Electrostaticspray preparation and properties of RDX/DOS composites
Jian Yao,Jian Liu,Yong-xu Wang,Bin Li,Li-feng Xie
School of Chemical Engineering,Nanjing University of Science and Technology,Jiangsu,Nanjing 210000,PR China
Electrostaticspray preparation and properties of RDX/DOS composites
Jian Yao*,Jian Liu,Yong-xu Wang,Bin Li,Li-feng Xie
School of Chemical Engineering,Nanjing University of Science and Technology,Jiangsu,Nanjing 210000,PR China
A R T I C L E I N F O
Article history:
31 March 2017
Accepted 10 May 2017
Available online 10 May 2017
RDX
DOS
Electrostaticspray
Composites
Insensitive
A composite explosive based on 1,3,5-trinitro-1,3,5-triazinane(RDX)was prepared by electrostaticspray method with dioctyl sebacate(DOS)as desensitizer.After preparation,the particle size and crystal structure were characterized and chemical features,such as chemical bonds,functional groups, thermal decomposition parameters and mechanical sensitivity were investigated as well.In terms of the morphologies of the composites,the particle sizes were in the range of 1-3μm.Compared with RDX,the crystal types,chemical bonds and functional groups of the RDX/DOS composites were unchanged.The activation energy of the composites was lower than that of raw RDX,and the 3wt%DOS composites had the lowest activation energy.The impact sensitivity and friction sensitivity of the RDX/DOS composites were lower than those of raw RDX,and the 10wt%DOS composites had the highest H50(125.9 cm)and the lowest friction sensitivity(8%).
©2017 Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
The improvement in the energy of one explosive always brings the increase in its sensitivity,which results to the development of compound explosives.RDX is one of the common explosives with great power and high mechanical sensitivity.How to reduce sensitivity of RDX as well as maintain a high energy is a challenging question[1-7].DOS,an oily substance,can be used to coat RDX and the excellent sensitivity of the RDX/DOS composites has been proved[8,9].In the production of RDX-based composite explosives, it is common to combine RDX and DOS together with traditional mixing method(mixing and stirring)[10-14],but the uniformity of mixture cannot be maintained well and fundamental changes on the characteristics of mixture components were never found.It is meaningful to develop a new method to update the mixing process.
In this paper,we demonstrated the preparation of the RDX/DOS composites by electrostaticspray method,which was used to break molten liquid or solution into tiny droplets by the electrostatic field force,had successfully been used to produce uniform nanometersized particles[15-21].However,insuf ficient researches had been conducted into electrostaticspray method in the field of energetic materials.In this work,RDX-based composites with DOS were prepared byelectrostaticspray.Furthermore,the properties of the composites were characterized and analyzed in detail.
2.Materials and methods
2.1.Materials
Raw RDX(99.9%)was provided by Jiangsu Yongfeng Machinery Co.Ltd.;DOS(AR,97%)was provided by Aladdin Reagent (Shanghai)Co.Ltd;acetone(AR,99.5%)was provided by Shanghai Lingfeng Chemical Reagent Co.Ltd.
2.2.Preparation of the RDX/DOS composites
RDX and DOS(1000 mg in total,and the mass ratios of F2604 to RDX were 1:99,3:97,5:95 and 10:90,respectively)were dissolved in acetone(25 ml)to form a solution.As shown in Fig.1,the RDX/ DOS composites were prepared by electrostaticspray method.The solution was sprayed in four injection syringes by the TYD01-02 Syringe Pump(Lead Fluid Ltd.,China).The voltage was 19 kV, which was provided by the high voltage direct-current power supply(Dongwen High Voltage Power Supply(Tianjin)Ltd.,China), the flow rate of solution in the injection syringe was 1 ml h-1,and the receiving distance was 10 cm.The composite particles were collected on aluminum foils.
