Solubility of 4,4′-Methylene Diphenyl Diisocyanate in Supercritical Carbon Dioxide
, , ,
(College of Chemistry and Materials Science,South-Central University for Nationalities,Wuhan 430074,China )
Solubility of 4,4′-Methylene Diphenyl Diisocyanate in Supercritical Carbon Dioxide
YangHaijian,GengYongchao,LiuYanyan,XuLingxiao
(College of Chemistry and Materials Science,South-Central University for Nationalities,Wuhan 430074,China )

4,4′-methylene diphenyl diisocyanate; supercritical carbon dioxide; solubility; Bartle and Chrastil models; partial molar volume
In the past decades,supercritical carbon dioxide (SF-CO2),as a significant supercritical fluids (SCFs),has been arousing increasing interest for its versatile properties,such as nontoxicity,nonflammability,low cost,and especially for its moderate critical values[1,2].Besides,unlike traditional solvents,its properties such as density,dielectric constant,diffusion coefficient,and solubility parameter can be continuously tuned just by changing the pressure or temperature at the supercritical condition,especially in the near-critical region.Hence SF-CO2has the potentials to take the place of many traditional organic solvents for various industrial applications[3-5].
Nowadays 4,4′-methylene diphenyl diisocyanate (MDI),despite its toxicity[6],has been the most important resource for the synthesis of polyurethane,which is mainly applied as an excellent industrial adhesive[7].However,a typical polymerization procedure of MDI is conducted in a traditional solvent such as water or organic solvent,where high concentration of residual isocyanate can be detected after polymerization.Isocyanate is very hard to be separated from the traditional solvent and is considered very harmful to the environment regarding the toxicity of anion NCO-.Consequently,developing a polymerization process by using green solvent such as supercritical CO2is of great significance.Before developing a polymerization process,the solubility data of MDI in supercritical CO2would be interesting and useful.
To the best of our knowledge,solubility data of MDI in SF-CO2have never been reported.Hence,in our present work,the solubility of MDI were studied at the temperature ranging from 313 to 353 K and pressure over the range of 9.6 to 16.3 MPa.The tested results were correlated by two density-based correlations (Bartle and Chrastil models).Finally,the solubility data were utilized to calculate partial molar volumes of MDI at various temperatures in SF-CO2,based on Kumar and Johnston theory.
1 Experimental
1.1 Chemicals and Apparatus
CO2was purchased from Wuhan Steel Co.(mass fraction purity of 99.99%),and 4,4′-methylene diphenyl diisocyanate (mass fraction purity of 99.5%) was supplied by Shanghai Darui Co.and directly used without further purification.The apparatuses of supercritical CO2were bought from JASCO Corporation (Hachioji Japan): PU-1580-CO2CO2Delivery Pump and BP-1580-81 Back Pressure Regulator.
1.2 Procedure for Solubility Test in Super- critical CO2
A certain amount of MDI were added into the cell (7.11 mL) with two sapphire windows,then the cell was sealed immediately after the replacement of air by CO2.The solute was stirred and the temperature was controlled by using a temperature controller jacket with a circulator.CO2was gradually compressed into the cell (0.2 mL·min-1) again by delivery pump after the system was heated to the set temperature[8].When the compound disappeared and the system in the cell became transparent single phase,stirring was stopped and the state was kept for 5 h to fix the equilibria.The pressure was recorded and defined as dissolution pressure if the transparent single phase was still stable,and the experiment was repeated at least three times.The uncertainty of the dissolution pressure was ±0.2 MPa and the uncertainty for the dissolution temperature was± 0.1℃[9].
2 Results and Discussion
2.1 Solubility results
The solubilities of MDI in supercritical CO2were determined at 313 K,323 K,333 K,343K,353K and the pressure range of 9.6 to 16.3 MPa.The experimental results are shown in Fig.1.The mole fraction of MDI in SF-CO2could reach as high as 3.61 % at 11 MPa and 313 K,and all the mole fraction was reproducible within ±2 %.The solubilities of MDI increased with the increase of pressure at the same temperature,and the solubilities decreased with the increase of temperature at the same pressure.

