保乳手术阴性切缘切取次数与早期乳腺癌患者临床预后的相关性
钱炜伟, 倪 毅
南通市第三人民医院甲乳科, 南通 226001
·短篇论著·
保乳手术阴性切缘切取次数与早期乳腺癌患者临床预后的相关性
钱炜伟, 倪 毅
南通市第三人民医院甲乳科, 南通 226001
目的: 探讨早期乳腺癌保乳术中获得阴性切缘所需切取次数与患者预后的相关性。方法: 收集2009年1月至2016年10月在江苏省南通市第三人民医院行保乳治疗的早期乳腺癌患者的临床资料,回顾性分析获得阴性切缘切取次数与患者年龄、肿块大小、淋巴结状态、激素受体状态、HER-2状态、病理类型及患者预后的相关性。结果: 共入组287例接受保乳治疗的早期乳腺癌患者,其中191例(66.6%)经1次切取即获得阴性切缘,65例(22.6%)经2次切取获得阴性切缘,31例(10.8%)经3次及以上切取获得阴性切缘。肿块大小(P=0.010)、病理类型(P<0.001)及HER-2状态(P=0.034)与获得阴性切缘切取次数相关。中位随访5年的局部复发率在1次切取患者(6.3%)、2次切取患者(9.2%)及3次及以上切取患者(25.8%)间差异有统计学意义(P=0.002);而3组患者间中位随访5年的远处转移率(P=0.989)及总生存率(P=0.326)差异无统计学意义。结论: 肿块大小、病理类型、HER-2状态与保乳患者获得阴性切缘所需切取次数相关,切取次数与术后局部复发率正相关,但与患者远处转移及总生存率无明显相关。
早期乳腺癌;保乳手术;阴性切缘;切取次数;预后
乳腺癌是女性最常见的恶性肿瘤[1]。在中国,每年约有4万多例患者死于乳腺癌[2]。改良根治术和保乳手术是针对乳腺癌最主要的手术方式。研究[3-5]表明,保乳手术联合术后放疗可获得与改良根治手术相同的远期疗效,而保乳术保留了乳房外形,能够更好地满足患者的心理需求[6]。保乳手术成功的前提是切缘阴性,但首次切取并不一定能获得阴性切缘,部分患者需反复切取[7]。目前,经反复切取的保乳手术的安全性仍有待研究,切取次数的最高限定尚无统一的标准。因此,本研究回顾分析了2009年1月至2016年10月于本院行保乳手术的乳腺癌患者的临床资料,分析了影响保乳手术获得阴性切缘所需切取次数的可能临床病理因素及不同切取次数与患者预后的相关性。
1 资料与方法
1.1 一般资料 回顾分析2009年1月至2016年10月在江苏省南通市第三人民医院手术的1 483例乳腺癌患者的临床资料,保乳患者首次切除范围包括距肿块0.2~2 cm的正常乳腺组织,补充切缘时切取0.2~0.5 cm宽的乳腺组织。按下列标准进一步筛选:早期乳腺癌(T1~2N0~1M0)、切缘阴性(定义为切缘无浸润性肿瘤或导管原位癌浸润,且肿瘤距切缘大于1 mm)。排除标准:术前接受新辅助治疗、术后未接受放疗、随访数据不完整。符合上述标准的乳腺癌患者共有287例,所有患者在术后除接受放疗外,根据病情需要给予其他辅助治疗(化疗、内分泌治疗或靶向治疗)。1.2 临床数据采集 调阅病历系统,记录患者的年龄、术中切缘切取次数、术后病理、术后辅助治疗信息。
1.3 随访指标及方法 通过门诊及电话进行随访,术后前2年每3个月随访1次,以后每6个月随访1次,5年后每12个月随访1次,期间评估局部及全身情况。主要观察指标:局部区域复发(同侧乳房、腋窝及锁骨区淋巴结复发)、远处转移(骨、肺、肝、脑等)及总生存率。随访截止时间至2017年1月20日。