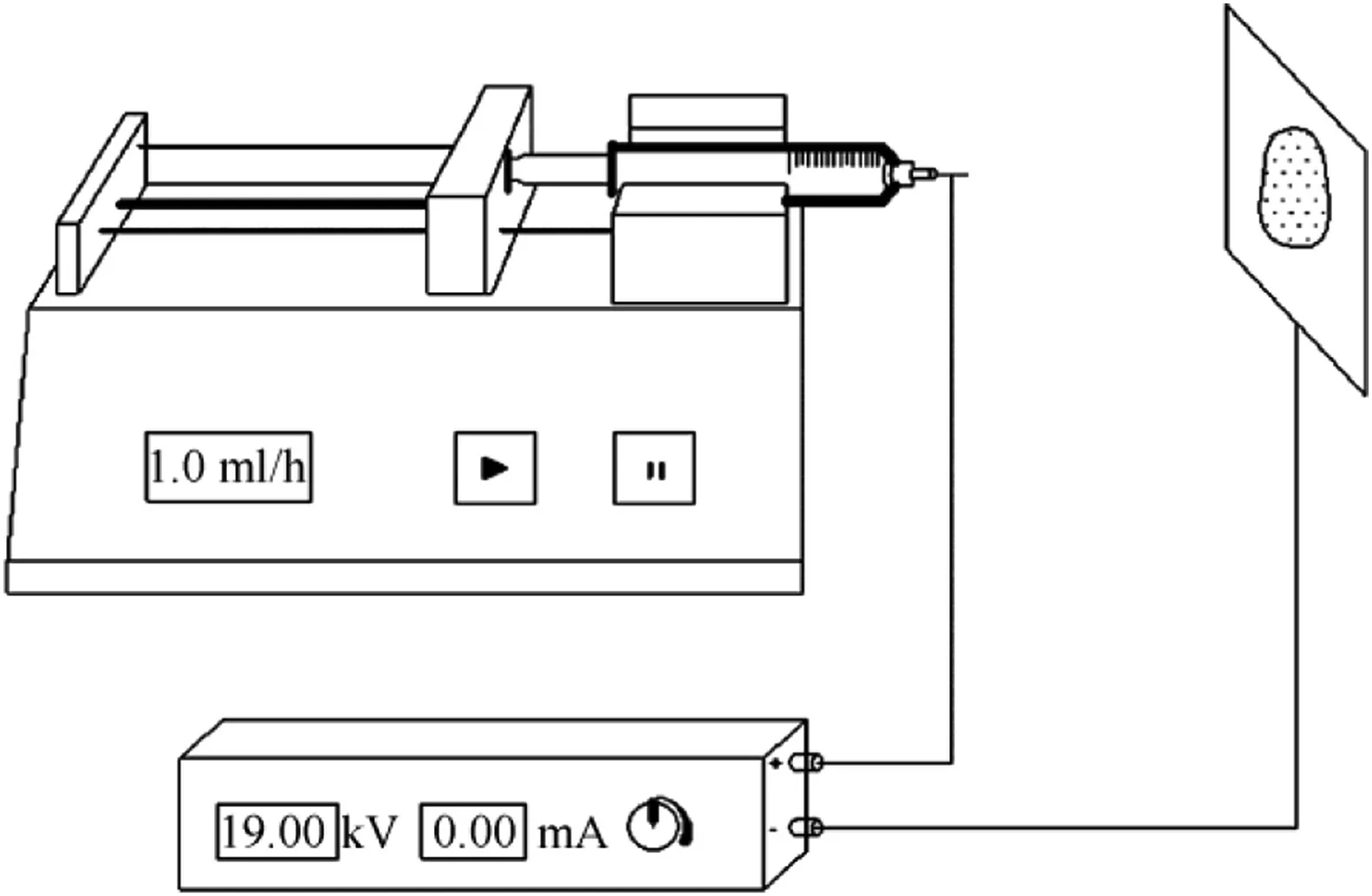
Fig.1.The experimental set up.
2.3.Characterization
The morphologies and sizes of the RDX/DOS composite particles were studied by QUANTA 250 FEG scanning electron microscope (SEM)(FEI Ltd.,America)with an electron beam spot of 2.0 and a generator voltage of 15 kV.
The chemical bonds and functional groups were studied by VERTEX70 fourier infrared spectrometer(FT-IR)(Bruker Ltd.Germany)with the spectral area of 500-4000 cm-1.
The crystal structure was studied by the D8 advance X-ray diffractometer(XRD)(Bruker Ltd.Germany)with Cu-Kαradiation at a generator voltage of 40 kV and a generator current of 200 mA. The scan range in 2θwas from 10°to 60°.
The thermal decomposition properties of the composites were studied by DSC1 differential scanning calorimeter(DSC)(Mettler Toledo Ltd.Switzerland)with closed stainless steel crucibles and about 0.7 mg of samples.The analysis was performed under a pure nitrogen atmosphere(50 ml min-1)at the heating rates of 1,2,4,10 K.min-1and the temperature was tested from 160°C to 280°C.
2.4.Tests of mechanical sensitivity
The impact sensitivity of the RDX/DOS composites was tested with a sample mass of 30±1 mg and a drop weight of 2 kg.Two groups of each sample and 25 same samples from each group were tested.The results were shown in terms of the critical drop-heightof 50 per cent explosion probability(H50).
