Electronic structure and photoluminescence property of a novel white emission phosphor Na3MgZr(PO4)3:Dy3+ for warm white light emitting diodes∗
Ge Zhu(朱革),Zhuo-Wei Li(李卓为),Chuang Wang(王闯),Fa-Guang Zhou(周发光), Yan Wen(温艳),and Shuang-Yu Xin(辛双宇),†
1 College of New Energy,Bohai University,Jinzhou 121000,China
2 School of Physics and Optoelectronic Engineering,Nanjing University of Information Science and Technology,Nanjing 210044,China
Electronic structure and photoluminescence property of a novel white emission phosphor Na3MgZr(PO4)3:Dy3+for warm white light emitting diodes∗
Ge Zhu(朱革)1,Zhuo-Wei Li(李卓为)1,Chuang Wang(王闯)1,Fa-Guang Zhou(周发光)1, Yan Wen(温艳)2,and Shuang-Yu Xin(辛双宇)1,†
1 College of New Energy,Bohai University,Jinzhou 121000,China
2 School of Physics and Optoelectronic Engineering,Nanjing University of Information Science and Technology,Nanjing 210044,China
To explore suitable single-phase white emission phosphors for warm white light emitting diodes,a series of novel phosphors Na3MgZr(PO4)3:x Dy3+(0≤x≤0.03)is prepared,and their phase purities as well as photoluminescence properties are discussed in depth via x-ray diffraction structure refinement and photoluminescence spectrum measurement. The electronic structure properties of the Na3MgZr(PO4)3host are calculated.The results reveal that Na3MgZr(PO4)3possesses a direct band gap with a band gap value of 4.917 eV.The obtained Na3MgZr(PO4)3:Dy3+phosphors are all well crystallized in trigonal structure with space group R¯3c,which has strong absorption around 365 nm and can generate warm white light emissions peaking at 487,576,and 673 nm upon ultraviolet excitation,which are attributed to the transitions from4F9/2to6H15/2,6H13/2,and6H11/2of Dy3+ions,respectively.The optimal doping content,critical distance,decay time,and Commission International de L’Eclairage(CIE)chromaticity coordinates are investigated in Dy3+ion-doped Na3MgZr(PO4)3.The thermal quenching analysis shows that Na3MgZr(PO4)3:Dy3+has a good thermal stability,and the thermal activation energy is calculated.The performances of Na3MgZr(PO4)3:Dy3+make it a potential single-phase white emission phosphor for warm white light emitting diode.
optical materials,optical properties,luminescence
1.Introduction
In recent years,white light emitting diodes(LEDs)have been considered as the next-generation solid-state light,substituting for the incandescent and energy saving lamps due to their unique advantages,such as high efficiency,environmentally friendly merit,long lifetime and energy saving.[1–4]Among many methods to obtain white LEDs,one method is by using the assembly of an ultraviolet(UV)LED chip with tri-color,namely blue,green,and red phosphors.This method can successfully avoid the inferior color rendering index(CRI)and unsuitable correlated color temperature(CCT) induced by the traditional combination of a blue LED chip with a yellow phosphor(Y3Al5O12:Ce3+).[5,6]However,the low luminescence efficiency caused by the reabsorption process and different degradation ratios within the tri-color phosphors as well as the complex manufacture process restrict their potential application. Therefore,the investigation of novel UV LED chip responded single-phase white light-emitting phosphor is still needed.[7,8]In such phosphor converted UV LEDs,the luminescent properties such as excitation and emission spectra,Commission International de L’Eclairage(CIE) chromaticity coordinates and the related color temperature (CCT)are important parameters for phosphors,which has great influence on luminesce spectrum,the CRI and the lumen efficiency of an LED lamp.As a result,the selection of luminescent centers and host materials of phosphors is of great importance.Among numerous luminescent centers,Dy3+ion is an important active ion,which has been widely used in phosphor for LED due to its versatile emissions in blue,yellow and red regions,which is attributed to the complex intra-configurational 4f states,typically its transitions4F9/2→6H15/2(∼480 nm),4F9/2→6H13/2(∼575 nm) and4F9/2→6H11/2(∼665 nm).[9,10]Thus,there is theoretical probability to achieve white light through the combination of these emissions from Dy3+ions.
