血管损伤反应对血管平滑肌合酶Ⅰ和转录释放因子表达的影响及机制
陈雪莹 姚 烨 周高适 陶诗婉 陈兆煜 谈 智
(1.中山大学中山医学院临床医学系,广州 510080;2.中山大学附属第三医院神经内科,广州 510630; 3. 中山大学中山医学院高血压研究所,广州 510080;4. 中山大学中山医学院生理教研室,广州 510080)
· 基础研究 ·
血管损伤反应对血管平滑肌合酶Ⅰ和转录释放因子表达的影响及机制
陈雪莹1姚 烨1周高适1陶诗婉1陈兆煜2谈 智3,4*
(1.中山大学中山医学院临床医学系,广州 510080;2.中山大学附属第三医院神经内科,广州 510630; 3. 中山大学中山医学院高血压研究所,广州 510080;4. 中山大学中山医学院生理教研室,广州 510080)
目的 探讨聚合酶Ⅰ和转录释放因子(polymerase Ⅰ and transcript release factor,PTRF or Cavin-1)在损伤血管后血管再狭窄病理过程的作用及分子机制。方法 将200~220 g雄性Sprague Dawley大鼠采用数字表法随机分为假手术组和血管损伤组,血管损伤组制备颈动脉损伤动物模型。用苏木精伊红染色显示损伤后颈动脉的结构,免疫荧光、Western blotting检测损伤后血管的Cavin-1蛋白表达,real-time RT-PCR方法检测损伤后血管的Cavin-1 mRNA表达,免疫组织化学、Western blotting、免疫共沉淀法检测颈动脉泛素化蛋白的表达。大鼠动脉平滑肌细胞实验分为①空白对照组(control,CTRL);②CHX组:用放线菌酮(cycloheximide, CHX,25 μmol/L)预处理1 h;③CHX+MG组:用CHX预处理1 h后,接着用MG132(蛋白酶体抑制剂,10 μmol/L)处理24 h;④CHX+CQ组:用CHX预处理1 h后,接着用氯喹(chloroquin,CQ,10 μmol/L)处理24 h。应用Western blotting检测细胞Cavin-1蛋白浓度。结果 动物模型中,球囊损伤后,血管壁明显增厚,Cavin-1表达显著下降(P<0.05),Cavin-1 mRNA表达差异不明显,泛素化蛋白表达显著上调(P<0.05)。细胞实验中,应用CHX预处理可明显降低平滑肌细胞Cavin-1的表达(P<0.05),而CHX+MG可以明显对抗CHX上述作用过程(P<0.05)。结论 球囊损伤颈动脉后,血管的Cavin-1蛋白表达下调,其机制可能与增加的泛素化-蛋白酶体降解途径有关。
血管损伤;平滑肌细胞;泛素化;血管再狭窄
经皮冠状动脉介入治疗(percutaneous coronary intervention,PCI)是目前治疗急性心肌梗死病人的冠状动脉再灌注的主要手段,但术后再狭窄严重影响PCI的远期疗效和病人预后[1]。血管再狭窄是一系列复杂的病理生理过程,其主要病因是PCI术程中支架植入及球囊膨胀扩张造成的血管损伤[2]。血管平滑肌细胞(smooth muscle cell,SMC)位于血管中膜,构成血管壁组织结构及维持血管张力的主要细胞成分,血管损伤后发生的增生、迁移及结构的改变是导致血管成形术后再狭窄等多种心血管病的细胞病理学基础[3]。