Enhanced propellant performance via environmentally friendly curable surface coating
Thelm Mnning,Jeffrey Wyckoff,Kenneth Klingmn,Virl Pnchl, Eugene Rozumov,John Bolognini,Ming Wng Young,Suhsh Ptel
aUS Army RDECOM ARDEC,Picatinny Arsenal,NJ,USA
bPolymer Processing Institute,Newark,NJ,USA
Enhanced propellant performance via environmentally friendly curable surface coating
Thelma Manninga,*,Jeffrey Wyckoffa,Kenneth Klingamana,Viral Panchala, Eugene Rozumova,John Bologninia,Ming Wang Youngb,Subhash Patelb
aUS Army RDECOM ARDEC,Picatinny Arsenal,NJ,USA
bPolymer Processing Institute,Newark,NJ,USA
A R T I C L E I N F O
Article history:
Surface coating of granular propellants is widely used in a multiplicity of propellants for small,medium and large caliber ammunition.All small caliber ball propellants exhibit burning progressivity due to application of effective deterrent coatings.Large perforated propellant grains have also begun utilizing plasticizing and impregnated deterrent coatings with the purpose of increasing charge weights for greater energy and velocity for the projectile.The deterrent coating and impregnation process utilizes volatile organic compounds(VOCs)and hazardous air pollutants(HAPs)which results in propellants that need to be forced air dried which impacts air quality.Propellants undergo temperature fluctuations during their life.Diffusion coef ficients vary exponentially with variations in temperature.A small temperature increase can induce a faster migration,even over a short period of time,which can lead to large deviations in the concentration.This large concentration change in the ammunition becomes a safety or performance liability.The presence of both polymeric deterrents and nitroglycerin(NG)in the nitrocellulose matrix and organic solvents leads to higher diffusion rates.This results in continued emissions of VOCs and HAPs.Conventional polymers tend to partition within the propellant matrix.In other words, localized mixing can occur between the polymer and underlying propellant.This is due to solvent induced softening of the polymer vehicle over the propellant grain.In effect this creates a path where migration can occur.Since nitrate esters,like NG,are relatively small,it can exude to the surface and create a highly unstable and dangerous situation for the war fighter.Curable polymers do not suffer from this partitioning due to“melting”because no VOC solvents are present.They remain surface coated.The smallscale characterization testing,such as closed bomb testing,smallscale sensitivity,thermalstability, and chemicalcompatibility,willbe presented.The 30 mm gun demonstration firing data at hot,cold,and ambient temperatures will also be presented.
Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
The objective of the research effort is to replace the current solvent based wet deterrent and impregnation coating technology, currently used in propellant production,with the environmentally friendly novel Light Emitting Diode Ultra-Violet(LED-UV)cured solvent-less advanced surface coating technology.This research effort work is to explore the use of LED UV curable polymers as deterrent coating materials,which do not suffer from this partitioning due to“melting”because of the highly adjustable and attainable network structure.The additional objectives are to increase performance without increasing maximum breech pressure by the slowed and inhibited burning and obtain progressivity at the grain and charge levels.The surface coating objective is also to achieve a flat temperature coef ficient by having a low glass transition temperature(Tg)ofcoating materials.The low Tgcan prevent the initiation,disrobing,cracking,ablation,penetration,and coating separation at cold temperatures.The improved mechanical properties across the ballistic temperature range are expected to improve Insensitive Munitions(IM)characteristics against thermal and spall threats.The crosslink LED UV coating polymer structure can inhibit/reduce migration which can prevent plasticizer migration and degradation ofperformance resulting from migration.Thismigration results in large concentration changes in ammunition which becomes a safety or performance liability[1].In addition,the recent advances in high power LED's ensures an abundant commercial availability of the LED UV light source.This makes it possible to greatly reduce the production cost by minimizing the production space requirements and energy usage,as well as, generating virtually no waste stream.