A new phosphate structural family called“Nasicon”has received significant attention,which is constructed by a flexible rhombohedral structure with possibilities of isomorphic institutions for different groups of elements.[11]For these reasons,these group compounds receive much attention for their potential applications in the field of ionic conductors and radioactive waste immobilization.[12,13]In recent years,many reports have focused on the luminescent properties of phosphors with Nasicon structure,such asEu0.5Zr2(PO4)3(blue emission),Cu0.5Mn0.25Zr2(PO4)3(blue and orange emission),Na4NbP3O12:Dy3+,Tb3+(white and green emission)Na1−xMg1−xSc1−x(MoO4)3:Eu3+(0≤x≤0.5)(red emission),etc.[14–20]Compound Na3MgZr(PO4)3(NMZP)belongs to the Nasicon system,which has been extensively studied because of its low thermal expansion and ionic conductivity.[21]However,reports on luminescent properties based on NMZP are limited till now.In this work,for some basic studies and promising applications in white LEDs, NMZP:Dy3+is prepared for the first time,the x-ray diffraction (XRD)structure refinement and photoluminescence properties are analyzed.
2.Experiment
Solid-state synthesis method is the most extensively used technique to prepare phosphors,which is easy,efficient,and suitable for mass production.In this work,samples of NMZP:Dy3+(0≤x≤0.03)are prepared via high temperature solid state method with analytical-grade Na2CO3, MgO,Zr(NO3)4·5H2O,(NH4)2HPO4,and Eu2O3as raw materials.The mixture is then placed into an alumina crucible and heated at 1150°C in air for 8 h and then cooled down to room temperature slowly with a cooling rate of 5°C/min.
The phase purity is identified by using a Rigaku D/Max-2400 x-ray diffractometer with Ni-filtered Cu Kα radiation. The luminescence spectra of the samples are measured by using an FL-1039(Horiba Jobin Yvon) fluorescence spectrophotometer equipped with a 450 W xenon light source. The PL decay curves are measured by using an FLS-920T fluorescence spectrophotometer equipped with a millisecond Flashlamp.High-temperature luminescence intensity measurements are tested by using an aluminum plaque with cartridge heaters,and the temperature is measured by thermocouples inside the plaque and controlled by a standard TAP-02 high-temperature fluorescence controller.The electronic structure is investigated by using the CASTEP package of Materials Studio software.[22]
3.Results and discussion
Figure 1(a)illustrates the XRD Rietveld refinement of NMZP:0.002Dy3+phosphor by Materials Studio and it shows that NMZP:Dy3+crystallizes well in a trigonal crystal structure with space group R¯3c and cell parameters a=b= 8.8469˚A,c=22.2668˚A.[23]The goodness-of-fit parameters(Rwp=12.43%,Rp=8.97%)guarantee the phase purity of NMZP.In each NMZP unit cell,the number of available cationic sites is 8,including 1 Na,2 Mg,1 Zr,and 4 P atoms, of which P atoms adopt tetrahedral groups(blue tetrahedron) while the two Na atoms named Na1 and Na2 occupy the irregular polyhedral cavities with coordination numbers of six and eight,respectively,as depicted in the inset of Fig.1(a). Mg/Zr atoms occupy the same sites with six-fold coordinated in an octahedral environment.According to the effective ionic radius,Dy3+ions are expected to enter Na+sites when they are introduced into NMZP host.[24]A series of XRD patterns of NMZP:x Dy3+(0≤x≤0.03)is plotted in Fig.1(b).The diffraction peaks are well fitted with the calculated XRD patterns and no second phase is observed,indicating that the addition of Dy3+has no significant change in the NMZP structure.

Fig.1.(color online)(a)XRD Rietveld structure analysis of NMZP:0.002Dy3+by using Materials Studio program The inset shows the crystal structure diagram of NMZP host(b)XRD patterns of NMZP:x Dy3+(0≤x≤0.03)and the calculated XRD patterns.