聚合酶Ⅰ和释放因子(polymerase I and transcript release factor,PTRF or Cavin-1)是一种细胞表面微囊的结构相关蛋白[4],已有文献表明其在癌细胞[5]和人成纤维细胞[6]的增生与迁移过程中发挥重要调控作用,Cavin-1的高表达能抑制细胞增生和迁移,但对SMC损伤后的增生和迁移的影响目前尚未有文献报道。为此,本研究探索Cavin-1在损伤后血管内膜增生的作用及可能的机制,为探讨抑制损伤后血管再狭窄提供新的靶点和研究思路。
1 材料与方法
1.1 实验材料
200~220 g雄性清洁级SD大鼠购于中山大学北校区动物实验中心[许可证号:SCXK(粤)-2013-0002],采用数字表随机分为假手术组、损伤组。DMEM培养基购于美国Hyclone公司。胎牛血清购于美国Gibco公司。Cavin-1抗体、MG132(蛋白酶体抑制剂)、放线菌酮(cycloheximide, CHX)、A/G plus-agarose、泛素抗体购于美国Santa公司。氯喹(chloroquin,CQ)购于美国ATCC公司。
1.2 颈动脉损伤动物模型的建立
200~220 g清洁级雄性SD大鼠经10%(质量分数)水合氯醛0.3 mL/100 g腹腔注射麻醉后,逐层分离颈部皮肤、筋膜、肌肉,找到左颈总动脉,肝素100 U/kg抗凝,经颈外动脉远心端切口送入2F Forgety导管至颈总动脉,充分充盈球囊,向左右各转动3次,损伤颈总动脉。逐层缝合筋膜、皮肤。术后注射青霉素80万单位抗感染3 d。
1.3 SMC细胞培养与分组给药
大鼠动脉平滑肌细胞来源于200~220 g SD大鼠主动脉原代培养获得的SMC,培养于含10%(体积分数)胎牛血清的DMEM培养基,置于5%(体积分数) CO2、37 ℃ 的温箱。
取成对数增生约第五代的SMC,接种于12孔培养板中,SMC生长至培养孔面积70%~80%时,实验分为4组:①空白对照组(control,CTRL)用含10%(体积分数)胎牛血清的DMEM培养基培养;②损伤模型组(以下简称CHX组)用25μmol/L CHX+含10%(体积分数)胎牛血清的DMEM培养基处理1 h;③CHX+MG组用25μmol/L CHX+含10%(体积分数)胎牛血清的DMEM培养基预处理1 h后,接着10 μmol/L MG 132+含10%(体积分数)胎牛血清的DMEM培养基处理24 h;④CHX+CQ组:用25μmol/L放线菌酮(CHX)+含10%(体积分数)胎牛血清的DMEM培养基预处理1 h后,用50 μmol/L CQ+含10%(体积分数)胎牛血清的DMEM培养基处理24 h。
1.4 苏木素伊红染色显示颈动脉结构
根据文献[7]动物模型方法,球囊损伤后14 d为最佳观察血管内膜增生的时间。球囊损伤14 d后,将假手术组和球囊损伤组大鼠处死,取颈总动脉分叉处下0.5 cm处血管,在血管分离时注意保护血管的完整性,不过多清除血管外膜周围结缔组织,制作石蜡切片,进行苏木素伊红(hematoxylin and eosin staining,HE)染色。
1.5 免疫荧光染色法检测颈动脉Cavin-1表达