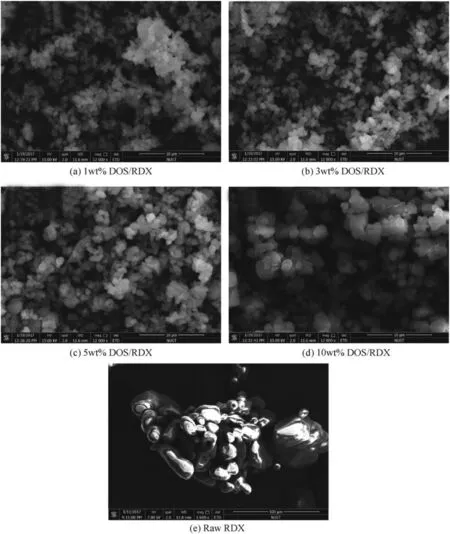
Fig.2.The SEM images of raw RDX and the RDX/DOS composites.
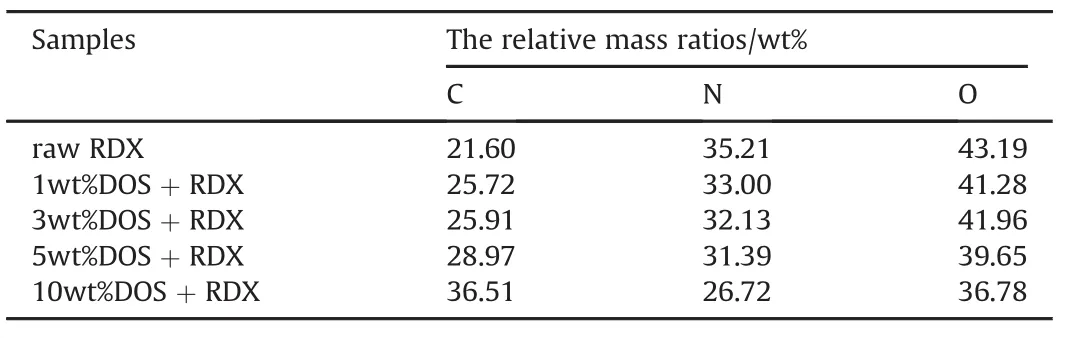
Table 1 The relative mass ratios of the elements in the samples.
The friction sensitivity of the RDX/DOS composites was tested with a sample mass of 20±1 mg,a pendulum weight of 1.5 kg,a swing angle of 90°,and a pressure of 3.92 MPa.Two groups of each sample and 25 same samples from each group were tested.The results were shown in terms of the explosion probability.
3.Results and discussion
3.1.SEM characterization
Fig.2 showed the SEM images of the RDX/DOS composites and raw RDX.In Fig.2(a-d),the RDX/DOS composite sizes were in the range of 1-3μm,while the size of raw RDX was in the range of 15-30μm.The distributions of the Carbon,Nitrogen and Oxygen elements in the composites’surface were tested by energy dispersive spectrometer(EDS)connected with the SEM,and the relative mass ratios of the elements were listed in Table 1.The relative mass ratio of Carbon increased as the increase of DOS quantity,while the relative mass ratio of Nitrogen decreased,which indicated the existence of DOS in the composites.
3.2.FT-IR characterization
As shown in Fig.3,compared with raw RDX and DOS,the RDX/ DOS composites had similar FT-IR spectra,which indicated that the chemical bonds and functional groups of composites were in accordance with those of raw RDX and DOS.The infrared absorption peaks near 2900 cm-1(the symmetrical stretching vibration and anti-symmetric stretching vibration of-CH3in DOS)and 1750 cm-1(the stretching vibration of C=O in DOS)were gradually getting stronger with the increase in DOS quantity.The results indicated the purity of the composites and the uniform distribution of DOS and RDX in the composites.