Figures 2(a)and 2(b)show the calculation results of electronic structure property of NMZP host based on the density functional theory.The local-density approximation(LDA)is used as the theoretical basis of the density function.The band structure calculation results indicate that the valence band (VB)of NMZP consists of two parts which are located in energy ranges from−15 to−23 eV and from 0 to−8 eV,which are mainly contributed from the s orbits of Na,s and p orbits of Zr,p orbits of P and s and p orbits of O atoms,respectively. The conduction band(CB)structure is located in an energy range from 4 to 10 eV and is mainly attributed to the p of P and s and p orbits of Zr atoms.The top of VB and bottom of CB are at the same point(point G)of the Brillouin zone,which indicates that NMZP has a direct band gap of about 4.971 eV.

Fig.2.(color online)(a)Calculated band structure and(b)properties of total and partial density of states of NMZP host.

Fig.3.(color online)(a)PL excitation spectra of NMZP:x Dy3+(0.002≤x≤0.03)monitored at 576 nm;(b)the emission spectra of NMZP:x Dy3+(0.002≤x≤0.03)excited at 365 nm;(c)the decay curve of NMZP:0.02Dy3+monitored at 576 nm and excited at365 nm;(d)the CIE 1931 diagram of NMZP:0.02Dy3+and the standard white light.
Figures 3(a)and 3(b)illustrate the excitation and emission spectra of NMZP:x Dy3+(0.002≤x≤0.03)phosphors. When the phosphors are monitored at 576 nm,the spectra of NMZP:x Dy3+(0.002≤x≤0.03)consist of a series of sharp peaks in a range of 280–500 nm,caused by the transitions from the ground state6H15/2to higher energy states of Dy3+as plotted in Fig.3(a).[7,8]The strong absorption around 365 or 400 nm indicates that it has potential applications in UV pumped white LEDs.Under 365 nm excitation, the emission spectra of NMZP:x Dy3+(0.002≤x≤0.03)contain three typical emission peaks located respectively at about 487,576,and 673 nm,which can be ascribed to the transitionsof Dy3+.[7,8]Normally,the structure of the host matrix has a great influence on the optical performance of the phosphor.If Dy3+takes a high symmetry site,the blue emission will be prominent,otherwise,the yellow emission will be dominant.[25]The NMZP:x Dy3+(0.002≤x≤0.03)phosphors each have a strong peak at 576 nm,which implies that Dy3+occupies a low symmetry site,consistent with the disordered lattice environment around Na+in NMZP host.With the increase of doping content x,both the excitation and emission intensities are gradually enhanced until x increases to 0.02, reaching the concentration quenching.Since the valence electrons of Dy3+are shielded by 5s and 5p outer electrons,the line emission shape mainly remains the same as Dy3+contents vary.[26]According to Blasse’s report,the critical distance Rcfor the relevant energy transfer between Dy3+ions can be es-ti mated from the following equation:
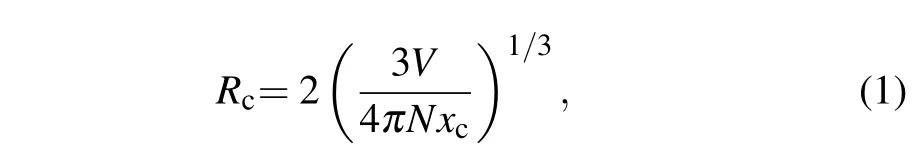
where xcis the critical concentration(xc=0.02),V is the unit cell volume(V=1506.99˚A3)and N is the available site number of the dopant in the unit cell(N=6)according to the XRD structure refinement results.Thus,Rccan be calculated to be 28.84˚A,suggesting that the energy transfer is through electric multipolar interaction exchange rather than interaction mechanism.[27]
The room-temperature decay curve of NMZP:0.02Dy3+is depicted in Fig.3(c).The decay curve can be well fitted into a double exponential equation I(t)=A1exp(−t/τ1)+ A2exp(−t/τ2)as reported and the average lifetime(τ)is calculated to be 1.63 ms.[28]The short decay time indicates that NMZP:Dy3+is suitable to serve as a potential phosphor in solid-state lighting.Besides,the CIE chromaticity coordinates of NMZP:0.02Dy3+are calculated to be(0.403,0.416)and the CCT is 3707 K as shown in Fig.3(d),which is warmer than that of the standard white light(0.33,0.33).The CIE and CCT results indicate that the warm white light could be obtained from Dy3+-doped NMZP phosphor.

