng AJ, Ma JY, Liu J (2017) Dried Rehmannia root protects against glutamate-induced cytotoxity to PC12 cells through energy metabolism-related pathways. Neural Regen Res 12(8):1338-1346.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81471308, 81271412; the Natural Science Foundation of Liaoning Province of China, No. 2014023052; and the Dalian Science and Technology Project Foundation of China, No. 2015SF11GH094.
Graphical Abstract
Rehmannia has been shown to be clinically ef f ective in treating neurodegenerative diseases; however, the neuroprotective mechanisms remain unclear. In this study, we established a model of neurodegenerative disease using PC12 cytotoxic injury induced by glutamate.e cells were treated with 20 mM glutamate in the absence or presence of water extracts of dried Rehmannia root of varying concentrations (70%, 50% and 30%).e dif f erent concentrations of Rehmannia water extract signif i cantly increased the activity of glutamate-injured cells, reduced the release of lactate dehydrogenase, inhibited apoptosis, increased the concentrations of NADH, NAD and ATP in cells, ameliorated mitochondrial membrane potential, and reduced the levels of light chain 3. Taken together, our fi ndings demonstrate that Rehmannia water extracts exert a cytoprotective ef f ect against glutamate-induced PC12 cell injuryviaenergy metabolism-related pathways.
nerve regeneration; Rehmannia water extracts; glutamate; PC12 cells; autophagy; energy metabolism; neural regeneration
Introduction
Neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, are a group of degenerative disorders of the central nervous system that primarily af f ect the elderly and severely reducing their quality of life. Glutamate is the principal excitatory neurotransmitter in the central nervous system, and modulates numerous physiological and pathophysiological processes (Durand et al., 2008). Excessive release of glutamate can directly lead to excitotoxicity in neurons through both ligand-gated ion channels, including the N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptors, and G-protein-coupled metabotropic glutamate receptors. Glutamate-mediated excitotoxicity is thought to play a role in many neurological diseases, such as brain trauma (Brittain et al., 2011), stroke (Brennan-Minnella et al., 2013), Alzheimer’s disease (Degoset al., 2013) and Parkinson’s disease (Hsieh et al., 2012).
Autophagy and energy metabolism-related signaling pathways play important roles in nerve injury (Koike et al., 2008; Wen et al., 2008; Cui et al., 2012).ere are no studies showing that Rehmannia root promotes nerve repair by regulating the cellular autophagy pathway. In the present study, we investigated the cytoprotective ef f ects of Rehmannia root against glutamate-induced cytotoxicity in PC12 cells using a perfused three-dimensional (3D) cell culture system. We also examined the cell and molecular mechanisms underlying the neuroprotective actions of the traditional Chinese medicine.
Materials and Methods
Ultra high performance liquid chromatography (UHPLC) of dif f erent Rehmannia extracts
Radix Rehmanniae (Huichuntang Co., Ltd., Anhui Province, China) was weighed, extracted with water, and then subjected to three serial extractions with AB-8 resin, consisting of 30%, 50% and 70% ethanol elutions (Table 1).e dif f erent extracts were weighed, transferred to 10 mL volumetric fl asks, ultrasonicated with methanol for 30 minutes, and centrifuged (15,000 r/min) twice for 5 minutes each.e resulting supernatant was fi ltered with a 0.45-µm microporous membrane.
olution preparation
Catalpol, 10.3 mg, was weighed and transferred to a 10-mL volumetric flask, according to the Chinese Pharmacopoeia (Chinese Pharmacopeia Commission, 2015) and dissolved in a 0.1% acetonitrile-phosphate solution.en, 1 mL of this liquidwas diluted to 100 mL.is stock solution was stored at 4°C.
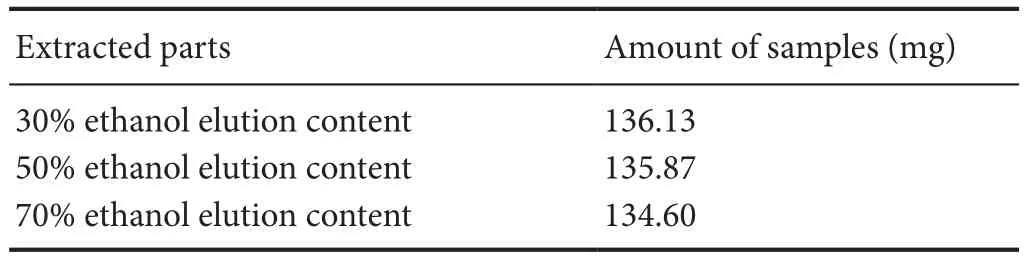
Table1 Rehmannia extracts from dif f erent parts and amount of dry product samples
Chromatographic conditions
A Shimadzu UHPLC system (Japan) was equipped with a Solvent Delivery Pump (Shimadzu LC-30AD), a vacuum degasser (DGU-20A), a Shimadzu UV-VIS spectrophotometric detector (SPD-20A) and ChemStation soware (Shimadzu). A Kromasil analytical column was used (5 µm, 150 × 4.6 mm). The mobile phase for UHPLC analysis, consisting of 0.1% acetonitrile-phosphate, was passed under vacuum through a 0.22-µm fi lter membrane and degassed by exposure to ultrasonic waves before use. UHPLC analysis with ultraviolet detection at 210 nm was performed at a fl ow rate of 1 mL/min.e column temperature was 35°C.e sample injection volume was 5 µL or 40 µL.e total run time was 55 minutes.
Cell culture
Rat PC12 (pheochromocytoma) cells were purchased from ATCC (Manassas, VA, USA). PC12 cells of passages 6–14 were cultured in 100-mm dishes in RPMI-1640 medium (Gibco, Waltham, MA USA) supplemented with 10% fetal bovine serum (Gibco) at 37°C in a humidif i ed atmosphere of 5% CO2.
Microbioreactors for parallel screening
Cells seeded on coverslips were placed in 4-well Tissue-Flex®(Zyoxel Ltd., Oxford, UK) microbioreactors (Figure 1) for parallel screening. Briefly, cells were plated at 2.4 × 106cells/cm2onto the coverslip in the wells of the microbioreactor, which was coated with 0.01% poly-L-lysine (Sigma-Aldrich, St. Louis, MO, USA), and cultured under perfusion conditions at 37°C. Culture medium (RPMI-1640 containing fetal bovine serum) was supplied continuously by a multi-channel peristaltic pump (TYD01-01, LeadFluid Technology Co., Ltd., Baoding, Hebei, China) at 0.5 µL/min. Aer 48 hours, the morphology of the cells was observed with an inverted microscope (Leica, Solms, Germany).