球囊损伤14 d后,将假手术组和球囊损伤组大鼠处死,取颈动脉制作石蜡切片,用Cavin-1特异性抗体进行染色,加入荧光工作液,容量以覆盖细胞为准。37 ℃孵育30 min。用PBS溶液洗涤细胞3次,以充分去除残留的工作液,然后加入荧光激发溶液覆盖细胞,用荧光倒置显微镜观察,并随机选取5个不重复区域采集图像。实验重复5次。
1.6 real-time RT-PCR检测颈动脉Cavin-1 mRNA
球囊损伤14 d后,将假手术组和球囊损伤组大鼠处死,取颈动脉,超声粉碎组织块,real-time RT-PCR法检测组织块Cavin-1 mRNA。
1.7 免疫组织化学染色法检测颈动脉泛素化蛋白的表达
球囊损伤14 d后,将假手术组和球囊损伤组大鼠处死,取颈动脉制作石蜡切片,进行Cavin-1免疫组织化学染色,染色完成后用光学倒置显微镜观察,并随机选取5个不重复区域采集图像。实验重复5次。
1.8 Western blotting法检测颈动脉Cavin-1、泛素化蛋白表达及SMC的Cavin-1蛋白表达
将建模完成的颈动脉组织块在相同部位称取相同质量的组织块,加入裂解液。SMC接种于12孔培养板中,培养至70%~80%满时,各组给予不同的处理因素,然后用预冷的PBS洗2次,加入裂解液。接着,都在4 ℃静置30 min,12 000 r/min离心10 min,取上清,采用BCA法进行蛋白定量。总蛋白经SDS-PAGE分离后,转移到PVDF膜上。用5%(质量分数)脱脂牛奶封闭60 min,随后加入一抗体4 ℃过夜,用TBST洗3遍,每遍10 min。将PVDF膜用发光试剂ECL显色,将图片以Tif格式保存并分析结果。按照以上步骤检测Cavin-1、泛素化蛋白的表达。利用Image-Pro plus 6.0图像分析软件检测蛋白灰度值与β-actin灰度值的比值表示目的蛋白的表达水平。
1.9 免疫共沉淀
免疫共沉淀的具体方法参见文献[8]。简而言之,从动物模型取得血管样品,提取出200 μg与1 μg的泛素抗体4 ℃孵育过夜。然后加入20 μL的A/G plus-agarose继续孵育4 h。然后1 000 g 4 ℃离心5 min,弃上清液。使用PBS清洗,离心3次。取得的样品加入40 μ L的电泳样品缓冲液,煮沸3 min,800 g 4 ℃离心5 min,取20 μL进行电泳,余下步骤同Western blotting。
1.10 统计学方法
2 结果
2.1 血管形态学分析
术后14 d,颈总动脉目的区域HE染色(图1)显示,假手术组仅见单层内皮细胞,无增生的内膜。损伤组切片可见内膜连续性中断,内弹力膜断裂破坏,大量平滑肌细胞增生,内膜增厚。

图1 14 d后假手术组与球囊损伤组的颈动脉HE染色结果
A:monolayer endothelial cells without neointima in the sham groups; B: intima lining interruption, elastic membrane damage, smooth muscle cells proliferation and intimal thickening in injury groups.
2.2 球囊损伤后,颈动脉Cavin-1的表达下调