1.4 统计学处理 采用SPSS 20.0软件对数据进行录入、分析,χ2检验分析获得阴性切缘的切取次数与临床病理因素及预后的相关性。检验水准(α)为0.05。
2 结 果
2.1 基本情况 287例接受保乳治疗的乳腺癌患者中,经1次切取、2次切取、3次及以上切取获得阴性切缘者分别为191例(66.6%)、65例(22.6%)、31例(10.8%),其中最多切取次数为4次,共有2例。
2.2 获得阴性切缘切取次数与临床病理因素的相关性分析 结果表明肿块>2 cm、原位癌及HER-2阳性的患者获得阴性切缘所需切取次数更多,差异有统计学意义(P< 0.05)。而患者年龄、淋巴结状态及激素受体状态与获得阴性切缘所需切取次数无明显相关性(表1)。
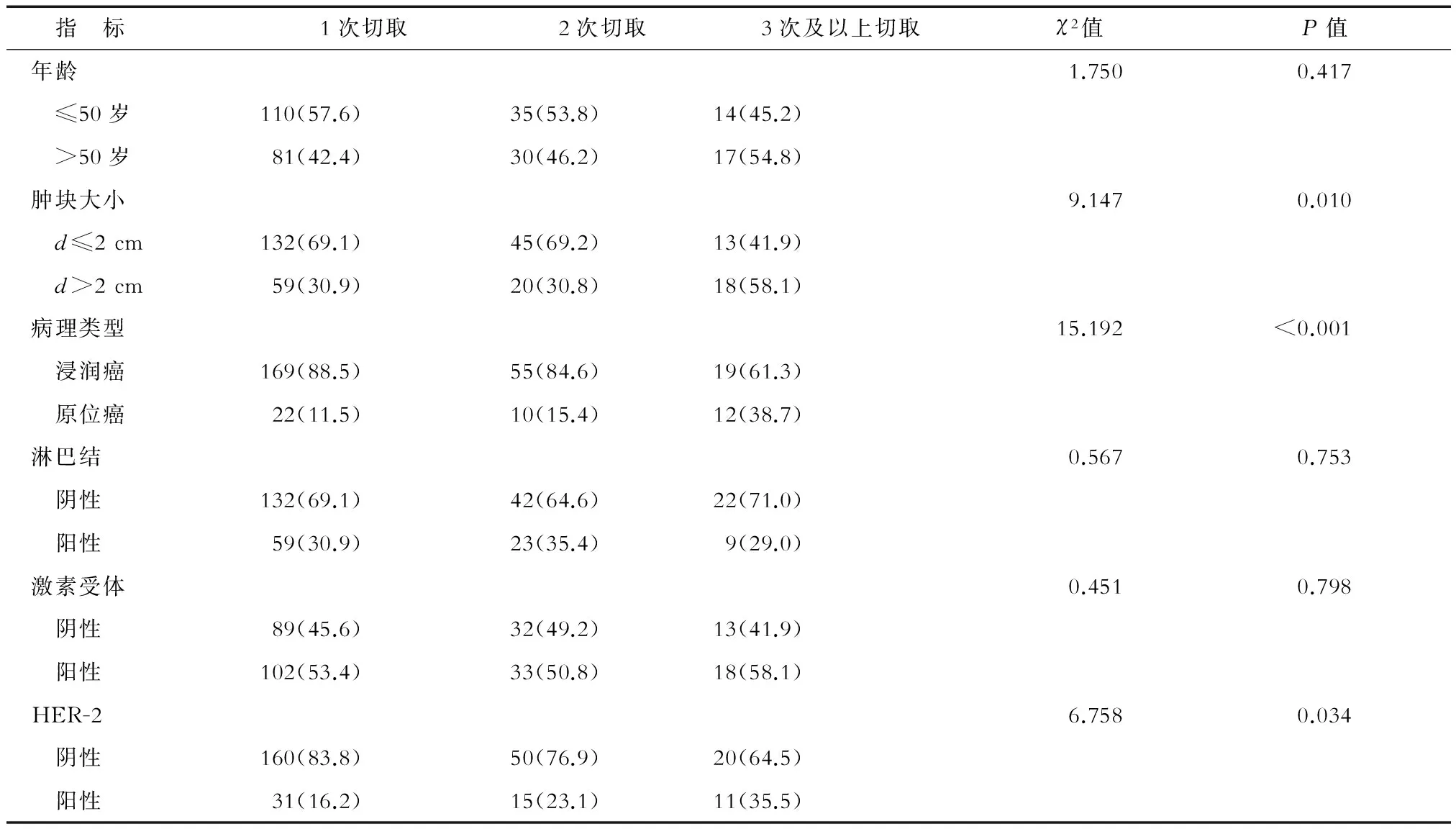
表1 保乳患者获得阴性切缘切取次数与临床病理因素的相关分析 n(%)
2.3 预后分析 术后随访3~95个月,中位随访时间61个月。3次及以上切取组局部复发率明显高于其他两组,差异有统计学意义(P< 0.05);3组间远处转移率差异无统计学意义(表2)。全组共16例患者死亡,1次切取组、2次切取组及3次及以上切取组分别有8例(4.2%)、5例(7.7%)及3例死亡(9.7%),组间总生存率差异无统计学意义(P=0.326)。
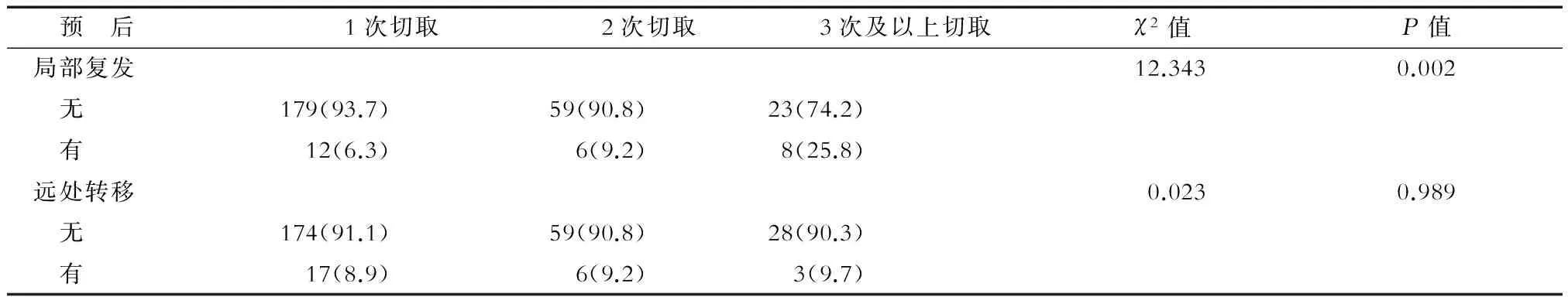
表2 保乳患者获得阴性切缘切取次数与复发转移情况的相关分析 n(%)
3 讨 论
切缘阴性是保乳手术的前提,但并非所有患者在首次切除时即可获得阴性切缘,研究[7-10]发现25%~40%乳腺癌患者在首次切除后切缘仍有肿瘤累及,因而需要再次切取送检切缘。较宽的切缘活检可明显降低切缘阳性的比例,但会影响患者术后乳房形态。Anees等[11]研究发现,切除肿块后进行残腔刮除比不刮除组的切缘阳性率明显降低(19%vs34%,P=0.01)。虽然切缘阳性时可以再次切取,但是目前对多次切取才获得阴性切缘的保乳安全性仍有质疑,切取次数的最高限定尚无统一的结论。因此,本研究回顾分析了影响保乳手术获得阴性切缘所需切取次数的可能临床病理因素,对不同切取次数与患者预后的相关性进行了分析。
Ramanah等[12]分析了206例保乳患者的数据,通过多因素分析发现原位癌较大是切缘阳性的危险因子。Hanna等[13]分析了美国国家癌症数据库中1 170 284例保乳患者的数据,发现肿块越大,出现切缘持续阳性的概率越高。本研究结果与既往报道相符,发现肿块较大时获得阴性切缘所需切取次数更多。对于肿块较大且有保乳意愿的患者,可以选择新辅助治疗来缩小肿块,以降低切缘阳性率[14]。Torabi等[15]分析了224例保乳患者的临床资料,结果发现31%的患者切缘阳性,且组织分级高、淋巴血管浸润及广泛导管内癌成分的患者更易出现切缘阳性。也有学者发现合并有导管内癌时,保乳患者切缘阳性或切缘距肿瘤小于1 mm的比例增加[16]。Subhedar等[17]研究发现,随访6年时12%的导管内癌患者出现复发。