Syntheses,Characterization and Radical Scavenging Activity of Two Copper(Ⅱ)Complexes Containing Pyrazoles
YANG HongGUO Li-Jun
(Department of Chemistry and Chemical Engineering,Taiyuan Institute of Technology,Taiyuan 030008,China)
Syntheses,Characterization and Radical Scavenging Activity of Two Copper(Ⅱ)Complexes Containing Pyrazoles
YANG Hong*GUO Li-Jun
(Department of Chemistry and Chemical Engineering,Taiyuan Institute of Technology,Taiyuan 030008,China)
By using a tripodal ligand tris(3,5-bimethyl-pyrazolylmethyl)amine(TMPzA)and a bridged ligand thiophenedicarboxylic acid(H2TPDC),two binuclear copper(Ⅱ)complexes have been synthesized and characterized.It has been found that TMPzA lost an arm and coordinated to copper with di(3,5-bimethylpyrazolylmethyl)amine(DMPzA)in complex[Cu2(DMPzA)2(TPDC)2]ClO4(1).The structure presented a microcycle with the Cu(1)…Cu(1A)distance of 0.786 84 nm.The complex[Cu2(TMPzA)2(TPDC)(H2O)2](ClO4)2(2)is a binuclear structure and with the TMPzA completely.The investigation in Feton system shows that complex 1 has better hydroxyl radicals scavenging property than that of complex 2.CCDC:1411232,1;1411233,2.
binuclear copper(Ⅱ)complexes;Feton system;hydroxyl radicals scavenging
0 Introduction
Oxygen-derived free radicals such as superoxide anion radical(O2·-)and hydroxyl radical(·OH)which can oxidize the proteins,carbonhydrates,nuclein and fats aremost active and harmful radicals in organisms[1-2]. The cells and organs would be injured and cause diseases such as coronary heart disease,rheumatoid arthritis and cancer[3]if these radicals cannot be removed just in time[4-6].So the study of scavenging of oxygen-derived free radicals is more valuable.
It is well-known that free radicals are controlled on human body by some enzymes such as superoxide dismutase and peroxidase[7],but the non-enzymes can also scavenge free radicals and play an important role toprotectbodiesfromfreeradicals.Thepolyfunctionalcoordinatedpolymerandmulti-nuclear complexes can be used to scavenge hydroxyl radicals[8-12].The hydroxyl radicals can be generated via a redox process from Feton system[13].Herein,we report two excellenthydroxylradicalsscavengingbasedon binuclear Cu(Ⅰ)complexes.The two complexes were added to the Feton system to compete the hydroxyl radicals with salicylic acid and the complexes showed good ability and they might be potential hydroxyl radical scavenger.
1 Experimental
1.1 Starting materials and general methods
All commercially available chemicals were of analytical grade and directly used without further purification.Infrared spectra were obtained(KBr disk, 4000~400cm-1)using a sp-100 spectrometer.Elemental analyses were performed in a PE240C elemental analyzer.UV-Vis spectra were carried out with a TU-1901 double beam instrument.
1.2 Synthesis of[Cu2(DMPzA)2(TPDC)2]ClO4(1) and[Cu2(TMPzA)2(TPDC)(H2O)2](ClO4)2(2)
A colorless aqueous solution of 2,5-Thiophenedicarboxylate(17.2 mg,0.1 mmol,deprotonated by reactingwithNaOH)wasaddedtoa10mL acetonitrile solution of Cu(ClO4)2·6H2O(74.2 mg,0.2 mmol)and TMPzA(68.2 mg,0.2 mmol)with stirring for 30 min,filtered and allowed to evaporate at room temperature.Green rectangular crystals of 1 and blue needle crystals of 2 were deposited after a few days, which were separated and air-dried.Yield:40%for 1; 30%for 2(based on copper).Anal.Calcd.for 1 (C36H41ClCu2N10O12S2,%):C,41.84;H,3.96;N,13.56. Found(%):C,41.72;H,4.03;N,13.67.Calcd.for 2 (C42H60Cl2Cu2N14O14S,%):C,41.48;H,4.94;N,16.13. Found(%):C,41.29;H,4.85;N,16.07.IR data(cm-1) for 1:3 434(m),2 925(w),1 639(m),1 585(s),1 554 (m),1 529(m),1 420(w),1 318(m),1 234(w),1 121(s), 1 061(m),808(w),774(w),625(w).For 2:3 435(s),2 925(w),1 627(s),1 557(m),1 468(w),1 393(m),1 349 (m),1 121(vs),797(w),727(w),624(w).