免疫荧光染色显示绿色的区域为Cavin-1表达阳性区域,亮度与Cavin-1表达量成正相关。术后14 d,颈动脉免疫荧光染色结果显示:损伤组颈动脉的Cavin-1表达区域更大,也表达于增厚的内膜区域,但单个细胞表达量低于假手术组(图2A)。为进一步量化SMC的Cavin-1表达,术后14 d,颈动脉组织Western blotting结果(图2B)显示:损伤组颈动脉的Cavin-1表达水平低于假手术组,差异有统计学意义(n=7,P<0.05)。
2.3 球囊损伤后,颈动脉Cavin-1 mRNA水平
术后14 d,用real-time RT-PCR检测假手术组与损伤组的颈动脉Cavin-1 mRNA水平(图3),2组差异无统计学意义(n=7,P>0.05)
2.4 球囊损伤后,颈动脉泛素化蛋白表达上调
被染成棕黄色区域显示为泛素化蛋白阳性表达区域,颜色深度与泛素化蛋白表达量成正相关。术后14 d,颈动脉免疫组织化学染色结果显示损伤组颈动脉的表达量上调,高于假手术组,详见图4A。为进一步量化泛素化蛋白表达量,颈动脉组织Western blotting结果显示损伤组颈动脉的泛素化蛋白表达水平高于假手术组,差异有统计学意义(n=7,P<0.05)详见图4B。免疫共沉淀(Co-immunoprecipitation,Co-IP)显示Cavin-1通过泛素化途径进行降解(图4C)。
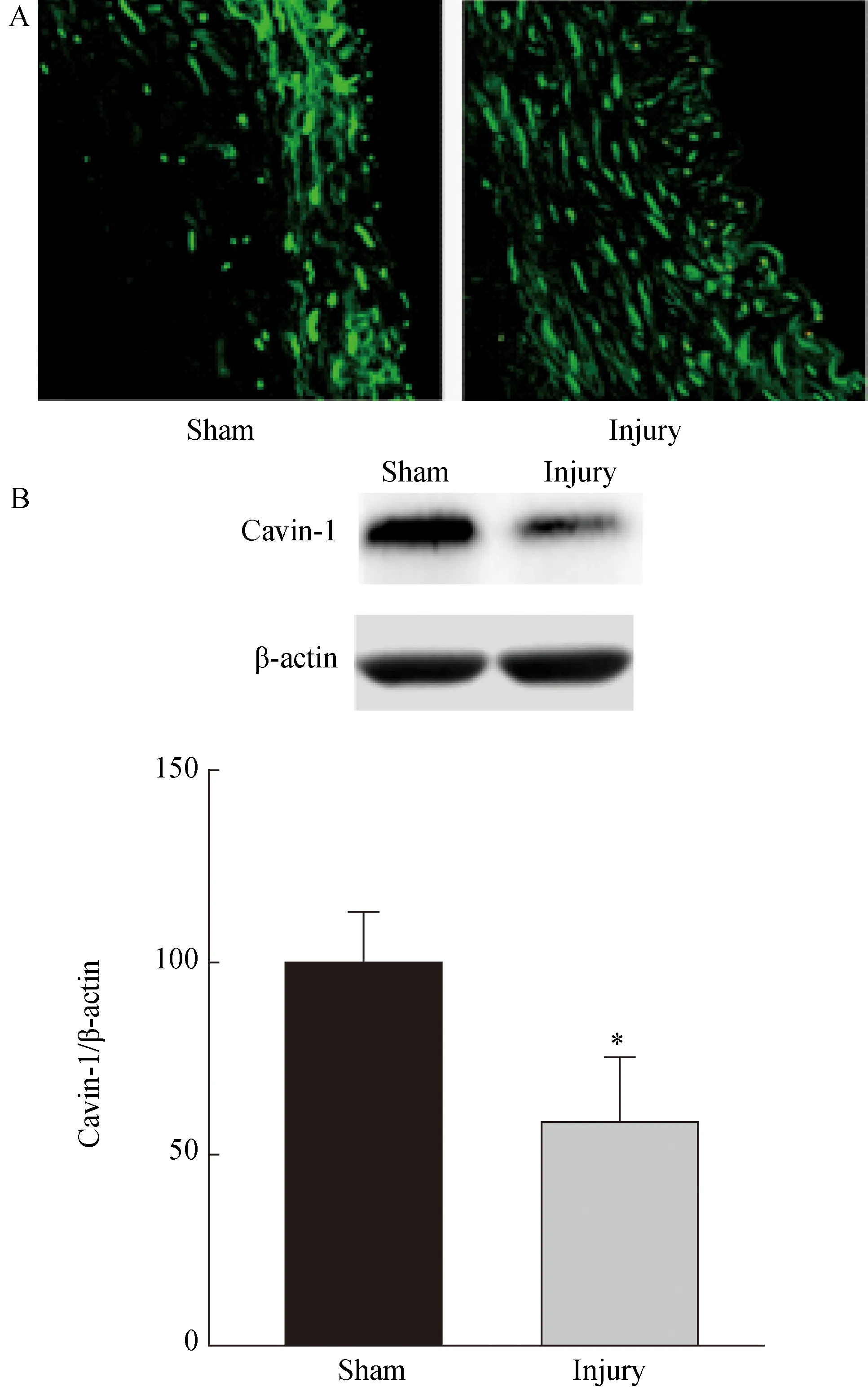
图2 Cavin-1表达的检测
A: Immunofluorescent staining showed that Cavin-1 expression from sham mainly exists in intima and larger range and lower percell level Cavin-1 expression from injury groups; B: Western blotting showed that Cavin-1 expression is much lower in injury groups compared with sham groups, which was significantly reduced by the balloon injury.n=7,*P<0.05vssham group; Cavin-1:polymerase Ⅰ and transcript release factor.
2.5 上调的泛素化-蛋白酶体途径使SMC的Cavin-1表达下调
大鼠主动脉SMC的Western blotting结果显示:与对照组相比,经25μmol/L CHX预处理1 h的SMC显著下调了Cavin-1的表达(P<0.05)。MG-132是蛋白酶体抑制剂,经25μmol/L CHX预处理1 h的SMC再用10μmol/L MG 132处理24 h,Cavin-1表达量高于CHX组,差异具有统计学意义(P<0.05)。CQ是溶酶体抑制剂,经25μmol/L CHX预处理1 h的SMC再用50 μmol/L CQ处理24 h,Cavin-1表达量与CHX组相比差异无统计学意义(P>0.05)(图5)。细胞实验提示SMC的Cavin-1表达量下调主要是通过调节蛋白酶体活性来上调泛素化降解途径来实现的。

图3 假手术组与球囊损伤组的颈动脉
Cavin-1:polymerase Ⅰ and transcript release factor.