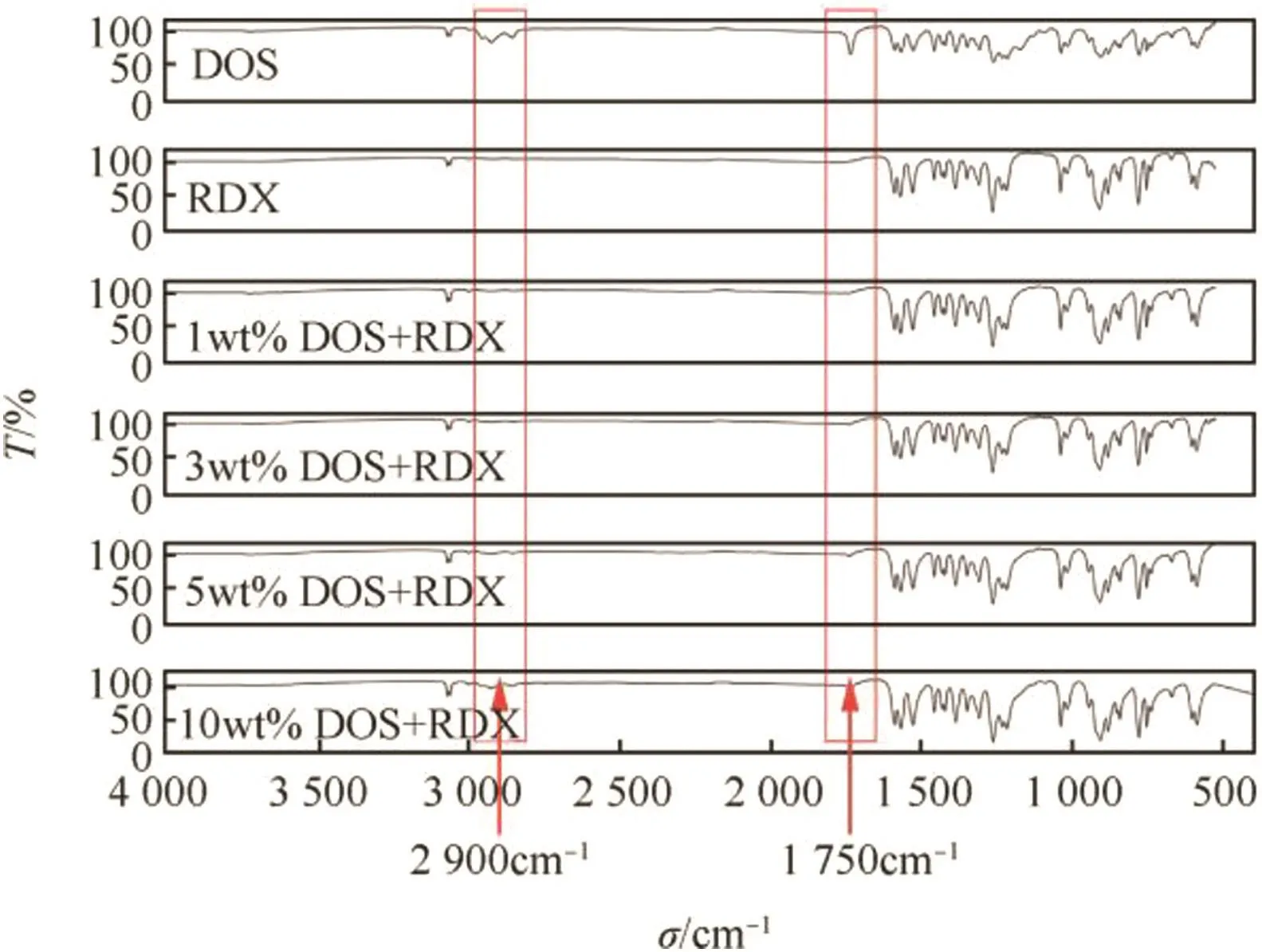
Fig.3.The FT-IR absorption spectra.
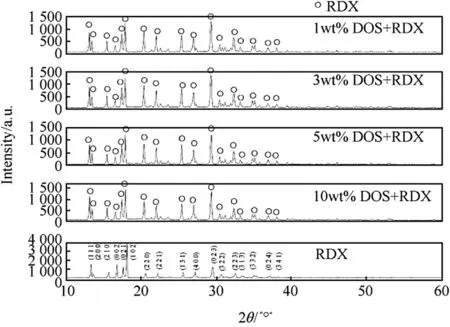
Fig.4.The XRD patterns.
3.3.XRD characterization
Fig.4 showed that the positions and shapes of the diffraction angles of the RDX/DOS composites were similar to those of raw RDX,which were in accordance with the referenced data of RDX in the PDF2-2004 card,and the crystal structure parameters of the samples were calculated by MDI Jade 6 software and were listed in Table 2.DOS had some small in fluences on the crystal structure of the RDX/DOS composites.The diffraction peaks of the composites were weaker(diffraction peak intensity below 1500 a.u.)than those of raw RDX(diffraction peak intensity below 40000 a.u.).It was because that the sizes of the composites were smaller than that of raw RDX.
3.4.DSC characterization
Fig.5(a-e)showed the DSC curves of raw RDX and the RDX/DOS composites at 1,2,4,10 K min-1heating rates respectively.The exothermic peak temperatures of the RDX/DOS composites were close to each other and higher than that of raw RDX.
The thermal decomposition kinetics parameters of raw RDX and the RDX/DOS composites could be calculated by the Kissinger method(Equation(1))[22].