1.3 Crystal structure determination
The X-ray diffraction measurements for 1 and 2 were performed on Bruker SMART APEXⅡCCD diffractometer with graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm)by using φ-ω scan mode at 100(2)K for 1 and 296(2)K for 2.The absorption corrections were applied using SADABS program[14]. All structures were solved by direct methods and refined by full matrix least-square on F2using SHELXTL program package[15].All non-hydrogen atoms were refined with anisotropic displacement parameters.All the hydrogen atoms bonded to carbon atoms were placed in calculated positions and treated in a ridingmodel approximation.The hydrogen atoms of the H2O in complex 2 were located in a difference Fourier map.In complex 1,the part of one DMPzA molecule (N3,N3A,N4,N4A,N5,N5A,C12,C12A,C13, C13A,C14,C14A,C15,C15A,C16,C16A,C17, C17A,C18 and C18A)were disordered by refining over two sides with occupancy of part 21.00 and -21.00.The crystal data and details of structural determination for the complex 1 and 2 are presented in Table 1.
CCDC:1411232,1;1411233,2.
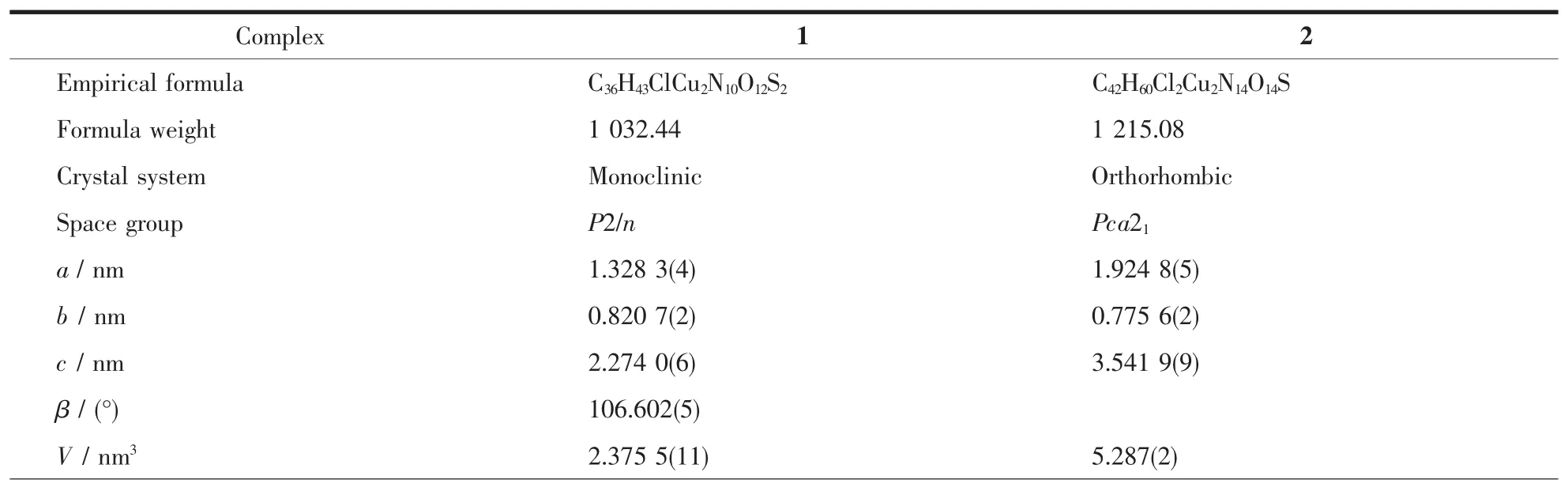
Table 1Crystallographic data and details for complexes 1 and 2
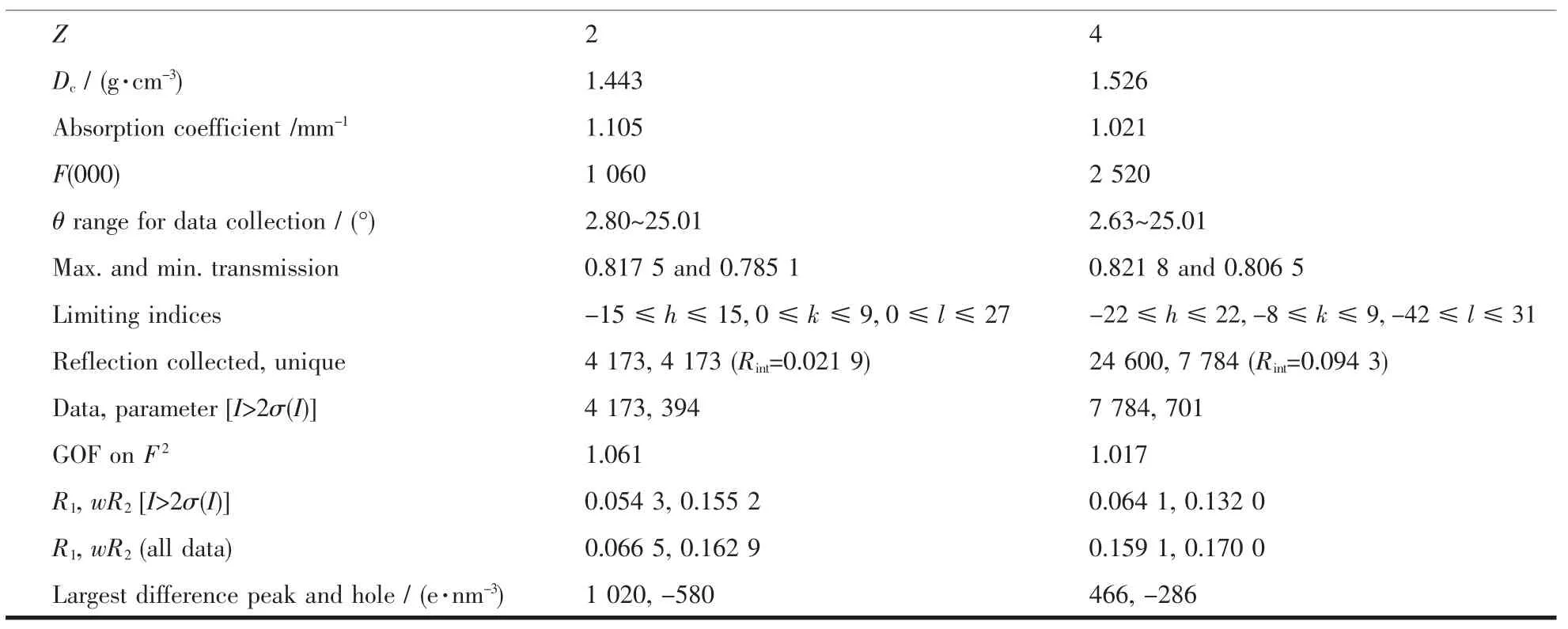
Continued Table 1
1.4 Hydroxyl radical-scavenging essay
1.4.1 Generation and trapping of hydroxyl radicals