2.Experimental
2.1.LED UV coating formulation development
There are four major attributes of the UV curable monomers which contribute to the final coating performance.They are: functionality,chemical backbone,chemical structure and molecular weight.The formulation development focused on two basic components in the make up of the LED UV curable compositions: Monomer and/or Reactive Diluents and Photo Initiators(PI).An increase in the functionality ofthe monomers usually speeds up the light curing reactions.The concentration of the photoinitiator was varied to achieve a dramatic effect on desired properties of LED UV cured coating.Monomer and Reactive Diluents utilized were: mono-functional i.e.SR256,2(2-Ethoxyethoxy)Ethyl Acrylate,difunctional,tri-functional(i.e.SR4942(2 Ethoxy ethoxy)Ethyl Acrylate and SR 9012:Tri-functionalacrylate ester)and tetrafunctional(i.e.SR 494 Ethoxylated(4)Pentaerythritol Tetraacrylate) monomers to achieve a three dimensional network.The functionalized monomers used are lower in viscosity,which facilitates a coating application with improved surface wetting,leveling and offering widely attainable physical properties derived from numerous available chemicalstructures.Reactive diluents in a LED UV curable formulation played a key role.It is a mono-functional liquid;and affected both cure speed and the extent of polymerization,as wellas the properties of the finalproduct.Increasing the monomer functionality leads to higher cure speed,higher Tg,higher crosslink density,higher shear strength,and greater chemical and thermal resistance,but lower flexibility and low conversion.
A photoinitiator(PI)is a compound that,upon absorption of light,undergoes a photoreaction and produces reactive species of free radicals.This induces cross linking between the unsaturated carbon to carbon double bonds(C=C)sites ofmonomers/oligomers. This accomplishes the cure process to generate polymer C-C bonds. Thus,PI transforms the physical energy of light into suitable chemical energy in the form of reactive intermediates.The photoinitiator package was optimized for a given coating thickness and LED UV dosage.The formulation development focused on formulating surface cure PIwith excellentsolubility and sound resistance to oxygen poisoning.At the same time it possesses suitable photon sensitivity to the selected LED UV wavelengths from 385 nm to 415 nm.Five different types of photoinitiators with concentrations from 0.01 g/l to 0.2 g/l were investigated to achieve an optimized LED UV absorbance as a function of light wavelengths[3].
SeveralLEDUV coating formulations were developed,optimized and characterized.The formulation characterization consisted of using the Photo Differential Scanning Calorimetry(Photo-DSC)and LED UV Coating Droplet Diameter Measurement for Wettability. The optimized light cured formulations were then applied by coating them on the surface ofan inert simulant.This incorporated a cellulose acetate binder with calcium carbonate powder and an energetic single base propellant(AFP-001)grains as the substrates and processed using the Mini Glatt Fluidized bed to be discussed in the next section.
2.2.Processing LED UV coating formulation
The processing consisted of using Ultra Violet(UV)curable coatings employing solvent-less and low viscosity reactive liquids. They can be applied to selected substrates,and converted into a solid adherent film within a fraction of a second when exposed to LED UV light[5].Fig.1(a)is a remotely operated Fluidized Bed Coater fitted with an explosion proof UV light source(medium density mercury lamps);its operating mechanism is illustrated in Fig.1(b).The coating ofa UV composition on a propellant surface in the fluidized bed could be described in four elementary steps,(1) Fluidization of propellant grains(2)Atomization of UV liquid through the spray nozzle,(3)Wetting fluidized propellants with UV liquid droplets,and formation of coating layer via spreading,(4) Rapid curing of UV liquid when exposed to the UV light through the glass window.
2.3.Vacuum thermal stability testing(VTS)
Prior to processing,compatibility tests were conducted to determine if the UV light cured monomers were compatible with nitrocellulose binder,plasticizer,and stabilizer in the AFP-001 propellant substrate.Using a Perkin Elmer Pyris 1 Differential Scanning Calorimeter(DSC),samples were run through temperature scans according to STANAG 4147.Temperature scans were performed from 50°C to 350°C.Samples were sealed in aluminum pans and run in duplicate.A decomposition peak temperature shift of 4°C or less was deemed compatible.Any shift towards a lower peak temperature of the materials was deemed to exhibit some degree of incompatibility,with the magnitude of the shift corresponding to the degree ofincompatibility.A peak temperature shift of more then 20°C indicates incompatibility.Peak temperature shifts between 4°C and 20°C indicate partial or potential incompatibility,in which case Vacuum ThermalStability(VTS)testing was required to con firm or deny incompatibility.This was done in accordance with STANAG 4556 ED.1(Explosives:Vacuum Stability Test).This standard testing procedure measures the stability of an explosive at an elevated temperature under vacuum.The candidate explosive and materials are tested alone as control subjects.The explosive is then mixed with each individual material and tested. The reactivity(compatibility)is then determined by comparing the gas evolved by the candidate explosive control,the material control,and the mixture.The materials were tested for 40 h at 100°C.