Cell viability analysis
Cell viability was measured with a quantitative colorimetric assay using 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma-Aldrich). Brief l y, PC12 cells were seeded onto 96-well plates at 1.0 × 104cells/well. Aer treatment with dif f erent concentrations of glutamate (0, 1, 5, 10 or 20 mM) for 24 hours, cells were incubated with MTT solution (0.5 mg/mL) for 4 hours at 37°C in the dark. A 100-µL volume of dimethyl sulfoxide was used to dissolve the crystals.e absorbance was measured spectrophotometrically using a microplate reader (BioTek, Vermont, USA) at 540 nm.e viability of treated cells was reported as a percentage of that of control cells.
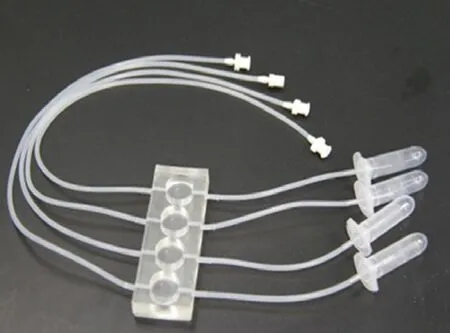
Figure 1e multiple parallel perfused microbioreactor (TissueFlex®) used for the experiments.
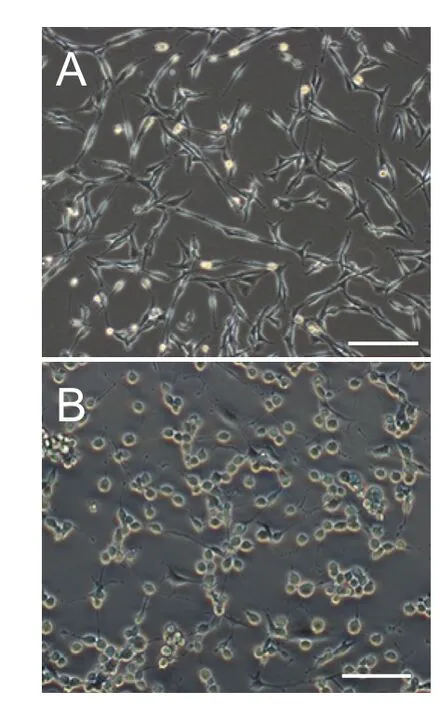
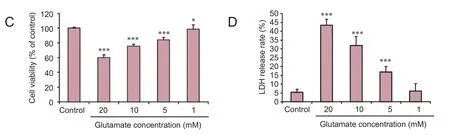
Figure 2 Morphology, viability and lactate dehydrogenase (LDH) release rate of PC12 cells before and aer treatment with glutamate.
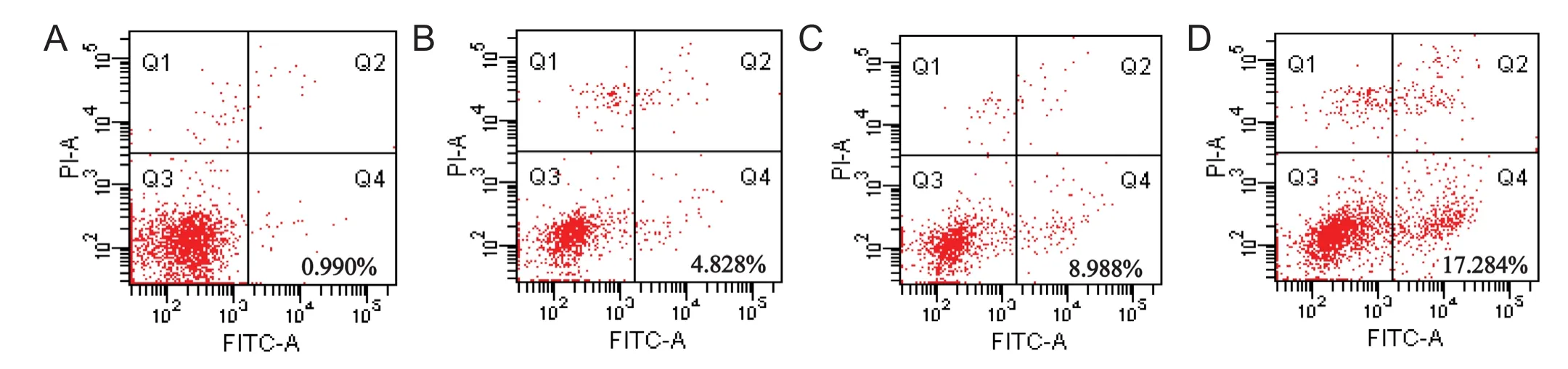
Figure 3 Dif f erent concentrations of water extract of dried Rehmannia root reduced the apoptosis rate.
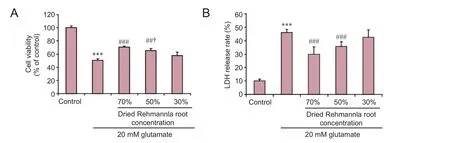
Figure 4 Ef f ects of dried Rehmannia root on PC12 cell injury induced by 20 mM glutamate.
Lactate dehydrogenase (LDH) release assay
LDH released from PC12 cells was assessed using the Cyto-Tox-ONE Homogeneous Membrane Integrity Assay (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Brief l y, cells were grown to 70% conf l uence in 96-well culture plates and exposed to 0, 1, 5, 10 or 20 mM glutamate for 24 hours.en, 50 µL of the culture supernatant was transferred to a separate assay plate.e LDH assay was allowed to proceed at room temperature for 10 minutes prior to the addition of 25 µL stop solution containing 10%sodium dodecyl sulfate per well. The contents of the wells were mixed by shaking the plates for 10 seconds prior to measuring resorufin fluorescence (560 nm excitation/590 nm emission).
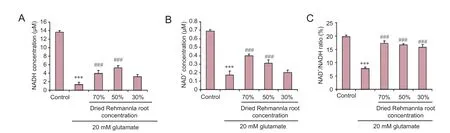
Figure 5 Ef f ects of dried Rehmannia root on energy metabolism in glutamate-treated PC12 cells.