3 讨 论
3.1 Cavin-1参与了损伤后血管再狭窄的过程
PCI治疗后血管再狭窄是当今临床治疗急性冠脉综合征的难题之一。目前认为血管平滑肌细胞增生及向内膜下迁移是血管再狭窄的主要因素,血管损伤后,炎性反应因子等刺激血管平滑肌细胞从收缩表型向合成表型转换,进入增生分泌旺盛阶段,向内膜迁移,在内膜增生[9]并合成分泌细胞外基质,参与内膜增厚、血管重塑病理过程[10]。大鼠颈动脉经球囊损伤后,血管壁明显厚于假手术组,管腔明显小于假手术组,模拟了临床中PCI术后再狭窄的现象。从HE染色可以看出,内弹力层破坏,平滑肌细胞增生,内膜增厚,SMC在血管壁中的增生、迁移中起到很重要的作用。目前关于Cavin-1的研究方向主要为细胞衰老、凋亡、肿瘤及物质转运等,尚未报道Cavin-1在心血管领域病理生理机制中的研究[11]。上述实验主要显示,Cavin-1参与了损伤后血管再狭窄的过程。且免疫荧光染色、Western blotting结果显示,Cavin-1在损伤后的血管中表达量显著下调,这提示Cavin-1可能是血管保护因子。
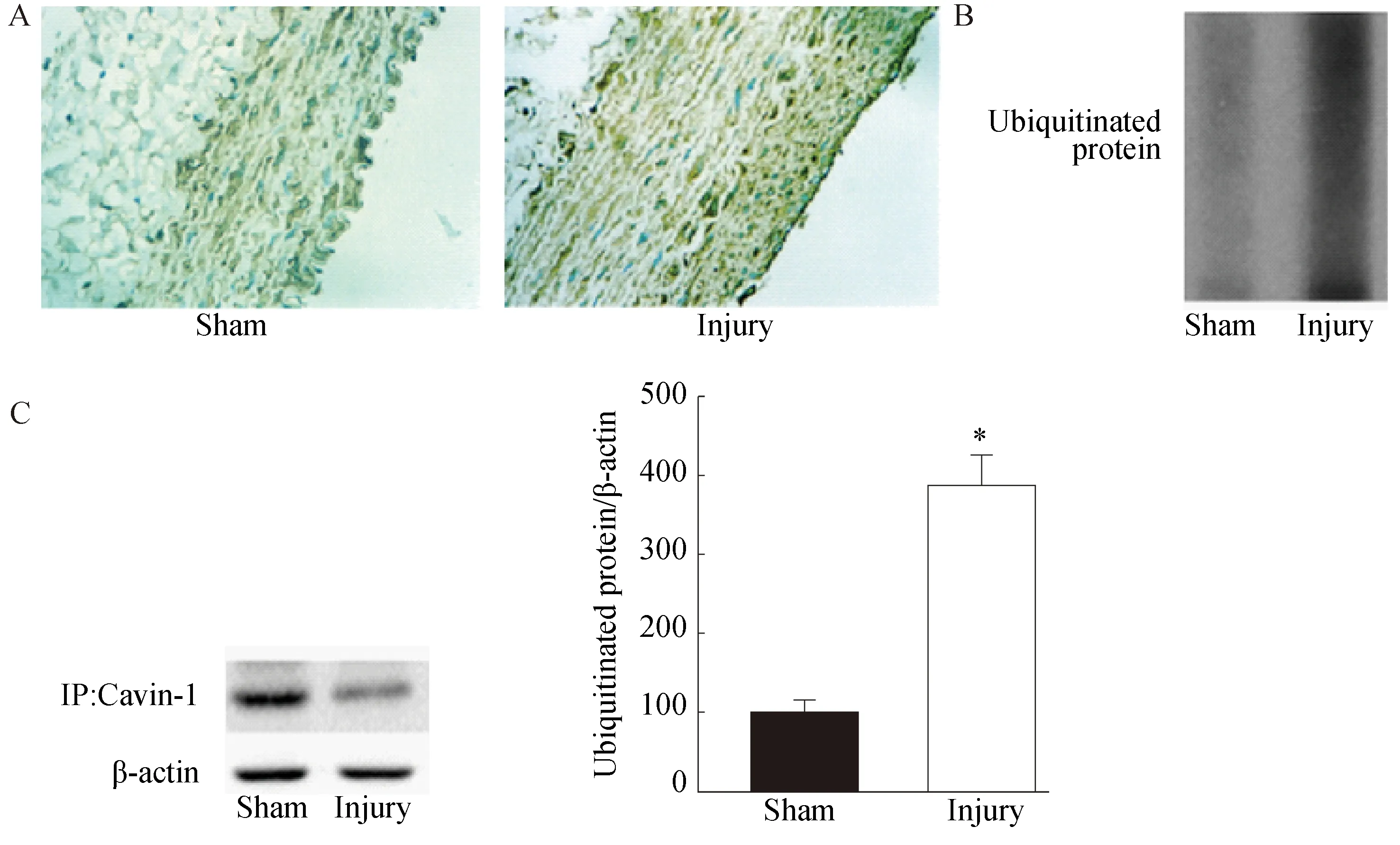
图4 颈动脉泛素化蛋白表达检测
A: Immunochemical staining of ubiquitinated protein in carotid artery from injury groups was higher than sham groups (400×).B: Western blotting revealed that ubiquitinated protein levels were higher in the injured carotid compared with sham group. C: Co-immunoprecipitation showed that Cavin-1 could be degraded by proteasomal pathway.n=7,*P<0.05vssham group; Cavin-1:polymerase Ⅰ and transcript release factor.

图5 上调的泛素化-蛋白酶体途径使SMC的Cavin-1表达下调
Rat aortic SMC were pretreated with CHX (25μmol/L) or not for 1 h, followed by treatment with proteasomal inhibitor MG 132(10 μmol/L)or lysosomal inhibitor CQ (50 μmol/L)for additional 24 h. Western blotting confirmed Cavin-1 protein degradation mainly through proteasomal pathway.n=5,*P<0.05vsCTRL;#P<0.05vsCHX or CHX+CQ; SMC:smooth muscle cell; CTRL:control; CHX:cycloheximide; MG:MG 132; CQ:chloroquin; Cavin-1:polymerase Ⅰ and transcript release factor.
3.2 Cavin-1参与损伤后血管再狭窄的可能机制
本研究显示,血管损伤后,Cavin-1表达量下调,与假手术组相比差异具有统计学意义。Cavin-1表达量的下调,笔者猜测可能通过目前已知的可能的机制来实现,包括Cavin-1基因转录、翻译、蛋白结构修饰、蛋白降解等一系列复杂的过程。但本实验real-time RT-PCR显示,Cavin-1在mRNA的水平差异无统计学意义,这说明Cavin-1量的下调并不是通过调节转录过程来实现的,而可能是通过转录后途径来调节的。颈动脉组织经免疫共沉淀后结果显示,Cavin-1可被泛素化降解,为进一步明确,需要细胞实验从细胞水平来证实。