The LEDUV lightcured monomers thatpassed the compatibility testing were then surface coated on the AFP-001 propellant substrate and the actual mixes were characterized for incompatibility via Vacuum Thermal Stability(VTS).The propellant surface coating formulations that passed the VTS test were then scaled up to 150 g surface coating.All of the new UV light cured monomers have shown compatibility with NC,plasticizer and stabilizer incorporated into the AFP-001 propellant formulation,according to both the DSC and VTS tests.The VTS criteria used are listed in Table 1.
2.4.Closed bomb test

Table 1 VTS criteria.
After the VTS analysis of those ingredients that proved to be compatible with AFP-001 propellant and plasticizer,several 150 g propellantformulations were surface coated using the UV Fluidized Bed Coater at ARDEC.They were then weighed out,grain dimensioned and density measured for closed bomb analysis at 21°C.The closed bomb test is designed to determine linear burning rates of energetic compositions at elevated pressures.The capacity for closed bomb used in this testing was 200 cc.Three 20-g propellant samples were tested for each gun propellant formulation using a loading density of 0.10.The propellant grains were single perforation(0.006′Perf.diameter)with an outer diameter of0.072′and a length of 0.088”.All tests generated pressures in excess of 15 KSI. The closed bomb test results were analyzed and plotted.Many of the new LED UV coating formulations have pressure exponents less than 1,like thatof JA2.JA2 is a legacy propellantthathas acceptable propellant characteristics.Burn rates in many of the surface coated AFP-001 with LED UV coating formulations such as the PAP-8604, 8605 and 8606 lot were identi fied as Picatinny Arsenal Propellant lots RDD14H-00194,RDD14H-00195,and RDD14H-00196.
Using the propellantthermochemicalproperties,burn rates and pressure exponent,IBHVG2 interior ballistics code was employed to predict the theoretical velocities of these novel surface coated AFP-001 with LED UV coating formulations such as the PAP-8604, 8605 and 8606 using a single perforated grain geometry.
3.Results and discussion
3.1.LED UV coating formulation development
For the approach,several Photoinitiators,monomers,reactive diluents and surface tension modi fiers for LED UV Curing were investigated,selected and characterized.
3.2.Characterization
3.2.1.Evaluation of photo-initiators(PI)
Photo-initiators are molecules that absorb the energy of light and act as donors by transferring this enenrgy to acceptor molecules[5,6].The molecules that receive the energy may in turn undergo reactions,such as polymerizations,isomerizations,couplings and others.A good photo-initiator,therefore,is not only a molecule that readily absorbs light energy,but also one that readily transfers complexes in molecules.The photo-intiators investigated were organic compounds,thatupon absorbing lightenergy,formed polymerization initiating species.Such species were free radicals. These molecules functioned as photo intiators.The PI used were mostly benzophenone and ketones.They absorb light energy and transfer it to another molecule and formα-β-cleavage to form initiating species when irradiated with light of the approriate wavelength.Five free radicalphotoinitiators were investigated and characterized.The UV absorbance spectra of the free radical photoinitiators are shown in Figs.2-6.
UV-VIS spectra of the five photo-initiators evaluated are shown in Figs.2-6.Based on the UV-VIS spectrum,KIP-100 showed 0.4 UV absorbance at 350-400 nm wavelength at the concentration of 0.2 g per liter when compared to the other photo initiators evaluated.
Table 2 shows the LED UV coating formulations evaluated for heat of reactions and percent conversion using the four different photo-initiators(see Table 3).
Each LED UV coating formulations were identi fied as Series 1,2,3,and 4.From the typicalphoto-DSC results as shown in Fig.7, the Heat of Reaction and percent conversions were determined for each LED UV coating formulaions.
Fig.8 showed that series 3 from Table 2 has the slowest reaction and had the slowest per cent conversion(see Fig.9).
3.2.2.Changes in contact angle(wettability)
After the photo initiator evaluations were completed,the wettability of the LED UV coating formulations were determined. An inert substrate using Cellulose Acetate Butyrate(CAB)binder with inert filler was used.The amount of 20 ul of the formulation mixture was applied on the CAB substrate plate.After 2 min,the diameters of the droplets were measured by caliper as shown in Fig.10.