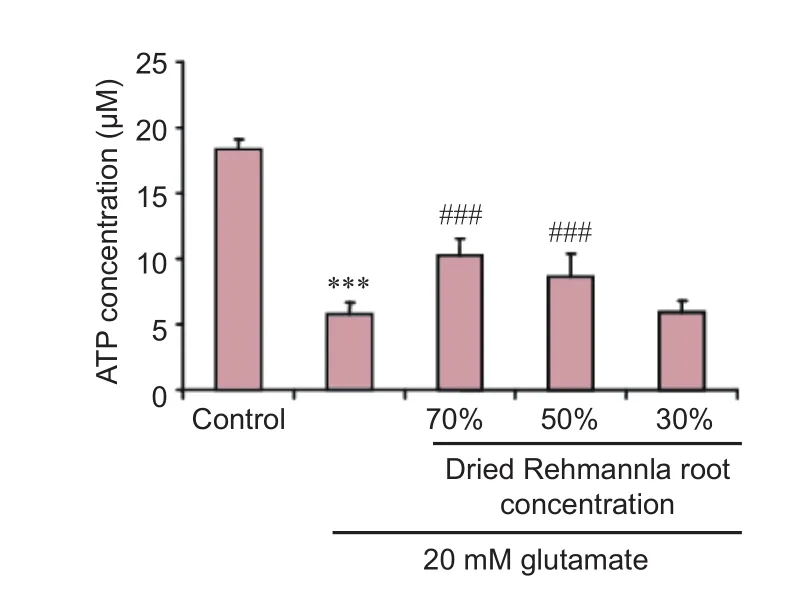
Figure 6 Ef f ects of dried Rehmannia root on adenosine triphosphate (ATP) concentration aer glutamate-induced PC12 cell injury (f l uorescence assay).
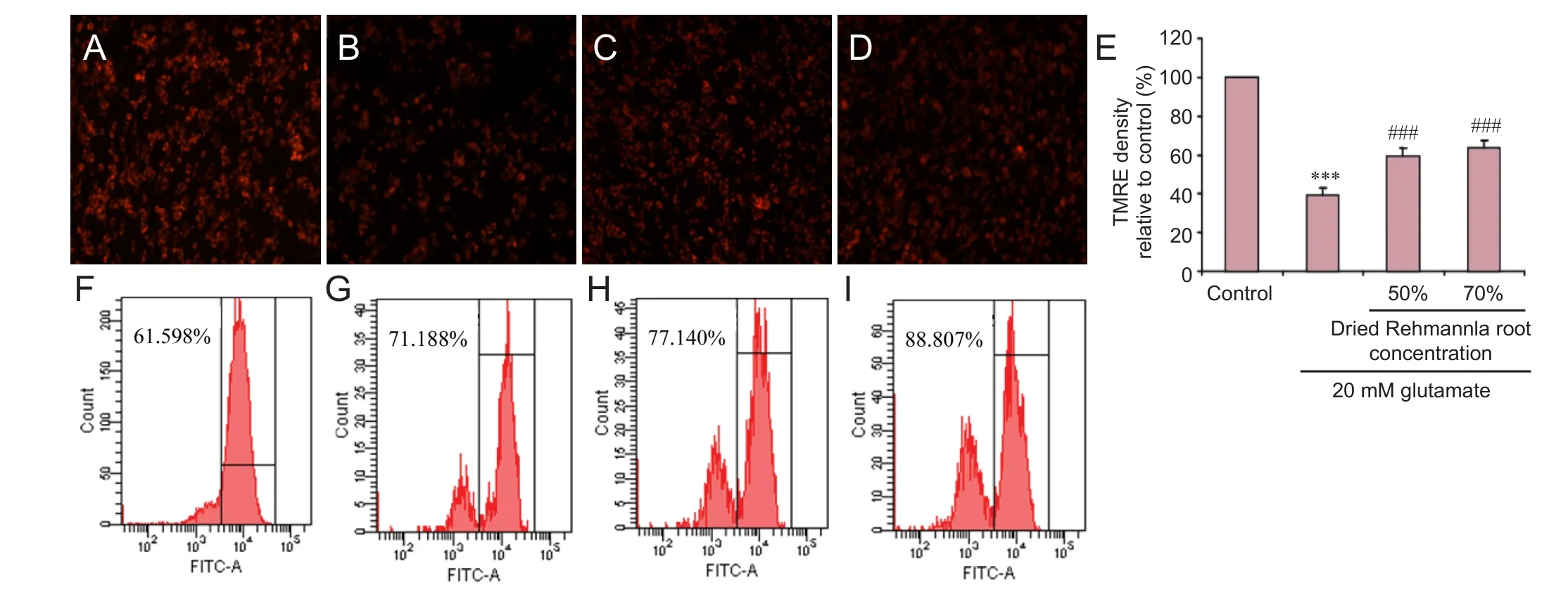
Figure 7 Mitochondrial membrane potential detected using the TMRE fl uorescent probe under the fl uorescence microscope.
Adenosine triphosphate (ATP) assay
Intracellular ATP levels were quantif i ed using an ATP Bioluminescence Assay Kit (Roche Applied Science, Basel, Switzerland) following the standard protocol. PC12 cellsexposed to 20 mM glutamate were treated with 0% (control), 70%, 50% or 30% dried Rehmannia root extract. Aer one wash with phosphate-buffered saline (PBS), the cells were lysed with the Cell Lysis Reagent (Beyotime Biotechnology, Shanghai, China), which was mixed with 50 µL Luciferase Reagent (Biovision, San Francisco, CA, USA). A plate reader (Biotek, Winooski, Vermont, USA) was used to detect the chemiluminescence of the samples.e ATP concentrations of the samples were calculated using an ATP standard curve, and normalized to the protein concentrations of the samples, which were determined using the bicinchoninic acid assay (Zhang et al., 2016).
Mitochondrial membrane potential (MMP) assay
MMP and superoxide levels were assessed after treating PC12 cells, plated at a seeding density of 1 × 105/cm2, with 20 mM glutamate. PC12 cells treated with 0%, 70%, 50% or 30% dried Rehmannia root for 24 hours (the 0% glutamate group served as the control). Cells were then incubated for 20 minutes at 37°C with 20 nM tetramethylrhodamine methyl ester (Life Technologies, Carlsbad, CA, USA) or 2.5 µM MitoSox (Life Technologies) (Pan et al., 2015). Six wells per treatment were analyzed, and the entire experiment was repeated on another batch of cells. Fluorescence intensity was analyzed using a CyAn ADP Analyzer (Beckman Coulter, Brea, CA, USA).
For measuring NAD+, mitochondria were isolated from conf l uent PC12 cells grown in T75 fl asks, lysed in extraction buffer from a commercial NADH/NAD determination kit (Biovision), and NAD was measured following the manufacturer’s protocol.e assay was performed in triplicate using three separate mitochondrial isolations.