在本研究中,3次及以上切取组的原位癌比例明显高于其他两组。导管内癌虽然预后比浸润癌更好,但其易沿着导管广泛分布,因此可能更难获得阴性切缘,需要的切取次数也就更多。Jia 等[18]发现HER-2阳性与切缘阳性密切相关,且HER-2阳性是局部复发的危险因子。van Deurzen等[19]的研究也证实了HER-2阳性的患者切缘阳性率更高,本研究结果与既往报道一致。一方面,由于HER-2阳性肿瘤本身生物学行为较差,易发生侵袭转移,增加了切缘阳性的概率;另一方面,导管内癌患者中HER-2阳性比例更高[20],也可能造成了3次及以上切取组中HER-2阳性的比例更高。
研究表明保乳术后放疗可使10年绝对复发转移风险降低15.7%[21]。本研究中所有患者都接受了术后放疗,大部分患者都有较满意的局部控制,但3次及以上切取组的局部复发率明显高于其他两组。其原因可能是切取次数多意味着肿瘤累及范围广,残留肿瘤的可能性及量也就越大,但放疗不能完全消灭肿瘤,所以更易出现局部复发。因此,临床医生在追求阴性切缘的同时,应考虑到切取次数≥3次时局部复发率会更高。一项三期临床研究[22]结果显示,全乳放疗后加量放疗(16 Gy)可增加保乳患者的局部控制。该研究的结果可能也适用于切取次数≥3次的保乳患者,在全乳放疗后加量放疗进一步降低局部复发。本研究中3次及以上切取组局部复发率虽然升高,但并未影响全身情况,组间远处转移率及总生存无差别。因此,对于获得阴性切缘切取次数多的患者,术后加强局部治疗(如放疗),而不用增加全身治疗的强度可能是一种更优的治疗策略。
综上所述,临床工作中对保乳患者进行选择时可能需要结合患者肿瘤大小、病理类型、HER-2状态等指标来进行综合考虑。如果切取2次仍未获得阴性切缘,考虑到患者术后局部复发风险的升高,术后放疗加量可能是更好的治疗策略。本研究结果为保乳切取次数上限的设定提供了参考依据,让临床医生更好地平衡保乳手术的获益与风险。
[1] TORRE L A, BRAY F, SIEGEL R L, et al. Global cancer statistics, 2012[J]. CA Cancer J Clin, 2015,65(2):87-108.
[2] CHEN W, ZHENG R, BAADE P D, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016,66(2):115-132.
[3] AGARWAL S, PAPPAS L, NEUMAYER L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer[J]. JAMA Surg, 2014,149(3):267-274.
[5] VILA J, GANDINI S, GENTILINI O. Overall survival according to type of surgery in young (≤40 years) early breast cancer patients: A systematic meta-analysis comparing breast-conserving surgery versus mastectomy[J]. Breast, 2015,24(3):175-181.