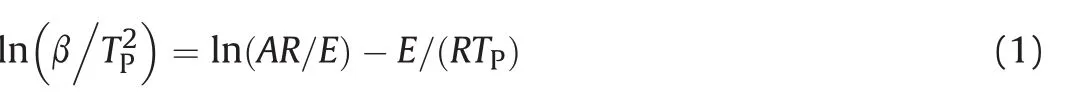
in whichβ,the heating rate,K.min-1;Tp,the temperature of the exothermic peak at heating rateβ,K;E,the activation energy, J.mol-1;A,the pre-exponential factor;R,the gas constant, 8.314 J mol-1K-1.
As shown from Fig.5(f-j),the activation energy and the preexponential factor of the RDX/DOS composites were obtained. The activation energy of the RDX/DOS composites were close to eachotherandlowerthanthatofrawRDX,whichwas194.614 kJ mol-1.Fig.6 showed that the 3wt%composites had the lowest activation energy,which was 152.186 kJ mol-1.
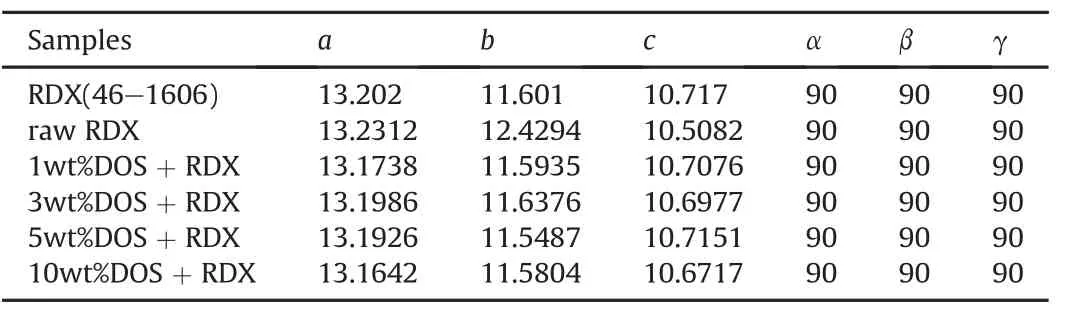
Table 2 The crystal structure parameters of the samples.
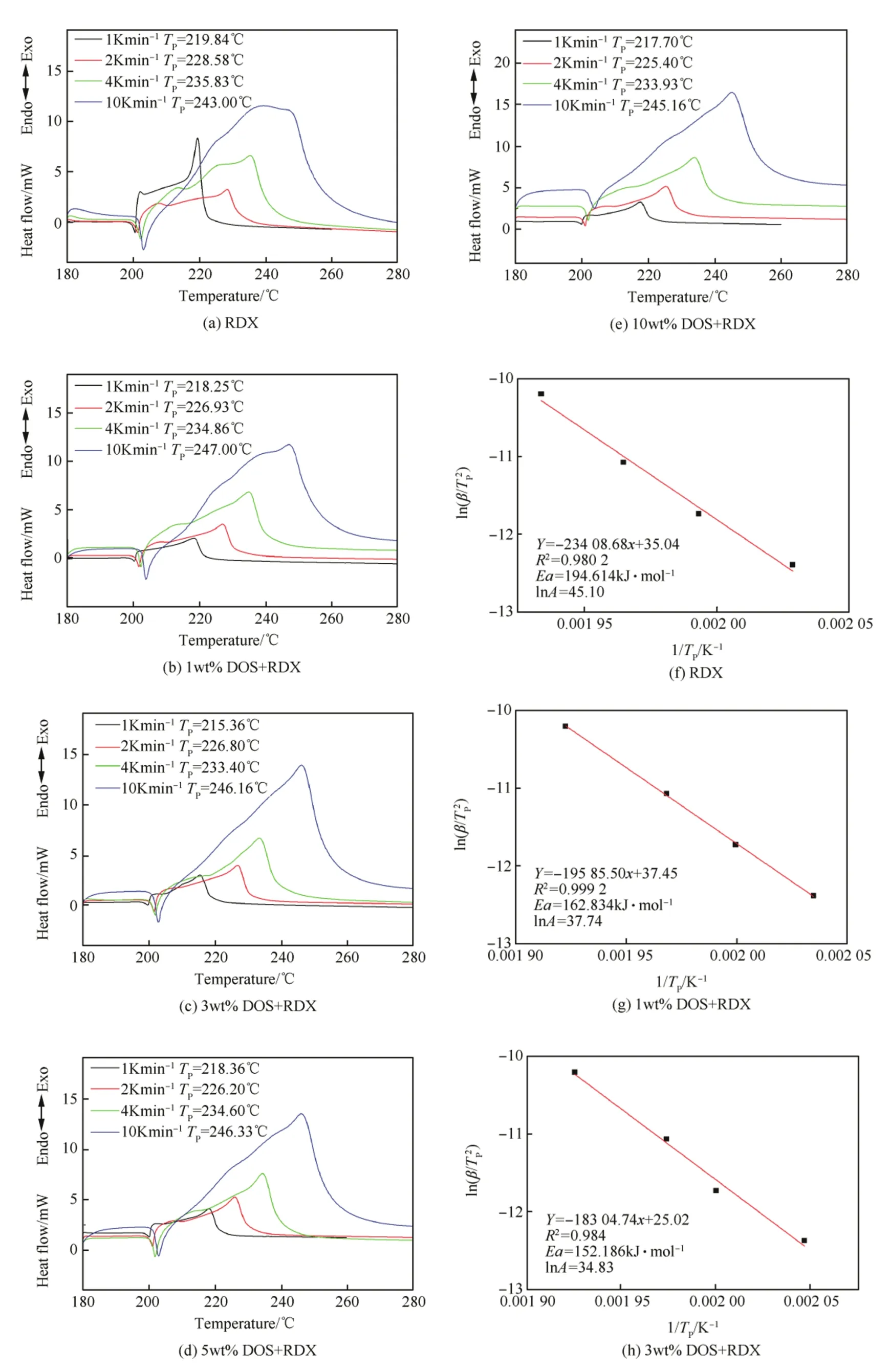
Fig.5.The DSC curves at different heating rates and Kissinger plots of the samples.
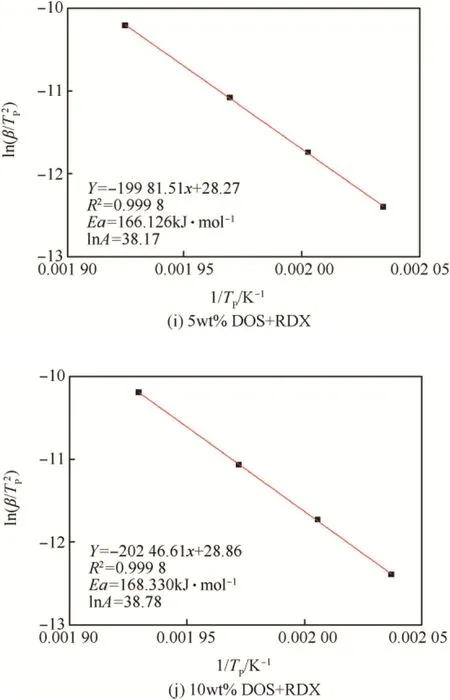
Fig.5.(continued).
On the one hand,the scales of the composites were smaller than that of raw RDX.On the other hand,the addition of DOS led to solvent effect[23]and insensitive effect.DOS was a polar plasticizer,which could decrease the stability of the crystal lattice of RDX by dissolving the surface layers of the nitramine crystal.When DOS mass ratio was less than 3wt%,the solvent effect made more difference in