细胞实验中,CHX是真核生物蛋白合成抑制剂[12],进而用CQ抑制细胞溶酶体活性,不能上调Cavin-1表达量,但用MG抑制蛋白酶体活性,SMC的Cavin-1表达上调,说明CHX引起的SMC中Cavin-1表达量下调,并不是通过调节溶酶体降解途径,而主要是通过蛋白酶体-泛素化途径实现的。Cavin-1表达量在合成与降解达到动态平衡,SMC细胞实验进一步证实了泛素化-蛋白酶体途径是Cavin-1表达量下调的主要原因,而不是溶酶体降解途径。泛素化-蛋白酶体途径不仅能降解增生相关基因[13]及蛋白包括细胞周期蛋白[14],还能降解炎性反应相关因子如核因子kappa B[15],此外还能降解凋亡相关分子如p53[16]。近年文献[17]指出,作为真核细胞内蛋白降解的三大途径之一,泛素化-蛋白酶体途径也能通过抗增生、抗炎、抗凋亡抑制血管再狭窄。本实验所有细胞模型说明Cavin-1可发生泛素化降解,损伤后血管再狭窄与Cavin-1经泛素化途径降解有关,但是本文观察到血管重构中有Cavin-1下调及泛素化水平上升,并不能说明Cavin-1泛素化降解参与血管重构,还需进一步实验验证其在血管再狭窄的病理生理机制的作用。
3.3 结论
血管平滑肌细胞在血管损伤的病理生理变化中发挥重要作用。目前Cavin-1对细胞的迁移和增生的影响主要针对癌细胞,对血管损伤后血管平滑肌细(vascular smooth muscle cells, VSMCs)的Cavin-1的表达以及Cavin-1在血管损伤后对VSMCs迁移和增生的影响尚未见报道。本文报道了在血管重构过程中,Cavin-1和泛素化水平的变化以及血管泛素化水平可以影响Cavin-1水平的这种现象。虽Cavin-1参与损伤后血管再狭窄已经有实验证据支持,其机制可能与泛素化-蛋白酶体途径降解有关,但至于Cavin-1表达量的变化是不是引起血管损伤后的再狭窄的原因或结果需要后续研究来证明,从而为研究与治疗临床PCI术后血管再狭窄提供理论依据。
总之,血管重构的病理生理过程中,Cavin-1表达下调和泛素化水平上调,且血管泛素化水平可以影响Cavin-1水平,可为研究血管损伤后血管再狭窄提供新的方向。
[1] Raina T, Iqbal J, Arnold N, et al.Coronary stents seeded with human trophoblastic endovascular progenitor cells show accelerated strut coverage without excessive neointimal proliferation in a porcine model[J]. EuroIntervention,2014,10(6):709-716.
[2] Han X J, He D, Xu L J, et al. Knockdown of connexin 43 attenuates balloon injury-induced vascular restenosis through the inhibition of the proliferation and migration of vascular smooth muscle cells[J]. Int J Mol Med,2015,36(5):1361-1368.
[3] Zuniga M C, White S L, Zhou W. Design and utilization of macrophage and vascular smooth muscle cell co-culture systems in atherosclerotic cardiovascular disease investigation[J]. Vasc Med,2014,19(5):394-406.
[4] Codenotti S, Vezzoli M, Poliani P L, et al. Caveolin-1, Caveolin-2 and Cavin-1 are strong predictors of adipogenic differentiation in human tumors and cell lines of liposarcoma[J]. Eur J Cell Biol,2016, 95 (8): 252-264.