[6] AERTS L, CHRISTIAENS M R, ENZLIN P, et al. Sexual functioning in women after mastectomy versus breast conserving therapy for early-stage breast cancer: a prospective controlled study[J]. Breast, 2014,23(5):629-636.
[7] LANDERCASPER J, WHITACRE E, DEGNIM A C, et al. Reasons for re-excision after lumpectomy for breast cancer: insight from the American Society of Breast Surgeons Mastery(SM) database[J]. Ann Surg Oncol, 2014,21(10):3185-3191.
[8] BIGLIA N, PONZONE R, BOUNOUS V E, et al. Role of re-excision for positive and close resection margins in patients treated with breast-conserving surgery[J]. Breast, 2014,23(6):870-875.
[9] MERRILL A L, COOPEY S B, TANG R, et al. Implications of new lumpectomy margin guidelines for breast-conserving surgery: changes in reexcision rates and predicted rates of residual tumor[J]. Ann Surg Oncol, 2016,23(3):729-734.
[10] WANIS M L, WONG J A, RODRIGUEZ S, et al. Rate of re-excision after breast-conserving surgery for invasive lobular carcinoma[J]. Am Surg, 2013,79(10):1119-1122.
[11] CHAGPAR A B, KILLELEA B K, TSANGARIS T N, et al. A randomized, controlled trial of cavity shave margins in breast cancer[J]. N Engl J Med, 2015,373(6):503-510.
[12] RAMANAH R, PIVOT X, SAUTIERE J L, et al. Predictors of re-excision for positive or close margins in breast-conservation therapy for pT1 tumors[J]. Am J Surg, 2008,195(6):770-774.