Fig.4.(color online)(a)Thermal quenching emission spectra of NMZP:0.02Dy3+excited at 365 nm;inset shows the comparison of thermal stability between NMZP:0.02Dy3+and commercial yellow phosphor YAG:Ce3+;(b)calculated activation energy for thermal quenching based on the linear fitting results.
The thermal quenching property is of importance for future applications in white LEDs.In order to study the relationship between temperature and luminescence properties,the thermal quenching spectra of NMZP:0.02Dy3+are measured in a temperature range from the room temperature to 230°C,and the results are shown in Fig.4(a). The temperature-dependent spectra of the commercial yellow phosphor YAG:Ce3+are measured and plotted in the inset of Fig.4(a)as well.It clearly shows that the emission intensities of both NMZP:0.02Dy3+and YAG:Ce3+decrease with increasing temperature.The emission intensity of NMZP:0.02Dy3+at 230°C is still about 62%of its initial value,suggesting that it has good thermal stability.To further discuss the thermal quenching phenomenon,the activation energy(ΔE)is calculated from the modified Arrhenius equation[29,30]

where I refers to the emission intensity,a function of temperature;I0is the initial emission intensity;C is a constant for the thermally activated escape;ΔE is the activation energy;k is the Boltzmann constant.[29,30]As shown in Fig.4(b),the results can be fitted linearly,which indicates that the temperature quenching process complies well with the Arrhenius-type activation model,and the activation energy ΔE is calculated to be 0.142 eV from the slope of the plot.
4.Conclusions
In this work,a series of warm white light emission phosphors NMZP:x Dy3+(0≤x≤0.03)is prepared via a high temperature solid-state reaction.The phase purity,electronic structure and the photoluminescence properties are investigated in detail.The XRD results indicate that each of the samples is of single phase and crystallizes well into a trigonal crystal system.Electronic structure property shows that NMZP possesses a direct band gap of about 4.917 eV.Photoluminescence property reveals that NMZP:Dy3+has strong absorption around 365 nm and could produce warm white emission,upon 365 nm excitation,with three emission peaks at 576,487,and 673 nm,which originate from the transitionsions.The optimal doping content is determined to be 0.02 and the critical distance Rcis calculated to be 28.84˚A.The decay time is measured to be 1.63 ms in Dy3+-doped NMZP.Moreover, NMZP:Dy3+phosphor shows stable color tone with CIE coordinates(0.403,0.416)and warm CCT of 3707 K.The thermal quenching property investigation shows that the NMZP:Dy3+phosphor has good thermal stability.The emission intensity of NMZP:Dy3+drops to 62%of its initial value at 230°C.The results show that the novel phosphor NMZP:Dy3+could be a potential white light emission phosphor for UV light pumped warm white LEDs.