the stability of the composites than the insensitive effect. Conversely,when DOS mass ratio was more than 3%,the insensitive effect made more difference.When the ratio was from 1wt%to 3wt %,the DOS dissolved the crystal surface layers of RDX gradually,the thermal stability as well as the activation energy decreased.When the DOS mass ratio reached 3wt%,DOS almost completely coated the crystal surface layers.However,when the DOS mass ratio went on increase,the lubrication of DOS began to affect the properties of the nitramine crystals,and the insensitive effect played a major role in the stability of the composites,which resulted to the increase on the activation energy.
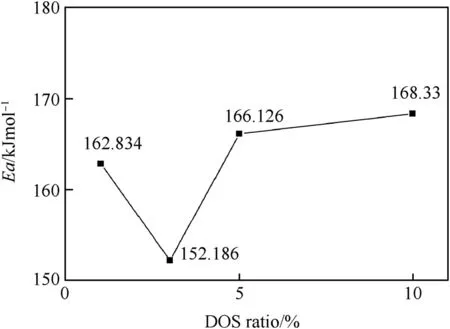
Fig.6.The changing curve of the activation energy.
3.5.Mechanical sensitivity
As shown in Fig.7(a),the H50s of the RDX/DOS composites were higher than that of raw RDX,which was 40.5 cm.The 3wt%DOS composites had the lowest H50,which was consist with the lowest activation energy.In Fig.7(b),the friction sensitivity of the RDX/ DOS composites decreased with the increase of DOS quantity,and lower than that of raw RDX,which was 100%.The results proved that DOS made a great difference in reducing the mechanical sensitivity of raw RDX.