[13] HANNA J, LANNIN D, KILLELEA B, et al. factors associated with persistently positive margin status after breast-conserving surgery in women with breast cancer: an analysis of the National Cancer Database[J]. Am Surg, 2016,82(8):748-752.
[14] GALIMBERTI V, TAFFURELLI M, LEONARDI M C, et al. Surgical resection margins after breast-conserving surgery: Senonetwork recommendations[J]. Tumori, 2016,2016(3):284-289.
[15] TORABI R, HSU C H, PATEL P N, et al. Predictors of margin status after breast-conserving operations in an underscreened population[J]. Langenbecks Arch Surg, 2013,398(3):455-462.
[16] THANASITTHICHAI S, CHAIWERAWATTANA A, PHADHANA-ANAKE O. Impact of using intra-operative ultrasound guided breast- conserving surgery on positive margin and re-excision rates in breast cancer cases with current SSO/ASTRO guidelines[J]. Asian Pac J Cancer Prev, 2016,17(9):4463-4467.
[17] SUBHEDAR P, OLCESE C, PATIL S, et al. Decreasing recurrence rates for ductal carcinoma in situ: analysis of 2996 women treated with breast-conserving surgery over 30 years[J]. Ann Surg Oncol, 2015,22(10):3273-3281.
[18] JIA H, JIA W, YANG Y, et al. HER-2 positive breast cancer is associated with an increased risk of positive cavity margins after initial lumpectomy[J]. World J Surg Oncol, 2014,12:289.
[19] VAN DEURZEN C H. Predictors of surgical margin following breast-conserving surgery: a large population-based cohort study[J]. Ann Surg Oncol, 2016,23(Suppl 5):627-633.
[20] TOT T. Early (<10 mm) HER2-positive invasive breast carcinomas are associated with extensive diffuse high-grade DCIS: implications for preoperative mapping, extent of surgical intervention, and disease-free survival[J]. Ann Surg Oncol, 2015,22(8):2532-2539.
[21] Early Breast Cancer Trialists′ Collaborative Group (EBCTCG), DARBY S, MCGALE P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials[J]. Lancet, 2011,378(9804):1707-1716.
[22] BARTELINK H, MAINGON P, POORTMANS P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial[J]. Lancet Oncol, 2015,16(1):47-56.
[本文编辑] 廖晓瑜, 贾泽军
Correlation between the re-excision frequency of breast-conserving surgery negative margins and clinical prognosis of patients with early-stage breast cancer
QIAN Wei-wei, NI Yi
Department of Thyroid and Breast Surgery, The Third People’s Hospital of Nantong, Nantong 226001, Jiangsu, China
Objective: To analyze the correlation between re-excision frequency of breast-conserving surgery negative margins and clinical prognosis of patients with early-stage breast cancer. Methods: We collected the clinical information of early breast cancer patients treated with breast conserving therapy in the Third People's Hospital of Nantong City, Jiangsu Province, from January 2009 to October 2016. We analyzed the relationship between the frequency of re-excision and patient age, tumor size, lymph node status, hormone receptor status, Her-2, pathological type and prognosis of patients retrospectively. Results: In this study, 287 patients with early breast cancer who underwent breast-conserving therapy were included. 191 (66.6%) patients acquired negative margins by one excision, 65 (22.6%) patients by twice excision, and 31 (10.8%) patients by thrice or more. Tumor size (P=0.010), pathological type (P< 0.001), and HER-2 state (P=0.034) corrected with the frequency of re-excision. The median follow-up time was 5 years. Local recurrence rates were 6.3%, 9.2%, and 25.8% for one excision group, twice excision group, and thrice or more excision group, respectively and statistical difference was found among the three groups (P=0.002). No statistical difference was found among the three groups with regard to distant metastasis rates (P=0.989) and overall survival rates (P=0.326). Conclusions: Tumor size, type of pathology, HER-2 status were correlated with the frequency of re-excision required for negative margins in the breast-conserving patients. The frequency of re-excision is positively correlated with local recurrence rates, but had no significant relationship with distant metastasis and overall survival rates.
early breast cancer; breast conserving surgery; negative margin; frequency of re-excision; prognosis
2017-03-04 [接受日期] 2017-05-15
钱炜伟,主治医师. E-mail: 1340223090@qq.com
10.12025/j.issn.1008-6358.2017.20170175
R 737.9
A