Annexin V/propidium iodide (PI) assay using fl ow cytometry
Flow cytometry was performed to determine the levels of early-stage apoptosis, late-stage apoptosis and necrosis using the ApoScreen Annexin V kit (SouthernBiotech, Birmingham, AL, USA) according to the manufacturer’s protocol. Briefly, PC12 cells exposed to various concentrations of glutamate were digested with 0.25% trypsin, washed with cold PBS, and resuspended in cold 1 × binding buf f er at concentrations between 1.0 × 106and 1.0 × 107cells/mL. A 5-µL aliquot of labeled Annexin V was added to 100 µL of the cell suspension.e number of stained cells was assessed immediately on a fl ow cytometer (FACS Aria II, BD Biosciences, Franklin Lakes, NJ, USA).
Immunof l uorescence staining
PC12 cells cultured on slides were rinsed once with PBS, fi xed with 4% paraformaldehyde overnight, and then incubated with a rabbit anti-light chain 3 (LC3) polyclonal (1:100; Novus Biologicals, Los Angeles, CA, USA) primary antibody for 90 minutes at room temperature. The cells were then washed with PBS and incubated with rabbit FITC-conjugated secondary antibody (1:70; Abcam, Cambridge, MA, USA) for 60 minutes at 37°C. Nuclei were stained with Hoechst 33258 (Sigma-Aldrich) for 5 minutes at room temperature, and then mounted with glycerol. Images were captured on an Axioskop 2 fl uorescence microscope (Zeiss, Oberkochen, Germany).
Western blot assay
Protein concentrations were determined with the Bradford method (Redmile-Gordon et al., 2013). Proteins were separated by 12% SDS-PAGE and transferred electrophoretically onto nitrocellulose membranes (Bio Basic, New York, USA). The membranes were then reacted with rabbit anti-LC3 polyclonal antibody or rabbit anti-P-AKT polyclonal antibody (both 1:1,000; Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight, followed by horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA) at 37°C for 12 hours.e signals were detected with enhanced chemiluminescence detection kits (GE Healthcare, Chicago, IL, USA).e gray scales of the bands were quantified by scanning densitometry using Quantity One 4.5.0 soware (Bio-Rad, Hercules, CA, USA).
Autophagy inhibitor treatment
To examine whether the changes in expression of LC3 induced by the dried Rehmannia root extract were associated with an effect on autophagy, PC12 cells were treated with 3-MA (an autophagy inhibitor, 5 mM, Sigma-Aldrich) simultaneously with the dried Rehmannia root for 24 hours.
Statistical analysis
Data are expressed as the mean ± SD for all cells from at least three independent experiments. The significance was determined with one-way analysis of variance followed by paired Student’st-test.P< 0.05 was accepted as statistically signif i cant.
Results
UHPLC fi ngerprint analysis
From the UHPLC chromatogram, retention time and area, there were no obvious chromatographic peaks at retention times of 3.6, 8.3, 9.0, 12.0, 14.0, 28.6, 29.5, 33.5 or 39.0 minutes in the ethanol extract. In comparison, peaks were observed in the water extract at retention times of 10.0, 26.0 and 34.0 minutes. A larger number of chromatographic peaks with greater areas were observed in the water extract, water extract passed through the AB-8 resin and eluted with water, and the water extract passed through the AB-8 resin and eluted with 30% ethanol. More chromatographic peaks with a greater area were observed in the water extract passed through the AB-8 resin and eluted with 50% ethanol compared with 70% ethanol.ere were characteristic chromatographic peaks at retention times of 5.0, 5.7, 15.0, 16.2 and 18.6 minutes, compared with ethanol extraction at retention times of 20.7, 28.0 and 33.0 minutes.
Glutamate-induced PC12 cell injury
Screening for the optimal concentration of dried Rehmannia root using the perfused microbioreactor 3D cell culture system
Apoptosis is a major mode of cell death triggered by cytotoxic drug treatment (Smilansky et al., 2015). To evaluate the induction of apoptosis, the cells were stained with Annexin V-FITC and PI aer treatment with dif f erent concentrations of water extract of dried Rehmannia root.e apoptosis rate of cells treated with 70%, 50% and 30% concentrations of the dried Rehmannia root extract was 0.990 ± 0.20% (Figure 3A), 4.828 ± 0.16% (Figure 3B) and 8.988 ± 0.15% (Figure 3C), respectively.e rate of apoptosis in PC12 cells treated with glutamate, but without Rehmannia root, was 17.284 ± 2.20% (Figure 3D). These findings suggest that 70% dried Rehmannia extract protects PC12 cells from glutamate-induced apoptosis in the 3D bioreactor system.
Ef f ects of dried Rehmannia root on glutamate-induced cytotoxicity to PC12 cells
Ef f ects of dried Rehmannia root on energy metabolism in glutamate-treated PC12 cells
To assess whether glutamate-induced injury to PC12 cells affects energy metabolism, the levels of NAD+, NADH and NAD+/NADH were evaluated.e NADH concentration decreased from 13.63 µM to 1.33 µM, the NAD+concentration decreased from 0.69 µM to 0.37 µM, and the NAD+/NADH ratio decreased from 9.70% to 3.59% aer treatment with 20 mM glutamate. All three concentrations of dried Rehmannia root ameliorated glutamate-induced cellular injury, and the 70% and 50% concentrations had signif i cant ef f ects (Figure 5).
Ef f ects of dried Rehmannia root on ATP concentration in PC12 cells exposed to glutamate
To study whether dried Rehmannia root protects PC12 cells from glutamate-induced injury by impacting ATP levels, we measured ATP concentrations. All three concentrations of dried Rehmannia root alleviated glutamate cytotoxicity to varying degrees. In addition, ATP levels were significantly higher aer treatment with 70% and 50% concentrations of dried Rehmannia root compared with non-treated (0% Rehmannia) cells (Figure 6).
Ef f ects of Rehmannia on MMP in glutamate-treated PC12 cells
We next examined the ef f ects of Rehmannia on MMP.e various concentrations of Rehmannia enhanced MMP (Figure 7A–E). Flow cytometry assay conf i rmed these fi ndings (Figure 7F–I).
Ef f ects of Rehmannia on LC3 protein levels in glutamate-treated PC12 cells
Immunofluorescence and western blot assay were used to evaluate LC3 protein expression. To confirm whether the changes in expression of LC3 produced by Rehmannia were caused by an effect on autophagy, the autophagy inhibitor 3-MA was added simultaneously with the Rehmannia preparation for 24 hours. Immunof l uorescence assay showed that LC3 protein expression was enhanced after treatment with glutamate (Figure 8B). Aer treatment with 70% dried Rehmannia root for 6 hours, LC3 protein expression was lower compared with the non-treated group (Figure 8C). Western blot assay showed that LC3 protein levels were signif i cantly higher at 12 hours compared with the other time points.