4.Conclusions
The composites,which contained RDX and DOS,were prepared successfully by the electrostaticspray method.The particle sizes were in the range of 1-3μm in size.The crystal structure and FT-IR spectra of the RDX/DOS composites were similar to those of raw RDX.The activation energy of the RDX/DOS composites was lower than that of raw RDX.The 3wt%composites had the lowest activation energy and H50.The impact sensitivity and friction sensitivity of the RDX/DOS composites were lower than those of raw RDX.These results suggested that the RDX/DOS composite was an insensitive explosive with excellent properties.
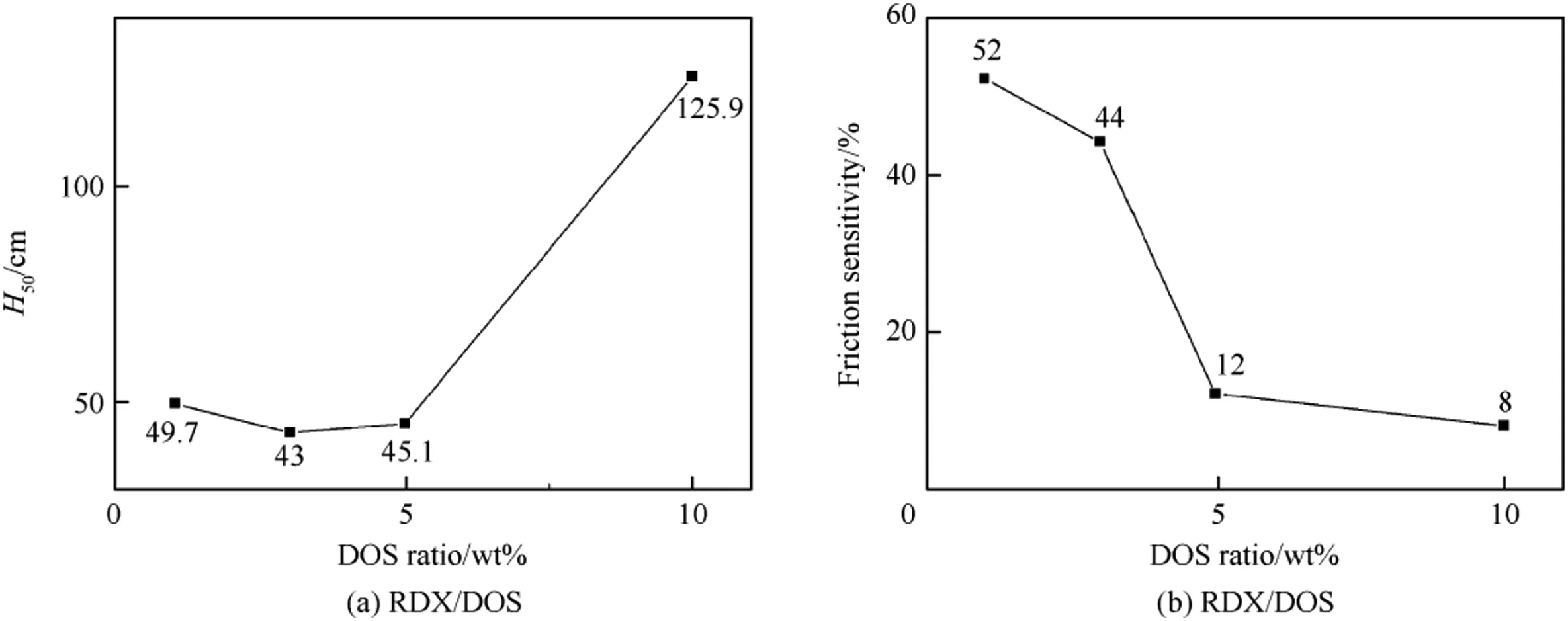
Fig.7.The mechanical sensitivity trends of the samples.
[1]Balzer JE,Field JE,Gifford MJ,et al.High-speed photographic study of the drop-weight impact response of ultra fine and conventional PETN and RDX. Combust Flame 2002;130(4):298-306.
[2]Elbeih A,Zeman S,Jungova M,et al.Effect of different polymeric matrices on the sensitivity and performance of interesting cyclic nitramines.Central Eur J Energetic Mater 2011;9(2):131-8.
[3]Qiu H,Stepanov V,Stasio ARD,et al.RDX-based nanocomposite microparticles for signi ficantly reduced shock sensitivity.J Hazard Mater 2011;185(1): 489-93.
[4]Elbeih Ahmed,Pachman Jiri,Zeman Svatopluk,et al.Detonation characteristics of plastic explosives based on attractive nitramines with polyisobutylene and poly(methyl methacrylate)binders.J Energetic Mater 2012;30(4): 358-71.