Fig.1 Solubility of MDI in supercritical CO2 at various temperatures图1 不同温度下MDI在超临界CO2中的溶解度
2.2 Bartle model
The experimental solubility data of MDI were correlated according to the following equation[10]:
ln(xP/Pref) =A+C(ρ-ρref) ,
(1)
A=a+b/T,
(2)
wherexis the mole fraction of MDI;Pis the pressure;Prefis 0.1 MPa;ρis the density of pure CO2under experimental conditions;ρrefis 700 kg·m-3; andA,C,aandbare constants.
In order to predict the solubility of MDI,the values ofa,bandCare required to be calculated by using Eq.(1) and Eq.(2).Consequently,the first step was to obtain the values ofCparameter at five temperatures (313 to 353 K),which could be achieved by plotting ln (xP/Pref) against (ρ-ρref) (Fig.2) and regarding the corresponding slopes asC.Then the five slopes were averaged to gainAparameters at different temperatures by using Eq.(1).Secondly,according to Eq.(2),we fitted the plots ofAversus 1/Tinto a straight line to get the interceptaand slopeb(Fig.3),and the relevant values ofa,bandCwere shown in Tab.1.In Battle model,bdepends on the enthalpy of sublimation of MDI,ΔsubH,and their relationship can be expressed asΔsubH=-Rb,whereRis the gas constant.

x: mole fraction of compound; P:CO2 pressure; Pref: 0.1MPa; ρ: density of pure CO2; ρref:700 kg·m-3Fig.2 Plots of ln (xP/Pref) vs (ρ-ρref) for MDI at various temperatures图2 MDI在不同温度下关联的ln (xP/Pref) 对 (ρ-ρref)拟合数据

Fig.3 Plots of A vs 1/T for MDI at various temperatures by Bartle model图3 不同温度下关联的 A 对 1/T Bartle模型拟合数据
Finally,the AARD from experimental data was used to test the correlation results and calculated with the following Eq.(3):
AARD=1/nΣ| (xi,calc-xi,expt)/xi,expt|×100 %,
(3)
wherenis the number of experimental points,andxi,calcandxi,exptare the calculated and experimental data,respectively.The values of AARD were in the range of 4.56% to 10.68 %.

Tab.1 Results of the solubility data correlation by Bartle and Chrastil model
n: number of data points used in the correlation;a,bandC: parameters of Bartle model ;ΔsubH:the enthalpy of sublimation of the solid solute; -: undone data
2.3 Chrastil model
As one of the most frequently-used and traditional density-based models,the Chrastil[11]model relates the solute solubility (S,g·L-1) in SF-CO2,the density of SF-CO2(ρ,g· L-1),and temperature (T,K).Chrastil suggested a hypothesis that when solute A has just been completely dissolved in SF,one of its molecule is attached withkmolecules of solvent B,and this causes a phase equilibrium between the resulting solvate-complex ABkand the system[12-14].Therefore,the experimental solubility of the solute can be correlated by studying the relationship between the solubility of the solute and the density of the supercritical solvent,and it can be specified as Eq.(4):
lnS=klnρ+ (α/T) +β,
(4)
whereρ(kg·m-3) is the density of pure CO2obtained from NIST[18];Tis the experimental temperature;kis the association number of CO2;αis a constant,defined as -ΔH/R(whereΔHis the sum of the enthalpies of vaporization and salvation of the solute andRis the gas constant),andβdepends on the molecular weights of the solute and solvent.Sis the solubility of the solute (g·L-1),which could be calculated by Eq.(5):
S= (ρM2x) /[M1(1-x)],
(5)
wherexis the molar fraction of MDI;M1andM2are the molecular weights of CO2and MDI,respectively.The values of constantsα,βandkare estimated via conducting a multiple linear regression on the experimental solubility data in Fig.4.
Consequently,parameters for the Chrastil equation were listed in Tab.1.Evidently,the AARD between experimental data and correlated results indicated that good consistency between them was observed by using the Chrastil equation.

Fig.4 Plots of lnS vs lnρ for MDI at various temperatures图4 MDI在不同温度下lnS 对 lnρ 曲线
2.4 Estimation of the partial molar volumes of the solutes
Normally,at the given temperature and pressure,adding a certain mount of solute into a solvent would lead to a variety in the system′s volume,which is called partial molar volume and considered very essential for the evaluation of the solute′s solubility.Despite the fact that the values of MDI′s partial molar volumes are not accessible from other works,it will still be referential for other future solubility researches.Thus,the partial molar volumes of MDI were estimated in accordance with Kumar and Johnston′s theory[12-15].To study this,the relationship between the solute′s solubility and partial molar volume was investigated in the vicinity of the critical density of SF-CO2,and it can be shown as the Eq.(6):
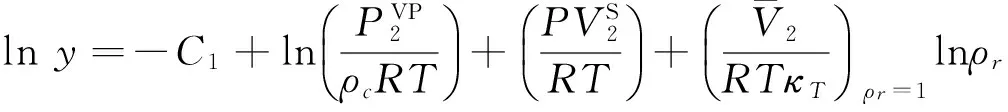
(6)