[1]Yu X,Wang T,Xu X,Jiang T,Yu H,Jiao Q,Zhou D and Qiu J 2014 RSC Adv.4 963
[2]Bian L,Wang T,Song Z,Liu Z,Li J and Liu Q 2013 Chin.Phys.B 22 077801
[3]Liu W Q,Chao K F,Wu W J,Bao F Q and Zhou B Q 2016 Acta Phys. Sin.65 207801(in Chinese)
[4]Zhuo N Z,Zhang N,Li B C,Li W Q,He Q Y,Shi F H,Zhu Y H,Xing H D and Wang H B 2016 Acta Phys.Sin.65 058501(in Chinese)
[5]Roushan M,Zhang X and Li J 2012 Angew.Chem.Int.Ed.124 451
[6]Du Y,Shao C,Dong Y,Yang Q and Hua W 2015 Chin.Phys.B 24 0117801
[7]Lin C C and Liu R S 2011 J.Phys.Chem.Lett.2 1268
[8]Ci Z,Zhang J and Wang Y 2010 Chin.Phys.B 19 057803
[9]Liu B,Kong L J and Shi C S 2007 J.Lumin.122 121
[10]Vijay S,Zhu J,Gundu Rao T K,Manoj T and Pan H 2005 Chin.Phys. Lett.22 03182
[11]Orlova A I 2002 Radiochemistry 44 423
[12]Park Y U,Seo D H,Kim B,Hong K P,Kim H,Lee S,Shakoor R A, Miyasaka K,Tarascon J M and Kang K 2012 Scientific Reports 2 704
[13]Oota T and Yamai I 1986 J.Am.Ceram.Soc.69 1
[14]Masui T,Koyabu K,Tamura S and Imanaka N 2006 J.Alloys Compd. 418 73
[15]Mouline A,Alami M,Brochu R,Olazcuag R,Parent C and Flem G L 2000 J.Solid State Chem.152 453
[16]Sobha K C and Rao K J 1996 J.Phys.Chem.Solids 57 1263
[17]Kozhevnikova N M and Tsyretarova S Y 2015 Inorg.Mater.51 494
[18]Koguma I,Oishi K,Takase S and Shimizua Y 2009 Ecs Transactions 16 81
[19]He Y,Quan B,Wang Y,Cheng Y and Wang B 2007 Mater.Lett.61 4519
[20]Kim Y H and Im W B 2015 28th ECS Meeting,Phoenix,AZ
[21]Kumar B V,Velchuri R,Prasad G and Vithal M 2009 Ceram.Int.35 2719
[22]Clark S J,Segall M D,Pickard C J,Hasnip P J,Probert M J,Refson K and Payne M C 2005 Zeitschrift fuer Kristallographie 220 567
[23]Miura H,Ushio T,Nagai K,Fujimoto D,Lepp Z,Takahashi H and Tamura R 2003 Cryst.Growth Des.3 6
[24]Shannon R 1976 Acta Crystallogr.32 751
[25]Xiu Z,Liu S,Ren M,Liu J,Pan J and Cui X 2006 J Alloy Compd.425 261
[26]Ye S,Xiao F,Pan Y X,Ma Y Y and Zhang Q Y 2010 Mater.Sci.Eng. R 7 1
[27]Xin S,Wang Y,Wang Z,Zhang F,Wen Yand Zhu G 2011 Electrochem. Solid-State Lett.14 H438
[28]Tian Y,Chen B J,Hua R N,Sun J S,Cheng L H,Zhong H Y,Li X P, Zhang J S,Zheng Y F,Yu T T,Huang L B and Yu H Q 2011 J.Appl. Phys.109 053511
[29]Liu C Y,Xia Z G,Molokeev M S,Liu Q L and Guo H 2015 J.Am. Ceram.Soc.98 1870
[30]Wang X C and Wang Y H 2015 J.Phys.Chem.C 119 16208
19 January 2017;revised manuscript
17 April 2017;published online 31 July 2017)
10.1088/1674-1056/26/9/097801
∗Project supported by the Doctoral Research Fund of Liaoning Province,China(Grant No.201601351),the National Natural Science Foundation of China (Grant No.51502142),and the General Program of Natural Science Foundation of the Jiangsu Provincial Higher Education Institutions,China(Grant No.15KJB430021).
†Corresponding author.E-mail:xinshuangyu@bhu.edu.cn
©2017 Chinese Physical Society and IOP Publishing Ltd http://iopscience.iop.org/cpb http://cpb.iphy.ac.cn
- Chinese Physics B的其它文章
- Improved control for distributed parameter systems with time-dependent spatial domains utilizing mobile sensor actuator networks∗
- Geometry and thermodynamics of smeared Reissner–Nordström black holes in d-dimensional AdS spacetime
- Stochastic responses of tumor immune system with periodic treatment∗
- Invariants-based shortcuts for fast generating Greenberger-Horne-Zeilinger state among three superconducting qubits∗
- Cancelable remote quantum fingerprint templates protection scheme∗
- A high-fidelity memory scheme for quantum data buses∗