[5] Meng F, Joshi B, Nabi I R. Galectin-3 overrides PTRF/Cavin-1 reduction of PC3 prostate cancer cell migration[J]. PLoS One,2015,10(5):e126056.
[6] Salle-Teyssieres L, Auclair M, Terro F, et al. Maladaptative autophagy impairs adipose function in Congenital Generalized Lipodystrophy due to cavin-1 deficiency[J]. J Clin Endocrinol Metab,2016,101 (7): 2892-2904.
[7] Yang J, Fan Z, Yang J, et al. MicroRNA-24 attenuates neointimal hyperplasia in the diabetic rat carotid artery injury model by inhibiting Wnt4 signaling pathway[J]. Int J Mol Sci,2016, 17(6):765.
[8] Tan Z, Zhou L J, Mu P W, et al. Caveolin-3 is involved in the protection of resveratrol against high-fat diet-induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats[J]. J Nutr Biochem, 2012,23(12):1716-1724.
[9] Sun L, Zhao R, Zhang L, et al. Prevention of vascular smooth muscle cell proliferation and injury-induced neointimal hyperplasia by CREB-mediated p21 induction: an insight from a plant polyphenol[J]. Biochem Pharmacol,2016,103:40-52.
[10]Borges B E, Appel M H, Cofre A R, et al. The flavo-oxidase QSOX1 supports vascular smooth muscle cell migration and proliferation: evidence for a role in neointima growth[J]. Biochim Biophys Acta,2015,1852(7):1334-1346.
[11] Low J Y, Nicholson H D. Emerging role of polymerase-1 and transcript release factor (PTRF/ Cavin-1) in health and disease[J]. Cell Tissue Res,2014,357(3):505-513.
[12]Tan X, Gao J, Shi Z, et al. MG132 induces expression of monocyte chemotactic protein-induced protein 1 in vascular smooth muscle cells[J]. J Cell Physiol, 2017,232(1):122-128.
[13]Keller K E, Wirtz M K. Working your SOCS off: the role of ASB10 and protein degradation pathways in glaucoma[J]. Exp Eye Res,2017,158:154-160.