The contact angles of the monomers on the CAB substrate surfaces changes depend on the type ofthe acrylates and the degree of functionalization.Tables 4-6 below,show the effects of photoinitiators and reactive diluents on the wettability of various formulations.From Tables 4-7,it can be seen that the contact angle may increase or decrease in diameterwith the addition ofthe photo initiator and reactive diluents[5].Table 7 shows the formulations selected for Photo-DSC study.The wettability of UV coating formulation is ranked the best when the droplet diameter is the largest in size when compared to the other LED UV coating formulations when the photo-initiator and reactive diluents are added in the UV coating formulations.
The recommended LED UV coating formulations shown in Table 8 were selected based on the rate ofreaction/heatofreactions and per cent conversions shown in Figs.11 and 12(a)and(b).After the selection of the LED UV coating formulation shown in Table 8, for the proof-of-concept,the initial UV coating formulation development effort started with an inert simulant consisting of an inert filler and Cellulose Acetate Binder(CAB).The UV coating formulation,concentration and processing parameters were varied as shown in Fig.13.The surface coating showed a blotchy coat and not uniform on CAB surface when seen under a microscope as shown in Fig.14.Although the LED UV coating were not uniformly spread on the inert propellant grain surface,the results gave us a direction to modify the LEDUV coating formulations and optimized the processing parameters to achieve better wettability on the propellant surface.
3.2.3.Glass transition temperature
The glass transition temperatures(T g)of the LED UV coating formulations listed in Table 8 were determined.The temperature sweep showed a T g=-31.50°C with PAP-8606 from-60 to+50C as shown in Fig.15(c).There were no visible Tg in that range for the other samples,PAP-8604 and PAP-8605 LED UV coating formulations as shown in Fig.15(b)and(c),respectively.These other samples could have a T g below-60C but our instrument is only accurate to-60C.
3.2.4.LED UV surface coating with energetic AFP-001 propellant
The UV surface coating formulations down-selected,listed in Table 8,was processed using the fluidized bed UV coater previously shown in Fig.1 using the energetic AFP-001 propellant substrate. The LED UV coated and uncoated AFP-001 propellant grains shown in Fig.15 were characterized by closed bomb testing.Burn rates were determined by closed bomb analysis.Samples were then weighted out,grain dimensioned and density measured for closed bomb analysis at 21°C.The closed bomb test is designed to determine linear burning rates of energetic compositions at elevated pressures.The closed bomb was a 200 cc vessel filled to a 0.1 g/cc loading density with AFP-001 single perforated grains. Three 20-g propellant samples were tested for each LED UV coated formulations.The uncoated AFP-001 propellant grains were single perf(0.006 inch perf.diameter with an outerdiameter of0.083 inch and a length of 0.096 inch).Alltests generated pressures in excess of15 kpsi.For the down-selected LED UV coating formulations,one testtemperatures were utilized:21°C.The closed bomb test results were plotted and are shown in Figs.18-24.The uncoated AFP-001 single perforated propellant has a digressive linear burning rate which followed the form function geometry.The new LED UV surface coated AFP-001propellants have pressure exponents less than 1.The apparent burn rates in many of the new LED UV surface coated AFP-001 propellants appear to burn linearly.The burn ratespressure coef ficients and pressure exponents are tabulated in Table 9.LED UV coating formulations 8606-4 and 8606-2 in AFP-001 burn much faster compared to all the other LED UV coating formulations shown in Fig.25.
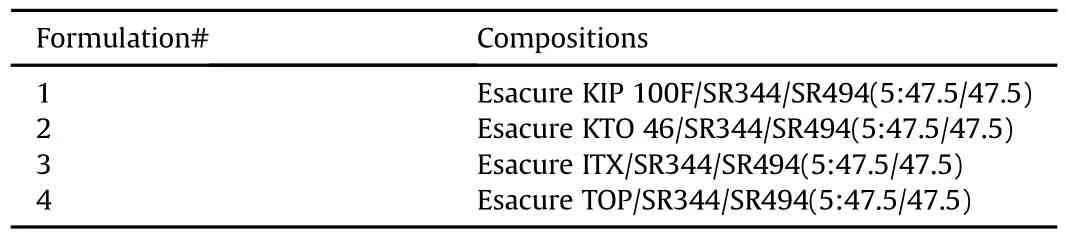
Table 2 UV LED chemical compositions.
PAP-8604-4 burns much slower when compared to the baseline uncoated AFP-001 and the other LEDUV coated formulations 8604-2,8605-4,and 8605-2,as shown in Fig.25.