[5]Elbeih A,Zeman S,Jungova M,et al.Effect of different polymeric matrices on some properties of plastic bonded explosives.Propellants Explos Pyrotech 2012;37(6):676-84.
[6]Pelikan V,Zeman S,Yan QL,et al.Concerning the shock sensitivity of cyclic nitramines incorporated into a polyisobutylene matrix.Central Eur J Energetic Mater 2014;11(2):219-35.
[7]Jangid SK,Singh MK,Solanki VJ,et al.1,3,5-Trinitroperhydro-1,3,5-triazine (RDX)-based sheet explosive formulation with a hybrid binder system.Propellants Explos Pyrotech 2016;41:377-82.
[8]Balzer JE,Proud WG,Walley SM,et al.High-speed photographic study of the drop-weightimpactresponseofRDX/DOSmixtures.CombustFlame 2003;135(4):547-55.
[9]Hou C,Wang J,Zhang J.Study on testing method of Explosive's internal compatibility.Initiators Pyrotech 2010;3:51-3.
[10]Kim JW,Shin MS,Kim JK,et al.Evaporation crystallization of RDX by ultrasonic spray.Industrial Eng Chem Res 2011;50(21):12186-93.
[11]Zhang S,Zhao T,Luo G,et al.HPLC approach to evaluate the degree of coverage of polymer-coated hexahydro-1,3,5-trinitro-1,3,5-triazine. Chromatographia 2012;75(19):1199-204.
[12]Shuai Z,Huang Hui,Luo Guan,et al.Characterization of the coverage of polymer-coated RDX.Hanneng Cailiao/chinese J Energetic Mater 2014;22(1): 57-61.
[13]Yang Z,Ding L,Wu P,et al.Fabrication of RDX,HMX and CL-20 based microcapsules via in situ,polymerization of melamine-formaldehyde resins with reduced sensitivity.Chem Eng J 2015;268:60-6.
[14]Shi X,Wang J,Li X,et al.Preparation and properties of RDX-based composite energetic microspheres.Hanneng Cailiao/chinese J Energetic Mater 2015;23(5):428-32.
[15]Zeleny J.The electrical discharge from liquid points,and a hydrostatic method of measuring the electric intensity at their surfaces.Phys Rev 1914;3(2): 69-91.
[16]Taylor G.Disintegration of water drops in an electric field.R Soc Lond Proc 1964;280(1382):383-97.
[17]Chen DR,Pui DYH,Kaufman SL,et al.Electrospraying of conducting liquids for monodisperse aerosol generation in the 4 nm to 1.8μm diameter range. J Aerosol Sci 1996;27(4):963-77.
[18]Wang H,Jian G,Shi Y,et al.Electrospray formation of gelled nano-aluminum microspheres with superior reactivity.Acs Appl Mater Interfaces 2013;5(15): 6797-801.
[19]Wang H,Jian G,Egan GC,et al.Assembly and reactive properties of Al/CuO based nanothermite microparticles.Combust Flame 2014;161(8):2203-8.
[20]Diego F,Marco Z,Luigi C.Polystyrene microspheres and nanospheres produced by electrospray.Macromol Rapid Commun 2006;27(23):2038-42.
[21]Almería B,Deng W,Fahmy TM,et al.Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery.J Colloid& Interface Sci 2010;343(1):125-33.
[22]Kissinger HE.Reaction kinetics in differential thermal analysis.Anal Chem 1957;29(11):1702-6.
[23]Shu Y.Thermal decomposition of nitamine high explosives.4th ed.Beijing: National Defence Industry Press;2010.
13 January 2017
*Corresponding author.
E-mail addresses:yaojian216@163.com(J.Yao),1121270251@qq.com(J.Liu), 876002529@qq.com(Y.-x.Wang),wrilber@sina.com(B.Li),xielifeng319@sina. com(L.-f.Xie).
Peer review under responsibility of China Ordnance Society.
http://dx.doi.org/10.1016/j.dt.2017.05.002
2214-9147/©2017 Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
in revised form
- Defence Technology的其它文章
- The uncertainty propagation analysis of the projectile-barrel coupling problem
- Studies on composite solid propellant with tri-modal ammonium perchlorate containing an ultra fine fraction
- Study on the effect of RDX content on the properties of nitramine propellant
- Simulation of two-dimensional interior ballistics model of solid propellant electrothermal-chemical launch with discharge rod plasma generator
- The in fluence of nozzle diameters on the interaction characteristic of combustion-gas jets and liquid
- Projected area and drag coef ficient of high velocity irregular fragments that rotate or tumble