Ef f ects of the autophagy inhibitor 3-MA on apoptosis in PC12 cells
Next, we sought to confirm whether the autophagy pathway is involved in the cytoprotective ef f ect of Rehmannia. PC12 cells were treated with the autophagy inhibitor 3-MA and/or Rehmannia, and apoptosis was assessed with fl uo-3 AM (calcium fluorescent probe) and acridine orange staining. In PC12 cells treated with both the autophagy inhibitor 3-MA and Rehmannia root, apoptosis was reduced compared with cells treated with Rehmannia root alone (Figure 9).
Discussion
The positive clinical outcomes and substantial efficacy of traditional Chinese medicines have brought hope for the treatment of Parkinson’s disease. Accumulating evidence indicates that traditional Chinese medicines not only alleviate Parkinsonian symptoms, but also reduce levodopa-induced dyskinesia and other complications (He et al., 2004). Current studies suggest that most Parkinsonian symptoms and levodopa-induced dyskinesia are the results of def i cient stirring wind pattern (Sheng et al., 2011; Park, 2017).e Rehmannia formulation was developed based on clinical experiences.is formulation has satisfactory clinical ef fi cacy in the treatment of Parkinson’s disease and levodopa-induced dyskinesia (Ge et al., 2012; Qiu et al., 2014).
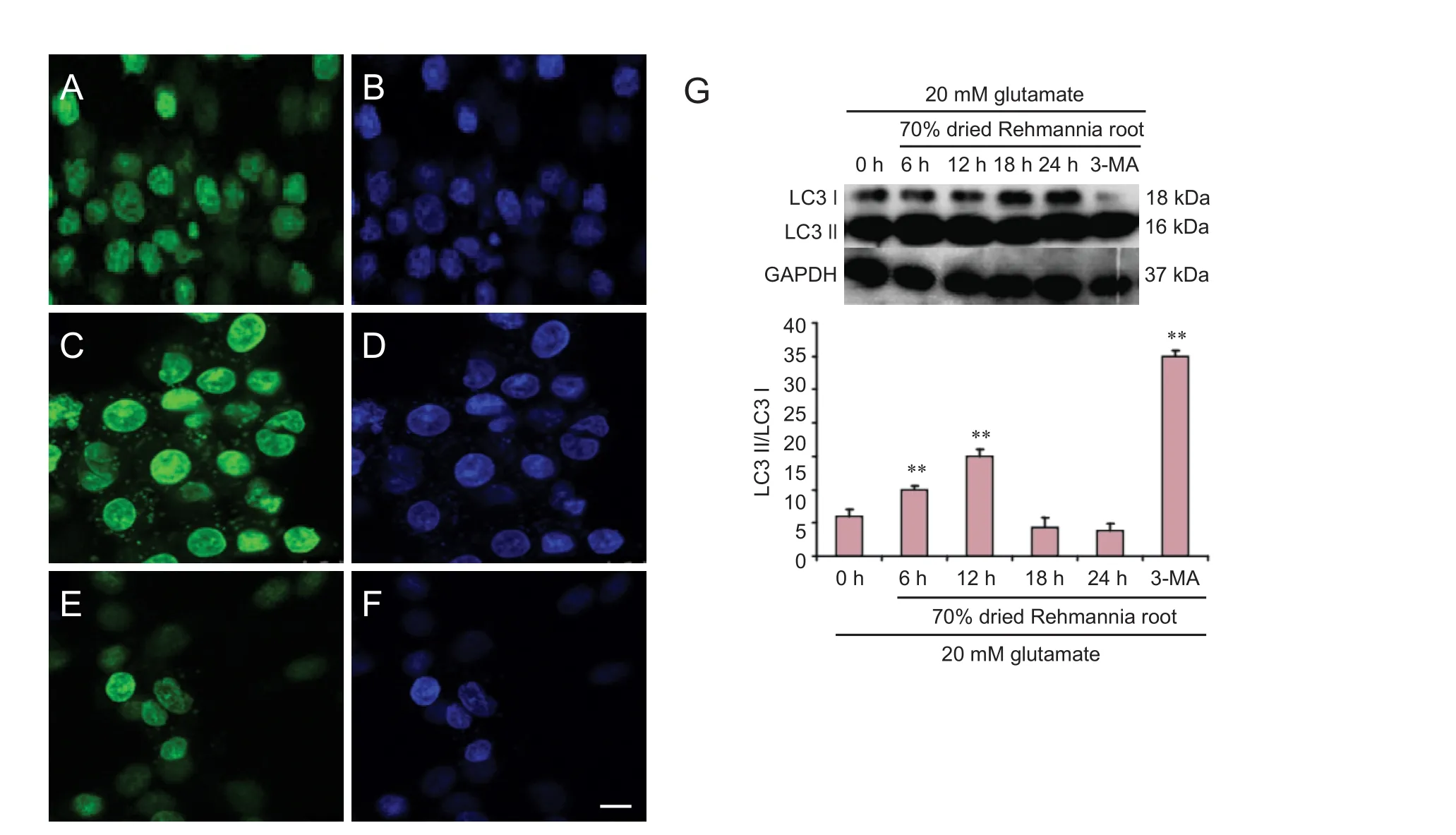
Figure 8 Autophagosomal LC3 protein and nuclei observed with laser confocal microscopy.
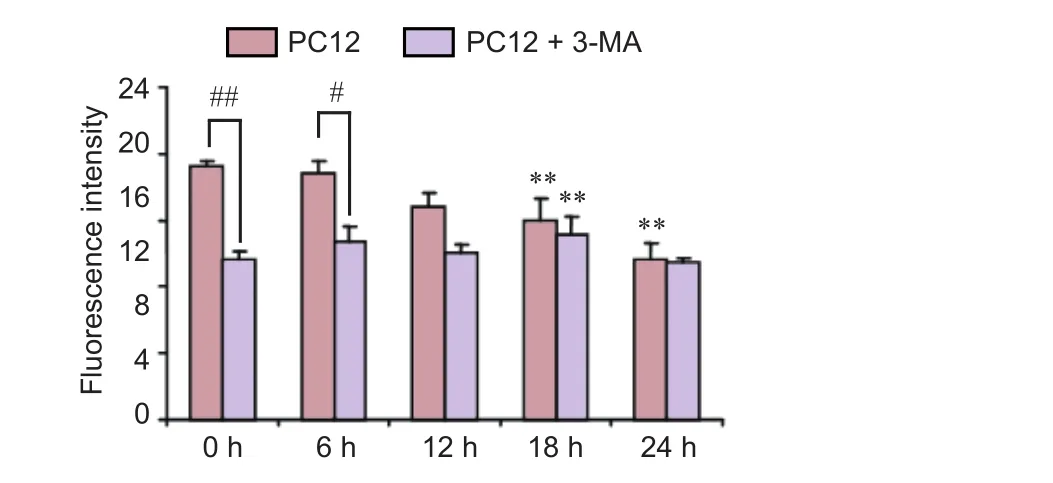
Figure 9 Ef f ects of the autophagy inhibitor 3-MA on the cytoprotective action of dried Rehmannia root in PC12 cells.