To provide a visualcomparison of the relative performance,the dynamic vivacity has been reviewed.One should note thatthere are three parts to the propellant vivacity curve.The first is sensitive to the rate of flame spread.The third is the result of flame spread time and splintering,spreading web burnout in time and therefore pressure.The middle part ofthe curve has a slope that is a function ofthe instantaneous surface area to originalpropellantvolume,and composition.The characteristic slope starts when allthe propellant surface area is in flamed.The end of this slope is easier to de fine when dealing with progressive geometries.The comparison of vivacity curves for the three LED UV coating formulations candidates are shown in Figs.18(b)-24(b).Since the LED UV coating formulations are experimental,an inspection ofthe vivacity curves was made to determine anomalous effects[4,7].Vivacity is an expression of the mass rate of combustion or the rate of gas generation[4,7].Under the assumption ofspatially constant propellant composition and LED UV coating formulations,a change in vivacity indicates a change in available surface area.The vivacity versus normalized pressures were plotted in Fig.26 to make a qualitative method to ascertain the nonstandard behavior of the propellantwith varying LED UV coating formulations.In these plots,the dynamic vivacity has been calculated and plotted against thenormalized pressure in the closed bomb(P/Pmax).The plots clearly show the in fluence on the burn speed for the three LED UV coating formulation candidates.Higher vivacity values are an indicator of higher gasi fication rates.Assuming the apparent burn rates are constant,the changes in the gasi fication are due to an increase in the burning surface area.The LED UV coating formulation 8606-4 and 8606-2 have a much higher vivacity followed by in decreasing order 8604-2,8605-2,AFP-001 and 8604-4 as shown in Fig.26.LED UV coating formulations 8606-4 and 8606-2 apparent burn rates were the highest when compared to the other LED UV coating formulations shown in Fig.25.The breakup of the LED UV surface coating were exposing fresh propellant surface area resulting in higher apparent burn rates and greater vivacity,resulting in the propellant progressivity as demonstrated by the LED UV coating formulations 8606-4 and 8606-2.

Table 3Kinetic data from four UV coating formulations.

Table 4 Effect of the photo-initiator.
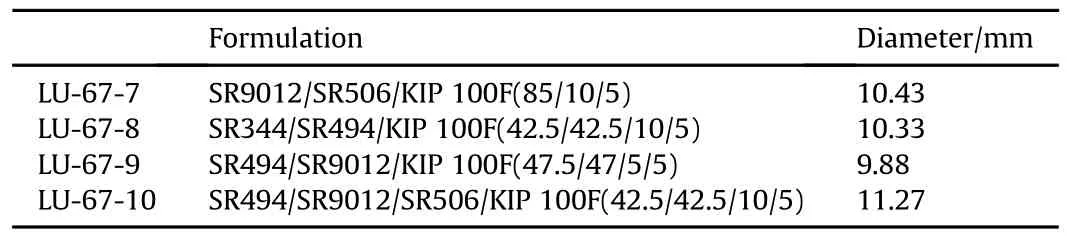
Table 5 Effect of reactive diluent SR 506A.
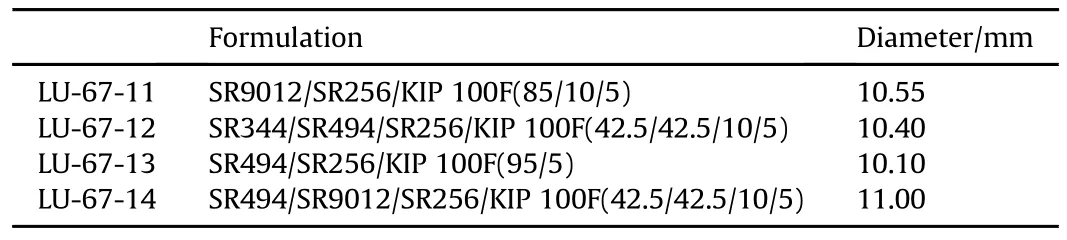
Table 6 Effect of reactive diluent SR 256.

Table 7 Formulations for Photo-DSC stud.

Table 8 Recommended formulations for coating test.
Using the data obtained from this test,the burn rate can be predicted using the Vielle's burn rate law shown in equation(1), wherein P is the pressure in the chamber,αis the burn rate coefficient,andβis the burn rate pressure exponent[2,4].