The hydroxyl radicals were generated by the well-known Fenton reaction using hydrogen peroxide and ferrous sulfate[16].If the salicylic acid is added to theFentonreactionsystem,thecharacteristic absorption bands at 504 nm will appear due to the hydroxyl radicals trapped and the products(a)and(b) formed,the reaction occurring as Scheme 1[17].The UV absorbanceat504nmwilldecreasewhenthe complexes which can scavenge the hydroxyl radical were added to the solution.That means we can determine the radical-scavenging activities of the complexes by measuring the absorbance of 504 nm.
1.4.2 Hydroxyl radical-scavenging rate measurement
The FeSO4solution(2 mL,1.8 mmol·L-1)was mixed with salicylic acid solution(2 mL,1.8 mmol· L-1)in five 10 mL volumetric flasks,then the solution of the two complexes with differentconcentrations was added to the mixture,respectively.After shaking, 1 mL H2O2was added andthe final volume was made up to 10 mL with double distilled water.The solutionsweretransferredtothecellforthe absorption measurement at 504 nm on a TU-1901 spectrophotometer with the temperature 37℃,the time 30 min and the pH 7.38.The absorption data of a and b(Scheme 1)by the action of the complexes with various concentrations can be obtained.The hydroxyl radical-scavenging activity was calculated.