[14]Gwak H, Kim Y, An H, et al. Metformin induces degradation of cyclin D1 via AMPK/GSK3beta axis in ovarian cancer[J]. Mol Carcinog,2016,56(2):349-358.
[15] Shukla S, Shankar E, Fu P, et al. Suppression of NF-kappaB and NF-kappaB-regulated gene expression by apigenin through IκBα and IKK pathway in TRAMP mice[J]. PLoS One,2015,10(9):e138710.
[16]Gao K, Wang C, Jin X, et al. RNF12 promotes p53-dependent cell growth suppression and apoptosis by targeting MDM2 for destruction[J]. Cancer Lett,2016,375(1):133-141.
[17]Meiners S, Laule M, Rother W, et al. Ubiquitin-proteasome pathway as a new target for the prevention of restenosis[J]. Circulation,2002,105(4):483-489.
编辑 孙超渊
Effect of vascular injury on the expression of polymerase Ⅰ and transcript release factor in vascular smooth muscle and its mechanism
Chen Xueying1,Yao Ye1, Zhou Gaoshi1,Tao Shiwan1,Chen Zhaoyu2,Tan Zhi3,4*
(1.DepartmentofClinicalMedicine,ZhongshanSchoolofMedicine,SunYat-senUniversity,Guangzhou510080,China; 2.DepartmentofNeurology,The3rdAffiliatedHospitalofSunYat-SenUniversity,Guangzhou510630,China; 3.InstituteofHypertension,ZhongshanSchoolofMedicine,SunYat-senUniversity,Guangzhou510080,China; 4.DepartmentofPhysiology,ZhongshanSchoolofMedicine,SunYat-senUniversity,Guangzhou510080,China)
Objective To investigate the role of polymerase Ⅰ and transcript release factor or Cavin-1 in vascular restenosis after vascular injury and its molecular mechanism. Methods 200-220 g Sprague Dawley(SD)male rats were randomly divided into sham and injury groups with injury groups establishing balloon injury models. Cellular content from carotid taken after the sham and balloon injuries were shown by hematoxylin and eosin staining. Expression Cavin-1 protein from above groups were determined by Western blotting and immunofluorescence. The Cavin-1 mRNA from above groups was detected by real-time RT-PCR, respectively. Immunochemical staining, Western blotting and Co-immunoprecipitation revealed ubiquitinated protein in carotid artery from sham and injury groups. Rat aortic smooth muscle cells were divided into: ①control group(CTRL);②CHX group rats were pretreated with cycloheximide (CHX, 25μmol/L) for 1 h;③CHX+MG group rats were pretreated with CHX (25μmol/L) for 1 h followed by treatment with MG 132(10 μmol/L)for additional 24 hours.④CHX+CQ group rats were pretreated with CHX (25μmol/L) for 1 h followed by treatment with chloroquin(CQ,50 μmol/L)for additional 24 hours.Expression Cavin-1 protein from above groups were determined by Western blotting. Results As for animal models, the carotid vascular wall became thicker after the balloon injury and the expression of Cavin-1 protein was significantly decreased(P<0.05) while real-time RT-PCR showed no significant difference of Cavin-1 mRNA between sham and injury groups. Ubiquitinated protein levels were higher in the injured carotid compared with sham group(P<0.05) As for cell experiment, expression of Cavin-1 was decreased significantly from CHX group compared with CTRL(P<0.05).CHX+MG group can reverse the above effects (P<0.05). Conclusion Expression of Cavin-1 protein from balloon injury carotid arteries were reduced, and its mechanism may relate to up-regulated ubiquitination degradation pathway.
vascular injury;smooth muscle cell;ubiquitination;vascular restenosis
国家自然科学基金(81270377),广东省科技厅社会发展领域项目(2015A020212020),广东省自然科学基金项目(2014A030313062)。This study was supported by National Natural Science Foundation of China (81270377), Science and Technology Department of Guangdong (2015A020212020), Natural Science Foundation of Guangdong (2014A030313062).
时间:2017-07-16 17∶24 网络出版地址:http://kns.cnki.net/kcms/detail/11.3662.r.20170716.1724.038.html
10.3969/j.issn.1006-7795.2017.04.012]
R54
2016-11-21)
*Corresponding author, E-mail:tanzhi@mail.sysu.edu.cn