This burn rate was then used in the Interior Ballistic High Velocity Gun version 2(IBHVG2)code to model ballistic performance.This code was utilized to determine the ballistic grain geometry and web dimensions needed for 30 mm gun firings.
For the ballistic performance test,AFP-001 propellant surface coated with 2%PAP-8604 LED UV coating formulation was used. The propellant AFP-001is an Air Force fielded propellant.Its ballistic behavior with 2% PAP-8604 LED UV surface coating formulation is unknown in the 30 mm gun based on the IBHVG2 predictions.ARDEC surface coated 3000 g ofsingle perforated AFP-001 is shown in Fig.16.In Fig.17,the uncoated,and 2%and 4%AFP-001 LED UV coating formulation surface coated on the AFP-001 propellant is presented.The 2%PAP-8604 LED UV coating formulation was used in the 30 mm×173 mm sub-scale ballistic performance and sensitivity tests.
3.2.5.Gun firing
After the LEDUV surface coating process and preliminary closed bomb tests were completed,ballistic tests were conducted.A blend of uncoated and coated propellants was shot in an effort to meet the ballistic targets.The ballistic testing was conducted across the operating temperature envelope required by the cartridge speci fication,-60°F,70°F and+145°F.
For the first iteration 30 mm sub-scale gun firing (30 mm×173 mm),the LED UV coating formulation PAP-8604-2 was selected for the initial proof of concept.A final blend con figuration was chosen for a finalsetofballistic tests.The finalAFP-001 propellant blend was an uncoated and LED UV coated blend.Final ballistic results are shown in Fig.27.The muzzle velocities of AFP-001 surface coated with LED UV coating formulation PAP-8604-2 were comparable to the uncoated AFP-001as shown in Fig.28 except at hot temperatures.The muzzle velocity of LED UV coating formulation PAP-8604-2 at hot temperatures was higher than the uncoated AFP-001.The Breech Pressures as shown inFig.29 for LED UV Coating Formulation PAP-8604-2 were lower than the uncoated AFP-001 at cold,comparable at ambient and higher at hot temperatures.At ambient temperature,21°C,the apparent burn rates and the vivacity curves of surface coated with LED UV coating formulation PAP-8606-4 and 8604-4 were higher than the uncoated AFP-001 shown in Figs.25 and 26,the same behavior were observed for the muzzle velocities and breech pressures when shot.
4.Summary and conclusions
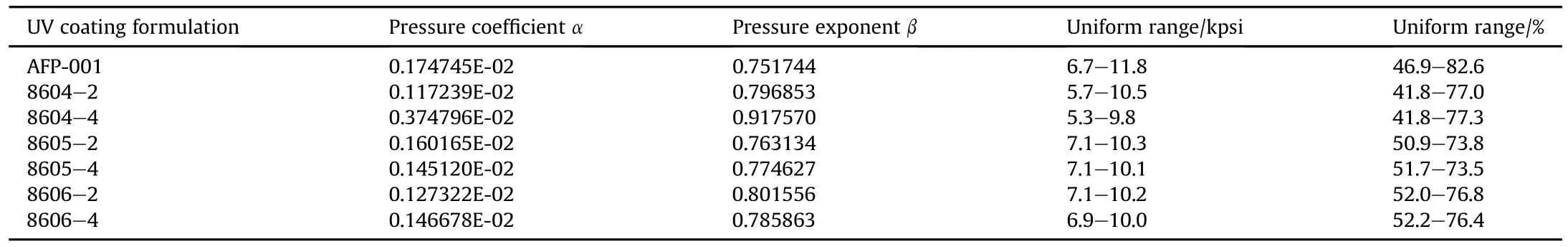
Table 9 Burn rate pressure exponent,pressure coef ficient,and pressure at uniform range.