To assess the bioactivity of dif f erent Rehmannia extracts, UHPLC was used in this experiment using macroporous resin adsorption equipment, and the Rehmannia root extracts were eluted with different concentrations of ethanol. The UHPLC fi ngerprint was used for quality control (Liu et al., 2014).
A perfused 3D cell culture system was used for its advantages over static two-dimensional cell culture systems (Sadri et al., 2014; Li and Han, 2017), particularly in overcoming nutrient limitations by continuous replenishing of the culture medium (Wu et al., 2006; Wu et al., 2008).ese bioreactors have been used successfully for engineered tissue culture (Seidel et al., 2004) and for maintaining the viability of organs for a def i ned period of time.e innovative 3D cell culture system mimics the brain microcirculation and ensures adequate drug concentrations (Bijonowski et al., 2013; Pollock, 2013; Caralt, 2015; Coakley et al., 2016). Here, a model of glutamate-induced PC12 cell injury was established with the 3D cell culture system, and different Rehmannia compositions were perfused in parallel to screen for the optimal concentration (Coecke et al., 2002; Lemmo et al., 2014).
In the present study, PC12 cells were treated with glutamate to establish an excitotoxic injury model (Wang et al., 2014).e ef f ects of dif f erent concentrations of glutamate on PC12 cells were assessed with cell viability and LDH experiments (Wei et al., 2014). A glutamate concentration of 20 mM was found to be ef f ective, consistent with other studies (Zhao et al., 2015; Jia et al., 2016).
The 70% Rehmannia extract had the best cytoprotective ef f ect in the bioreactor, based on apoptotic rates.e cell viability and LDH release assays conf i rmed that 70% Rehmannia had the greatest cytoprotective ef f ect in our model.
Mitochondrial ATP is the main energy source for intracellular metabolic pathways (Schapira, 2006; Hagl et al., 2015; Shindo et al., 2015). Mitochondria synthesize ATP from ADP in the matrix using the energy provided by the proton electrochemical gradient (Capaldi et al., 1994; Nijtmans et al., 1995; Zeviani and Di Donato, 2004). Energy metabolism is critical for numerous processes, including apoptosis (Lawet al., 2017; Zhao et al., 2017). We examined LDH release, NAD+ and ATP concentrations, as well as mitochondrial membrane potential, and found that all three concentrations of Rehmannia root af f ected these biochemical parameters in the 3D system (Gao et al., 2016; Hu et al., 2016).
To further assess the changes in the autophagy pathway, PC12 cells were treated with the 70% concentration of Rehmannia root for different time periods. Also known as LC3-associated phagocytosis, the intersection of autophagy and phagocytosis was initially described as a pathway that limited the proliferation of engulfed pathogens by expediting phagosome maturation (Ejlerskov et al., 2013; Mehta et al., 2014).e expression of LC3 protein was examined by western blot assay, which showed that 70% Rehmannia root suppressed autophagy in a time-dependent manner (Liu et al., 2017). In cells co-treated with the autophagy inhibitor 3-MA and Rehmannia root, apoptosis was reduced compared with cells treated with Rehmannia root alone, suggesting that autophagy might be involved in mediating the cytoprotective action of the traditional Chinese medicine.erefore, Rehmannia might have therapeutic potential for the treatment of nervous system diseases (Ahn et al., 2015; Jiang et al., 2015).
It has been reported that Akt is necessary for neuronal viability and for restoring neurological function following trauma or ischemia (Maiese, 2016). In our previous studies, levels of phosphorylated Akt were increased in glutamate-treated PC12 cells, suggesting that Akt is neuroprotective during PC12 cell injury (Lin et al., 2014; Wei et al., 2016). Moreover, Akt is also a critical autophagy regulator, with activated Akt signaling suppressing autophagy (Lu et al., 2017).
Taken together, our fi ndings suggest that dried Rehmannia root has a signif i cant cytoprotective ef f ect against glutamate-induced injury in PC12 cells.is cytoprotective ef f ect appears to involve energy metabolism-related pathways. Further studies using in vivo experiments are needed to validate the current results. Additional studies are also required to clarify the role of autophagic signaling pathways in the neuroprotective functions of Rehmannia root.
Author contributions:YL (Yong Liu) and LL conceived and designed the study. YL (Yong Liu), XXY, WJW, CH, and CHH performed the experiments. YL (Yang Liu) polished the language. YL (Yong Liu) wrote the paper. AJL, JYM and JL reviewed and edited the paper. All authors read and approved the fi nal version of the paper.
Conf l icts of interest:None declared.
Data sharing statement:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Ahn SM, Kim YR, Kim HN, Choi YW, Lee JW, Kim CM, Baek JU, Shin HK, Choi BT (2015) Neuroprotection and spatial memory enhancement of four herbal mixture extract in HT22 hippocampal cells and a mouse model of focal cerebral ischemia. BMC Complement Altern Med 15:202.
Bijonowski BM, Miller WM, Wertheim JA (2013) Bioreactor design for perfusion-based, highly-vascularized organ regeneration. Curr Opin Chem Eng 2:32-40.
Brennan-Minnella AM, Shen Y, El-Benna J, Swanson RA (2013) Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death. Cell Death Dis 4:e580.
Brittain JM, Chen L, Wilson SM, Brustovetsky T, Gao X, Ashpole NM, Molosh AI, You H, Hudmon A, Shekhar A, White FA, Zamponi GW, Brustovetsky N, Chen J, Khanna R (2011) Neuroprotection against traumatic brain injury by a peptide derived from the collapsin response mediator protein 2 (CRMP2). J Biol Chem 286:37778-37792.
Capaldi RA, Aggeler R, Turina P, Wilkens S (1994) Coupling between catalytic sites and the proton channel in F1F0-type ATPases. Trends Biochem Sci 19:284-289.
Caralt M (2015) Present and future of regenerative medicine: liver transplantation. Transplant Proc 47:2377-2379.
Chinese Pharmacopeia Commission (2015) Chinese Pharmacopoeia. Beijing: China Medical Science Press.