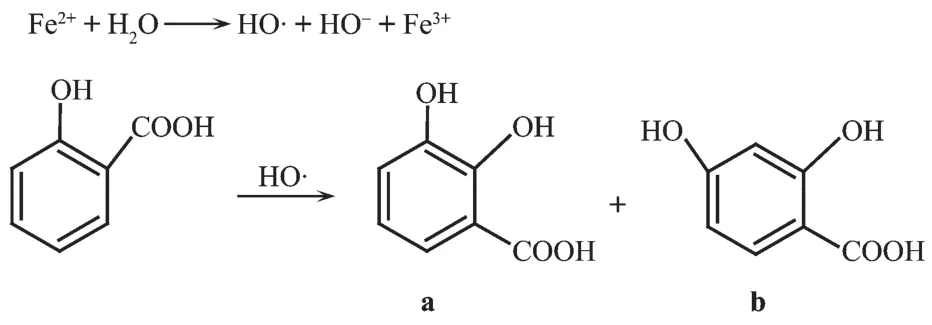
Scheme 1
2 Results and discussion
2.1 Descriptions of crystal structures
2.1.1 Crystal structure of[Cu2(DMPzA)2(TPDC)2]ClO4(1)
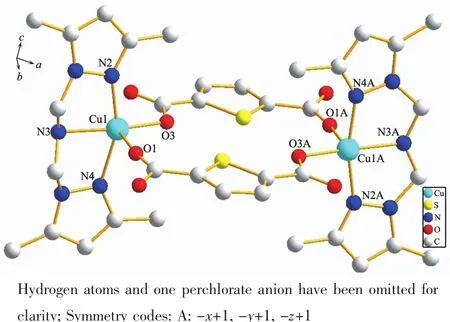
Fig.1Molecular structure of complex 1
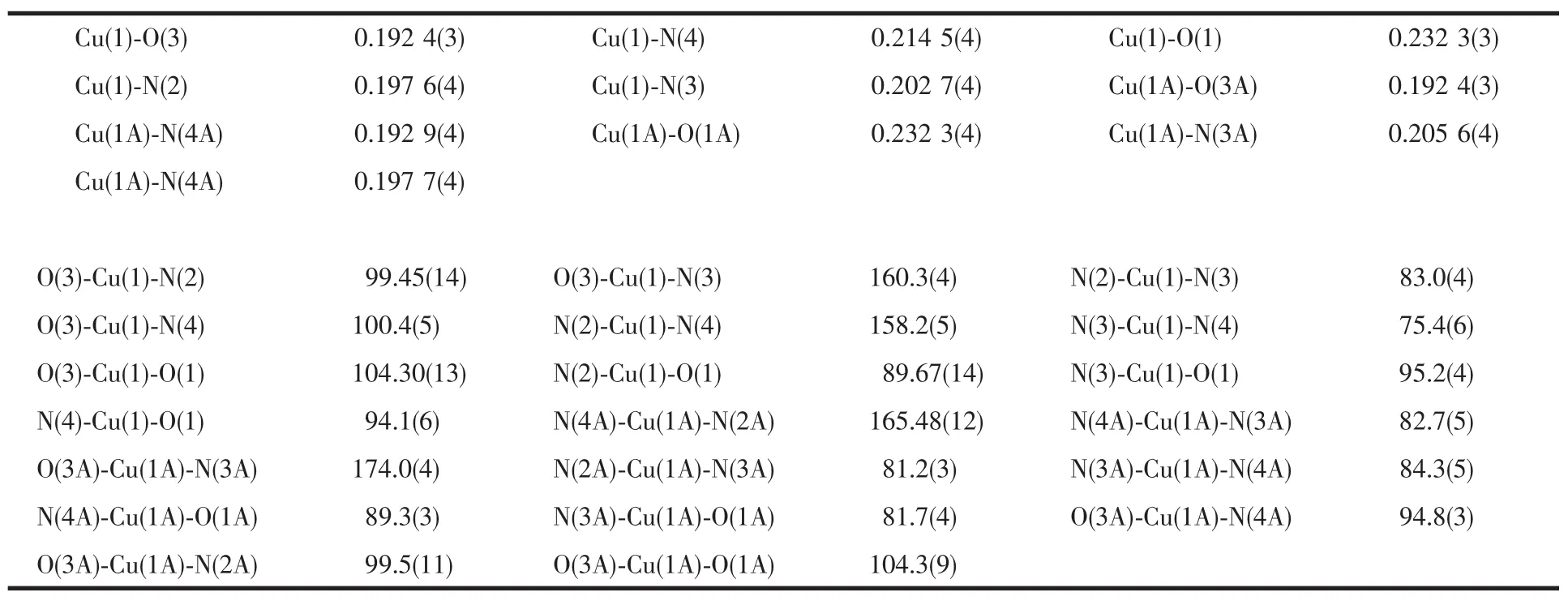
Table 2Selected bond lengths(nm)and angles(°)in 1
Complex 1 crystallizes in the monoclinic space group P2/n and has an independent molecule in the centrosymmetric unit,as shown in Fig.1.The tripodal ligand TMPzA lose a pendant arm and turn to DMPzA with the N atom of amine protonated partially,the possiblemechanismhasbeendiscussedinour previous works[18].The molecular structure of 1 consists of two Cu(Ⅰ)ions bridged through two Thiophenedicarboxylate molecular resulting in a microcycle with the intramolecular Cu(1)…Cu(1A)distance of 0.786 84 nm.The copper ions are coordinated with two Npyrazoleatoms,one Naminefrom a DMPzA molecule and two Ocarboxylatoms from two H2DPC molecules,forming a distorted square pyramidal geometry.The Cu-Namineis