The effects of photo-initiators and reactive diluents on the wettability of various LED UV coating formulations were demonstrated.The effects of the droplet size diameter for the LED UV coating formulations were larger when compared to the other LED UV coating formulations investigated.A larger droplet diameter represents spread-ability of the LED UV coating on the surface of the substrates.The first proofofthe phenomena is that the LED UV coating formulations 8606-4 and 8606-2 apparent burn rates were higher compared to the other LED UV coating formulations shown in Fig.25.The second proof of the phenomena is that the LED UV coating formulations 8606-4 and 8606-2 have a much higher vivacity curve which is an indication ofmore oxygen in the LED UV coating formulation compared to the other LED UV coating formulations shown in Fig.26.In addition,the Tg for PAP-8606 LED UV coating formulation was-31.52°C.During the propellant burning,the LED surface coating formulation PAP-8606 was breaking up exposing more surface area in so much controlled way exposing more propellant surface.This phenomena gives an increase in the apparent burn rate and vivacity resulting in progressivity.The LED UV coating formulation PAP-8606 do not contain any ofthe two reactive diluents,SR 256(SR256,2(2-Ethoxyethoxy) Ethyl Acrylate)and SR 506A.The vivacity curve shown in Fig.26 indicates that the mass rate of combustion or the rate of gas generation is higher for LED UV coating formulation 8606-4 and 8606-2,and followed by in decreasing order 8604-2,8605-2,AFP-001, and 8604-4.For the first iteration 30 mm sub-scale gun firing (30×173 mm),the LED UV coating formulation PAP-8604-2 was selected for the initialproof of concept.A finalblend con figuration was chosen for a final set of ballistic tests.The final AFP-001 propellant blend was an uncoated and LED UV coated blend.Final ballistic results are shown in Fig.27.The muzzle velocities of AFP-001 surface coated with LED UV coating formulation PAP-8604-2 were comparable to the uncoated AFP-001as shown in Fig.28 except at hot temperatures.The muzzle velocity of LED UV coating formulation PAP-8604-2 at hot temperatures was higherthan the uncoated AFP-001.The Breech Pressures for LED UV Coating Formulation PAP-8604-2 were lower than the uncoated AFP-001 at cold,comparable at ambient and higher at hot temperatures.At ambient temperature,21°C,the apparent burn rates and the vivacity curves of surface coated with LED UV coating formulation PAP-8606-4 and 8604-4 were higher than the uncoated AFP-001 shown in Figs.25 and 26,the same behavior were observed for the muzzle velocities and breech pressures when shot.
Future work
The LED UV coating formulations will need a second iteration formulation modi fication and further characterization.Additional 30 mm×173 mm sub-scale gun firings are necessary for all the three LEDUV coating formulations candidates listed in Table 8 to be able to verify if the goals and objectives previously discussed are met.
Acknowledgments
The authors would like to acknowledge the FREEDOMTech Base Program and the US Army RDECOM ARDEC for their support and the funding provided for this effort.
[1]Adam C,Rozumov R,Grau H.Investigation of the effects of dibutyl phthalate migration in propellant grains on the ballistic performance of small caliber weapons,60th JPM/9th modeling and simulation/7th liquid propulsion/6thspacecraft propulsion joint subcommittee meeting joint Army-Navy-NASA-air Force(JANNAF).Cheyenne Mountain Conference Center.29 April-2 May,2013.
[2]Fry RS,Peters ST.Burning rates of standard solid propellants for gun applications.Columbia,MD:CPTR 99-69,CPIA/JHU;Sep 1999.
[3]Irie M,Iga R.Macromolecules 1986;19:2480.
[4]Machalka,E.Dipl.-Ing,AVL Prof.List Ges.m.b.H.,Programs for the Determination of Propellant Characteristics in Conjunction with Closed Vessels.Special Edition of Proceedings,3rd International Symposium on Ballistics 02.
[5]Drobny JG.Radiation technology for polymers.2nd ed.CRC Press;2010.
[6]Ravve A.Light associated reactions of synthetic polymers.Springer Science; 2006.
[7]Klingaman KW,Domen JK.The role ofvivacity in closed vesselanalysis[JANNAF Propellant Development and Characterization Subcommittee Meeting,Patrick AFB,FL].1994.
30 January 2017
*Corresponding author.
E-mail address:thelma.g.manning.civ@mail.mil(T.Manning).
Peer review under responsibility of China Ordnance Society.
http://dx.doi.org/10.1016/j.dt.2017.04.007
2214-9147/Published by Elsevier Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Accepted 25 April 2017
Available online 27 April 2017
- Defence Technology的其它文章
- Impact-disrupted gunshot residue:A sub-micron analysis using a novel collection protocol
- Ignition and combustion of pyrotechnics at low pressures and at temperature extremes
- A comparative study of combustible cartridge case materials
- An approach for optimization of the wallthickness(weight)of a thickwalled cylinder under axially non-uniform internal service pressure distribution
- Trajectory optimization of a de flectable nose missile
- Improved theory of projectile trajectory reference heights as characteristics of meteo-ballistic sensitivity functions