Coakley DN, Shaikh FM, O’Sullivan K, Kavanagh EG, Grace PA, Mc-Gloughlin TM (2016) Design and evaluation of a novel subatmospheric pressure bioreactor for the preconditioning of tissue-engineered vascular constructs. Int J Artif Organs 39:77-83.
Coecke S, Eskes C, Gartion J, Vliet EV, Kinsner A, Bogni A, Raimondo L, Parissis N, Langezaal I (2002) Metabolism-mediated neurotoxicity: the signif i cance of genetically engineered cell lines and new three-dimensional cell cultures. Altern Lab Anim 30 Suppl 2:115-118.
Cui D, Wang L, Qi A, Zhou Q, Zhang X, Jiang W (2012) Propofol prevents autophagic cell death following oxygen and glucose deprivation in PC12 cells and cerebral ischemia-reperfusion injury in rats. PLoS One 7:e35324.
Cui YY, Hahn EJ, Kozai T, Paek KY (2000) Number of air exchanges, sucrose concentration, photosynthetic photon fl ux, and dif f erences in photoperiod and dark period temperatures af f ect growth of Rehmannia glutinosa plantlets in vitro. Plant Cell Tissue Organ Cult 62:219-226.
Degos V, Peineau S, Nijboer C, Kaindl AM, Sigaut S, Favrais G, Plaisant F, Teissier N, Gouadon E, Lombet A, Saliba E, Collingridge GL, Maze M, Nicoletti F, Heijnen C, Mantz J, Kavelaars A, Gressens P (2013) G protein-coupled receptor kinase 2 and group I metabotropic glutamate receptors mediate inf l ammation-induced sensitization to excitotoxic neurodegeneration. Ann Neurol 73:667-678.
Durand D, Pampillo M, Caruso C, Lasaga M (2008) Role of metabotropic glutamate receptors in the control of neuroendocrine function. Neuropharmacology 55:577-583.
Ejlerskov P, Rasmussen I, Nielsen TT, Bergstrom AL, Tohyama Y, Jensen PH, Vilhardt F (2013) Tubulin polymerization-promoting protein (TPPP/p25alpha) promotes unconventional secretion of alpha-synuclein through exophagy by impairing autophagosome-lysosome fusion. J Biol Chem 288:17313-17335.
Gao H, Chen Z, Fu Y, Yang X, Weng R, Wang R, Lu J, Pan M, Jin K, McElroy C, Tang B, Xia Y, Wang Q (2016) Nur77 exacerbates PC12 cellular injury in vitro by aggravating mitochondrial impairment and endoplasmic reticulum stress. Sci Rep 6:34403.
Ge J, He JC, Yuan CX (2012) Clinical Observation on treatment of Parkinson’s disease by nourishing liver and kidney. Shizhen Guoyi Guoyao 23:3070-3072.
Hagl S, Grewal R, Ciobanu I, Helal A, Khayyal MT, Muller WE, Eckert GP (2015) Rice bran extract compensates mitochondrial dysfunction in a cellular model of early Alzheimer’s disease. J Alzheimers Dis 43:927-938.
He JC (2005) On TCM Treatment of Parkinson’s Disease. Zhongyiyao Tongbao 4:12-14.
He JC, Yuan CX, Wei HC, Chen RX (2004) Ef f ects of chinese drugs for nourishing the liver and kidney and removing obstruction in collaterals and detoxication on tyrosine hydroxylase and its mRNA in parkinson’s disease model rats. Zhongyi Zazhi 45:140-142.
Hsieh MH, Ho SC, Yeh KY, Pawlak CR, Chang HM, Ho YJ, Lai TJ, Wu FY (2012) Blockade of metabotropic glutamate receptors inhibits cognition and neurodegeneration in an MPTP-induced Parkinson’s disease rat model. Pharmacol Biochem Behav 102:64-71.
Hu Y, Lv X, Zhang J, Meng X (2016) Comparative study on the protective ef f ects of salidroside and hypoxic preconditioning for attenuating anoxia-induced apoptosis in pheochromocytoma (PC12) cells. Med Sci Monit 22:4082-4091.
Jia J, Zhang L, Shi X, Wu M, Zhou X, Liu X, Huo T (2016) SOD2 mediates amifostine-induced protection against glutamate in PC12 cells. Oxid Med Cell Longev 2016:4202437.
Jiang B, Shen RF, Bi J, Tian XS, Hinchliffe T, Xia Y (2015) Catalpol: a potential therapeutic for neurodegenerative diseases. Curr Med Chem 22:1278-1291.
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y (2008) Inhibition of autophagy prevents hippocampal pyramidal neuron death aer hypoxic-ischemic injury. Am J Pathol 172:454-469.
Law BYK, Gordillo-Martinez F, Qu YQ, Zhang N, Xu SW, Coghi PS, Mok SWF, Guo J, Zhang W, Leung ELH, Fan XX, Wu AG, Chan WK, Yao XJ, Wang JR, Liu L, Wong VKW (2017) Thalidezine, a novel AMPK activator, eliminates apoptosis-resistant cancer cells through energy-mediated autophagic cell death. Oncotarget 8:30077-30091.
Lemmo S, Atef i E, Luker GD, Tavana H (2014) Optimization of aqueous biphasic tumor spheroid microtechnology for anti-cancer drug testing in 3D culture. Cell Mol Bioeng 7:344-354.
Li S, Han Y (2017) In vitro biomimetic platforms featuring a perfusion system and 3D spheroid culture promote the construction of tissue-engineered corneal endothelial layers. Sci Rep 7:777.
Lin SS, Zhu B, Guo ZK, Huang GZ, Wang Z, Chen J, Wei XJ, Li Q (2014) Bone marrow mesenchymal stem cell-derived microvesicles protect rat pheochromocytoma PC12 cells from glutamate-induced injury via a PI3K/Akt dependent pathway. Neurochem Res 39:922-931.
Liu J, Yang YX, Liu JH, Dong L, Liu LN, Li YJ (2014) Study on UIPLCDAD fi ngerprint of Disporum cantoniense. Zhong Yao Cai 37:1980-1983.
Liu L, Sun T, Xin F, Cui W, Guo J, Hu J (2017) Nerve growth factor protects against alcohol-induced neurotoxicity in PC12 cells via PI3K/ Akt/mTOR Pathway. Alcohol Alcohol 52:12-18.
Lu X, Lv S, Mi Y, Wang L, Wang G (2017) Neuroprotective effect of miR-665 against sevof l urane anesthesia-induced cognitive dysfunction in rats through PI3K/Akt signaling pathway by targeting insulin-like growth factor 2. Am J Transl Res 9:1344-1356.
Maiese K (2016) Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol 82:1245-1266.