somewhat longer than Cu-Npyrazoleand it is consistent with this class of complexes.The three N atoms(N2, N3,N4)and O3 define the bottom plane with the Cu atoms protrude out from it by 0.006 75 nm.The Cu1-O1 bond occupy the axial site and is significantly longer than other bonds may be due to the strain of the carboxylate ligand and the Jahn-Teller effect from the copper atom.Selected bond lengths and angles for 1 are listed in Table 2.
2.1.2 [Cu2(TMPzA)2(TPDC)(H2O)2](ClO4)2(2)
Be different from 1,the tripodal ligand TMPzA in 2 has not decomposed and coordinate to the Cu atoms with three Npyrazoleatoms and one Namineatom,as shown in Fig.2.The two Cu(Ⅰ)ions are also linked by the H2TPDC ligand and form a centrasymmetric structure.Each Cu ion is coordinated by four N atoms from TMPzA,one O atom form H2TPDC and one O atom from H2O molecule,forming a distorted octahedral coordination geometry.The three Npyrazoleatoms and O from H2O are located at the equatorial plane of Cu center,while the Namineand Ocarboxylatoms are located at the apical positions.The most distinguished feature of 2 lies in the Cu-Namine(Cu1-N3 0.206 4(7)nm;Cu2-N10 0.207 8(7)nm)bond lengths are significantly shorter than the Cu-Npyrazole(Cu1-N4 0.235 9(6)nm; Cu2-N8 0.238 0(7)nm)bonds,which is very rare in this class of complexes.Selected bond lengths and angles for 2 are listed in Table 3.
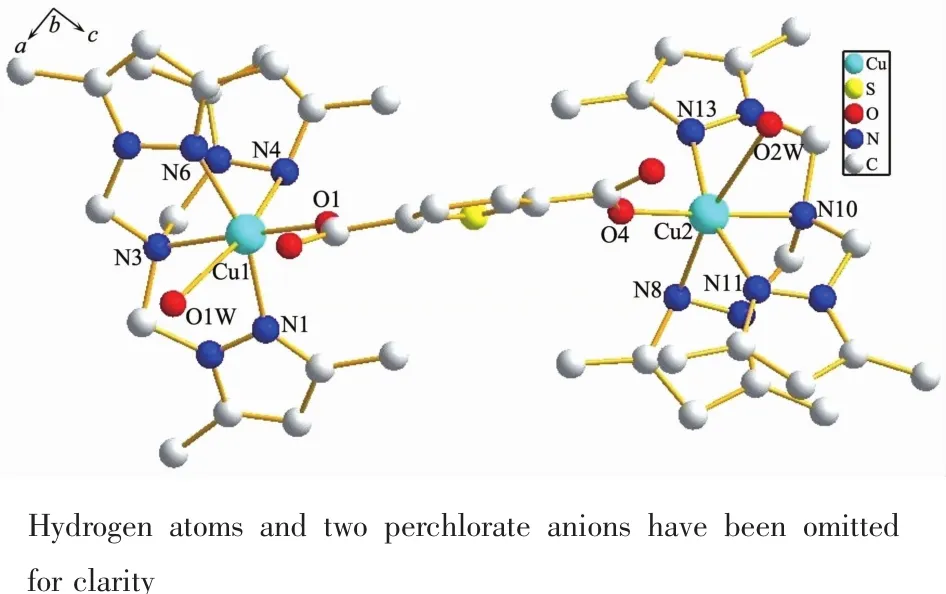
Fig.2Molecular structure of complex 2
2.2 Hydroxyl radical-scavenging studies
The absorbance with various concentrations of complexes 1 and 2,Ai,are summarized in Table 4 andtheradical-scavengingratesafteradding complexes are shown in Fig.3.The radical-scavenging rate(η)is calculated by the equation:η=1-and the plots of hydroxyl radical-scavenging rate vs the extent of complexes used can be made from the data, where A0(A0=0.533 5)represents the absorbance of the reaction system without any complex.It is found that the greater the concentration of 1 and 2,thelower the absorbance of a and b in the reaction system and the higher the hydroxyl