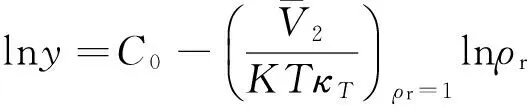
(7)
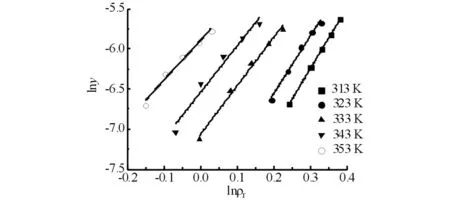
Fig.5 Plots of lny vs ln ρr for MDI at various temperatures图5 MDI在不同温度下lny 对 lnρr 曲线
Finally,the quality of the linear correlation is conveyed in the term ofσ2and all the results are summarized in Tab.2.As shown in Tab.2,the partial molar volume of MDI decrease when temperature goes up,which agree with Kumar and Johnston′s theory.
Tab.2 Slopes and partial molar volumes for MDP at different temperatures

表2 不同温度下的MDI的偏摩尔体积和相关参数
3 Conclusion

[1] Du Y,Cai F,Kong D L,He L N.Organic solvent-free process for the synthesis of propylene carbonate from supercritical carbon dioxide and propylene oxide catalyzed by insoluble ion exchange resins[J],Green Chem,2005,7(7): 518-523.
[2] Lamba N,Narayan R C,Modak J,et al.Solubilities of 10-undecenoic acid and geraniol in supercritical carbon dioxide[J].J Supercrit Fluids,2016,107: 384-391.
[3] Lin Y L,Yang H J,Zhou T.New CO2-philic propane derivatives: design,synthesis and phase behavior in supercritical carbon dioxide [J].Curr Org Chem,2013,17(2): 176-183.
[4] Yang Z,Yang H J,Tian J,et al.High solubility and partial molar volume of 2,2′-oxybis(N,N-bis(2-methoxyethyl) acetamide) in supercritical carbon dioxide[J].J Chem Eng Data,2011,56(4): 1191-1196.
[5] Zhang P Y,Yang H J,Xu L X.Solubilities and partial molar volumes of new CO2-philic propane derivatives in supercritical carbon dioxide[J].J Chem Thermodyn,2013,67(6): 234-240.
[6] Kumar A,Dongari D,Sabbioni G.New isocyanate-specific albumin adducts of 4,4′-methylenediphenyl diisocyanate(MDI) in rats[J].Chem Res Toxicol,2009,22(12): 1975-1983.
[7] Contraires E D,Udagama R,Lami E B,et al.Mechanical properties of adhesive films obtained from PU acrylic hybrid particles [J].Macromolecules,2011,44(8): 2643-2652.
[8] 杨海健,杨洪委,彭 静,等.联吡啶衍生物在超临界CO2中的溶解度和偏摩尔体积研究[J].中南民族大学学报(自然科学版),2013,32(3):8-12.
[9] Peng J,Yang H J,Yang H W.Solubilities and partial molar volumes of three bis(2-ethoxyethyl) ethanedioate derivatives in supercritical carbon dioxide[J].J Chem Thermodyn,2015,83: 33-42.
[10] Bartle K D,Clifford A A,Jafar S A,et al.Solubilities of solids and liquids of low volatility in supercritical carbon dioxide[J].J Phys Chem Ref Data,1991,20(4): 713-757.
[11] Chrastil J.Solubility of solids and liquids in supercritical gases [J].J Phys Chem,1982,86(15): 3016-3021.
[12] 杨海健,赵 璐,向 力,等.甲氧羰基乙酸己酯在超临界二氧化碳中溶解行为研究 [J].中南民族大学学报(自然科学版),2014,33(3): 1-5.
[13] 杨海健,张 宁,金 晶,等.乙二醇单醚在超临界二氧化碳中溶解度的研究及关联[J].中南民族大学学报(自然科学版),2011,30(3): 1-6.
[14] Luo J,Yang H J,Jin J.Solubilities and partial molar volumes ofN,N′-dibutyl-oxalamide,N,N′-dihexyl-oxalamide,N,N′-dioctyl-oxalamide in supercritical carbon dioxide[J].J Chem Thermdyn,2012,54(54): 339-345.
[15] Kumar S K,Johnston K P.Modeling the solubility of solids in supercritical fluids with density as the independent variable [J].J Supercrit Fluids,1988,1(1): 15-22.
2016-09-07
杨海健(1974-),男,教授,博士,研究方向:超临界二氧化碳,E-mail:yanghaijian@vip.sina.com
国家自然科学基金资助项目(51073175)
TQ426.81
A
1672-4321(2017)02-0001-05
4,4′-二异氰酸酯二苯甲烷在超临界CO2中溶解行为研究
杨海健,耿永超,刘艳艳,徐凌霄
(中南民族大学 化学与材料科学学院,武汉 430074)

4,4′-二异氰酸酯二苯甲烷;超临界CO2;溶解度;Bartle和Chrastil模型;偏摩尔体积