Mehta P, Henault J, Kolbeck R, Sanjuan MA (2014) Noncanonical autophagy: one small step for LC3, one giant leap for immunity. Curr Opin Immunol 26:69-75.
Nijtmans LG, Klement P, Houstĕk J, van den Bogert C (1995) Assembly of mitochondrial ATP synthase in cultured human cells: implications for mitochondrial diseases. Biochim Biophys Acta 1272:190-198.
Pan Q, Zhang W, Wang J, Luo F, Chang J, Xu R (2015) Impaired voluntary wheel running behavior in the unilateral 6-hydroxydopamine rat model of Parkinson’s disease. J Korean Neurosurg Soc 57:82-87.
Park D (2017) Correspondence: The pathophysiology of pramipexole-associated dystonia in patients with Parkinson’s disease. Parkinsonism Relat Disord 38:106-107.
Pollock CA (2013) Toward a bioartif i cial kidney: will embryonic stem cells be the answer? Kidney Int 83:543-545.
Qiu H, Fu P, Fan W, Zuo C, Feng P, Shi P, Cao L, Liu F, Zhou L, Chen F, Zhong H, Gou Z, Liang Y, Shi M (2014) Treatment of primary chronic glomerulonephritis with Rehmannia glutinosa acteosides in combination with the angiotensin receptor blocker irbesartan: a randomized controlled trial. Phytother Res 28:132-136.
Redmile-Gordon MA, Armenise E, White RP, Hirsch PR, Goulding KW (2013) A comparison of two colorimetric assays, based upon Lowry and Bradford techniques, to estimsate total protein in soil extracts. Soil Biol Biochem 67:166-173.
Sadri S, Khazaei M, Ghanbari A, Khazaei MR, Shah P (2014) Neuronal differentiation of PC12 and embryonic stem cells in two- and three-dimensional in vitro culture. Indian J Exp Biol 52:305-311.
Schapira AH (2006) Mitochondrial disease. Lancet 368:70-82.
Seidel JO, Pei M, Gray ML, Langer R, Freed LE, Vunjak-Novakovic G (2004) Long-term culture of tissue engineered cartilage in a perfused chamber with mechanical stimulation. Biorh 41:445-458.
Sheng HM, He JC, Wang WW, Ding HJ, Luo RJ (2011)e literature research of syndromes of TCM about Parkinson’s disease. Shizhen Guoyi Guoyao 22:967-969.
Shindo Y, Yamanaka R, Suzuki K, Hotta K, Oka K (2015) Intracellular magnesium level determines cell viability in the MPP(+) model of Parkinson’s disease. Biochim Biophys Acta 1853:3182-3191.
Smilansky A, Dangoor L, Nakdimon I, Ben-Hail D, Mizrachi D, Shoshan-Barmatz V (2015)e voltage-dependent anion channel 1 mediates amyloid beta toxicity and represents a potential target for alzheimer disease therapy. J Biol Chem 290:30670-30683.
Teng L, Hong F, Zhang C, He J, Wang H (2014) Compound Formula Rehmannia alleviates levodopa-induced dyskinesia in Parkinson’s disease. Neural Regen Res 9:407-412.
Wang D, Guo TQ, Wang ZY, Lu JH, Liu DP, Meng QF, Xie J, Zhang XL, Liu Y, Teng LS (2014) ERKs and mitochondria-related pathways are essential for glycyrrhizic acid-mediated neuroprotection against glutamate-induced toxicity in dif f erentiated PC12 cells. Braz J Med Biol Res 47:773-779.
Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM, Guan J, Bao XJ, Wu H, Han Q, Wang RZ, Zhao CH (2016) Protection of nerve injury with exosome extracted from mesenchymal stem cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38:33-36.
Wei S (2016) Potential therapeutic action of natural products from traditional Chinese medicine on Alzheimer’s disease animal models targeting neurotrophic factors. Fundam Clin Pharmacol 30:490-501.
Wei T, Tian W, Yan H, Shao G, Xie G (2014) Protective effects of phillyrin on H2O 2-induced oxidative stress and apoptosis in PC12 cells. Cell Mol Neurobiol 34:1165-1173.
Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH (2008) Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 4:762-769.
Wu MH, Urban JP, Cui Z, Cui ZF (2006) Development of PDMS microbioreactor with well-def i ned and homogenous culture environment for chondrocyte 3-D culture. Biomed Microdevices 8:331-340.
Wu MH, Huang SB, Cui Z, Cui Z, Lee GB (2008) Development of perfusion-based micro 3-D cell culture platform and its application for high throughput drug testing. Sens Actuators B Chem 129:231-240.
Zeviani M, Di Donato S (2004) Mitochondrial disorders. Brain 127:2153-2172.
Zhang J, An S, Hu W, Teng M, Wang X, Qu Y, Liu Y, Yuan Y, Wang D (2016)e neuroprotective properties of hericium erinaceus in glutamate-damaged dif f erentiated PC12 cells and an Alzheimer’s disease mouse model. Int J Mol Sci 17.
Zhao H, Tan J, Qi C (2007) Photosynthesis of Rehmannia glutinosa subjected to drought stress is enhanced by choline chloride through alleviating lipid peroxidation and increasing proline Accumulation. Plant Growth Regul 51:255-262.
Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, Zhang S, Huang Q, Shi M (2017) ROS signaling under metabolic stress: cross-talk between AMPK and AKT pathway. Mol Cancer 16:79.
Zhao ZD, Zhang LH, Xia L, Zhang XT, Fang CS, Ma JR (2015) Protective ef f ects of Ginkgolide N against glutamate-induced injury in PC12 cells. Zhong Yao Cai 38:1694-1698.
Copyedited by Patel B, Haase R, Yu J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Jing Liu, Ph.D., liujing.dlrmc@hotmail.com.
Jing Liu, Ph.D., liujing.dlrmc@hotmail.com.
orcid: 0000-0002-3132-5647 (Yong Liu)
10.4103/1673-5374.213556
Accepted: 2017-07-22
- 中国神经再生研究(英文版)的其它文章
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- A novel triple immunoenzyme staining enables simultaneous identif i cation of all muscle fi ber types on a single skeletal muscle cryosection from normal, denervated or reinnervated rats
- Optical coherence tomography and T cell gene expression analysis in patients with benign multiple sclerosis
- Impact of Pitx3 gene knockdown on glial cell linederived neurotrophic factor transcriptional activity in dopaminergic neurons
- Dexmedetomidine mitigates isof l urane-induced neurodegeneration in fetal rats during the second trimester of pregnancy
- Cold water swimming pretreatment reduces cognitive def i cits in a rat model of traumatic brain injury