radical-scavenging rate.Moreover,the radical-scavenging rate of 1 is somewhat higher than that of 2,which may be due to the different coordination environment of Cu ions.In complex 1,the Cu ions are five-coordinated with a potential coordination site that can combine with the hydroxyl radical while the Cu ions in complex 2 are six-coordinated.
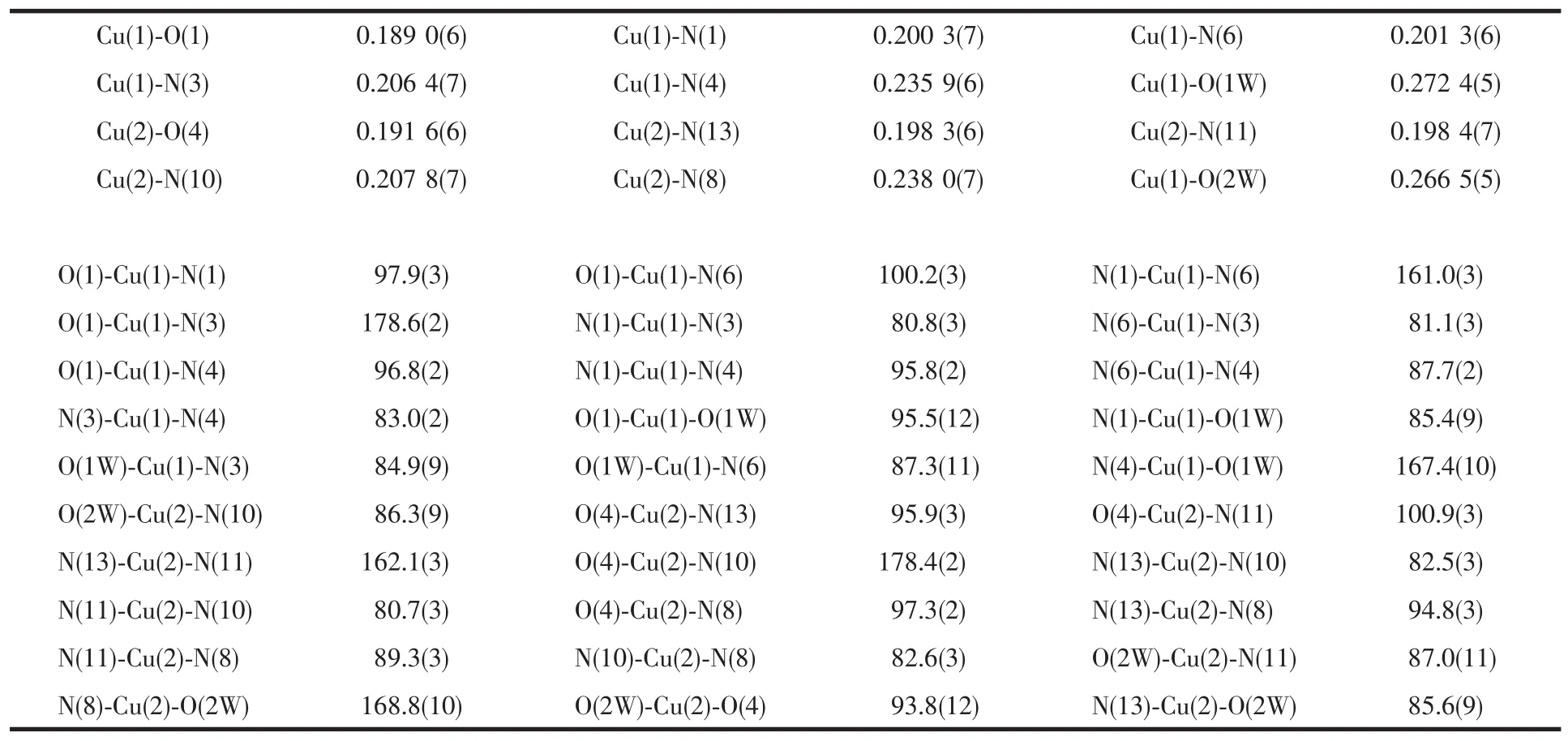
Table 3Selected bond lengths(nm)and angles(°)in 2
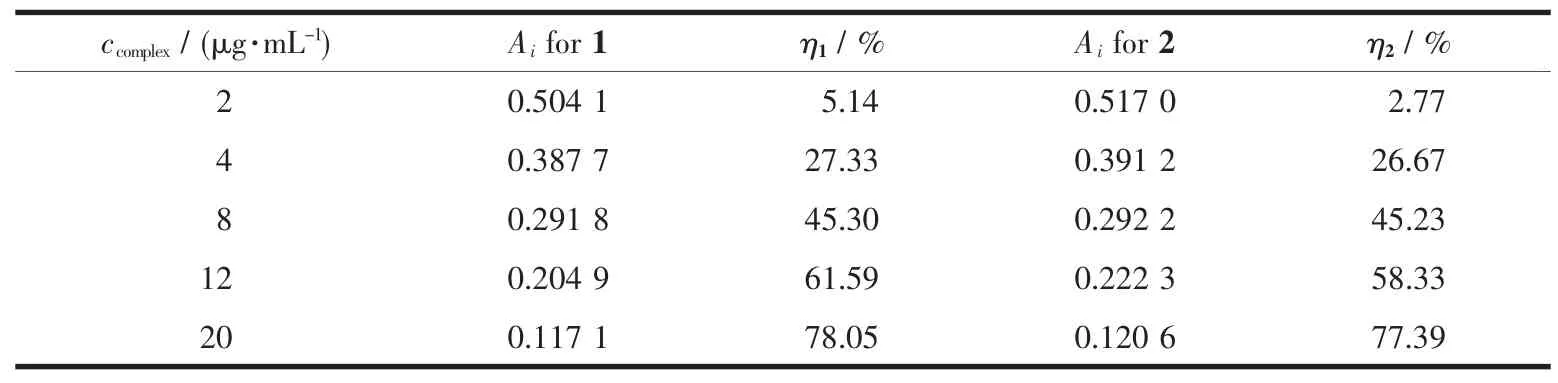
Table 4Hydroxyl radical-scavenging of 1 and 2
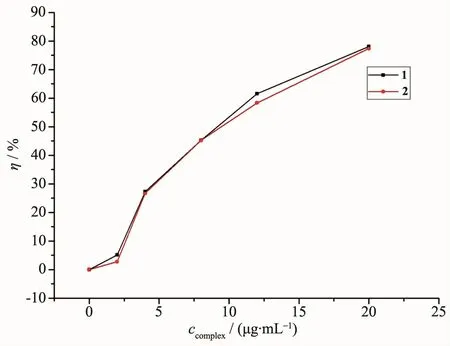
Fig.3Hydroxyl radical-scavenging rate of 1 and 2
3 Conclusions
Two binuclear copper(Ⅱ)complexes containing polyamine and bridged carboxyl ligands were prepared and characterized.In two complexes,the copper ions have square-pyramidal geometry and were bridged by thecarboxylligandviatheoxygenatomsin monodentate mode.The radical-scavenging activity of the two complexes was determined and the results indicate that two complexes present excellent radicalscavenging activities.
[1]JIANG Lin-Ling(蒋林玲),LI Bao-Lin(李宝林),WANG Li(王丽).J.Anal.Sci.Technol.(分析科学学报),2008,24(2):212-214
[2]Tavadyan L A,Sedrakyan G Z,Minasyan S H,et al.Transition Met.Chem.,2004,29(6):684-696
[3]Mittler R.Trends Plant Sci.,2002,7:405-410
[4]Halliwell B,Gutteridge J M C.Free Radicals in Biology and Medicine.Oxford:Clarendon Press,1989:110
[5]Vasquez J,Kalyanaraman B,Kennedy M C.J.Biol.Chem., 2000,275:14064-14069
[6]Harman D.Free Radicals in Molecular Biology,Aging and Disease.New York:Raven Press,1984:1-12
[7]Fattman C L,Schaefer L M,Oury T D.Free Radical Biol. Med.,2003,35(3):236-256
3.2.2 进行教学改革 首先,按照科学的标准进行课程设置。武进少体校必须以本校运动员的现实状况作为参考,根据运动员的时间和实际要求,结合苏教版教材的特点和学校实际情况,在不脱离国家义务教育背景的基本前提下,重新修改和制订一个既注重运动员基础文化知识和基本技能的传授,又利于学生德、智、体劳全面发展的课程体系。在此基础上,建议武进少体校能够编写与本校运动员的文化学习基础相适应的教学大纲和教材体系。
[8]Giokas D L,Vlessidis A G,Evmiridis N P.Anal.Chim.Acta, 2007,589(1):59-65
[9]Naughtona D P,Grootveld M.Bioorg.Med.Chem.Lett., 2001,11:2573-2575
[10]Senthil Raja D,Paramaguru G,Bhuvanesh N S P,et al. Dalton Trans.,2011,40:4548-4559
[11]Raja D S,Bhuvanesh N S P,Natarajan K.Dalton Trans., 2012,41:4365-4377
[12]Manikandan R,Viswanathamurthi P,Velmurugan K,et al. J.Photochem.Photobiol.B,2014,130:205-216
[13]Yan F F,An P D,Ying P H.Chin.Chem.Lett.,2009,20: 1235-1240
[14]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[16]Suttor H C,Winterbourn C C.Free Radical Biol.Med., 1989,6:53-60
[17]LI Yan(李艳),GONG Shi-Lei(巩士磊),CHE Ying(车影), et al.Chin.J.Appl.Chem.(应用化学),2015,32(8):948-954
[18]Yang H,Tang Y,Shang Z F,et al.Polyhedron,2009,28: 3491-3498
两个含吡唑环的双核铜配合物的合成、表征及清除自由基活性
杨红*郭丽君
(太原工业学院化学与化工系,太原030008)
以三(3,5-二甲基吡唑甲基)胺(TMPzA)及2,5-噻吩二羧酸为配体合成了2个双核Cu的配合物[Cu2(DMPzA)2(TPDC)2]ClO4(1)和[Cu2(TMPzA)2(TPDC)(H2O)2](ClO4)2(2)。在1中,配体TMPzA发生裂解,以二(3,5-二甲基吡唑甲基)胺(DMPzA)与Cu配位,并在2个Cu原子之间形成一个大环,2个Cu原子之间的距离为0.786 84 nm。清除自由基实验发现,配合物1比2具有较好的清除自由基活性。
双核铜配合物;Feton体系;清除羟基自由基
O614.121
A
1001-4861(2017)06-1059-06
2017-01-13。收修改稿日期:2017-04-18。
10.11862/CJIC.2017.125
国家自然科学基金(No.)资助项目。
*通信联系人。E-mail:hmily820805@163.com
- 无机化学学报的其它文章
- 过渡元素掺杂Fe3O4(001)表面磁电性能的理论研究
- 熔盐法合成电化学性能优异的富锂层状正极材料Li1.5Ni0.25Mn0.75O2.5
- Syntheses,Crystal Structures and Properties of Two d10Metal Complexes Constructed from 1,5-bis(2-ethyl-imidazolyl)pentane
- Efficient Synthesis and Application in Heck Reaction of Pd/Fe3O4Magnetic Nanoparticles
- Syntheses,Crystal Structures and Catalytic Activity of Rhenium Carbonyl Complexes Containing Aryl-Substituted Tetramethylcyclopentadienyl Ligands
- Characterization,Photocatalytic Property and Kinetics of ZnO Nanoparticles Synthesized by One Step Solid State Reaction

