多酸功能化储能材料的研究进展
赵广震,姜天尧,时君友
(东北电力大学 化学工程学院,吉林 吉林 132012)
多酸功能化储能材料的研究进展
赵广震,姜天尧,时君友
(东北电力大学 化学工程学院,吉林 吉林 132012)
与煤炭、石油等非再生能源不同,可再生能源(太阳能、风能)不能大规模直接储存,必须通过能量转化实现能源的储存,而储能技术是解决可再生能源发电非稳态特性的关键技术。开发高效、稳定的储能材料是突破储能技术瓶颈的有效途径之一。多酸具有较强的电子和质子转移及存储能力,因此多酸功能化储能材料引起越来越多的学者关注。该部分主要对多酸功能化储能材料的研究进行文献综述。
多酸;太阳能电池;锂离子电池;燃料电池;超级电容器
21世纪以来,能源问题是人们最关心的问题之一。煤炭、石油等非再生能源曾经是经济发展的重要保障,但现阶段非再生能源的枯竭、利用效率低以及造成的环境问题是制约经济发展的重要因素。而风能、太阳能等可再生能源具有丰富、廉价、清洁和地域分布广泛等优势,但其开发和利用程度还不成熟,现阶段还不能取代传统的化石能源,只能作为辅助能源。与非可再生能源相比,不能大规模直接储存,必须通过能量转化实现能量的储存。因此储能技术是可再生能源开发和利用的重要发展方向,而开发高效稳定的储能材料是突破储能技术的关键[1-4]。
多金属氧酸盐又称多酸(polyoxometalates,POMs),是前过渡金属离子的高氧化态(如V、Mo、W等)与氧形成的纳米级的金属-氧簇类化合物[5]。自1934年,学者们相继提出Keggin (XM12O40)、Dawson (X2M18O62)、Anderson (XM6O24)、Waugh (XM9O32)、Silverton (XM12O42)以及Lindqvist (M6O19)六种基本结构[5-9],具有以下特点[6-9]:
(1)结构多样性、可修饰性和可调变性,具有较强的电子和质子转移/存储能力;
(2)优异的氧化还原性能;
(3)热稳定性高,相对分子质量较大(103-104),易溶于水。
POMs可以与多种功能材料结合并实现材料之间的协同作用。目前,POMs功能化的材料在医药、磁性材料、环境保护、催化、能源转化和储能材料等尖端技术领域具有广阔的应用前景[10-19]。近年来,由于POMs功能化储能材料具有低成本、高效率、稳定性高、兼容性强等特性,有利于实现储能材料的工业化,因此受到科研工作者的极大关注[12,19]。该部分概述了POMs在功能化储能材料的制备及应用领域的研究进展。
1 POMs功能化材料的制备
POMs功能化储能材料主要制备方法包括:溶胶-凝胶法、电化学沉积法、分子层层组装法、吸附法等。
1.1 溶胶-凝胶法
溶胶-凝胶法是在互溶溶剂及酸或碱的条件下,金属或半金属醇盐发生水解和缩聚反应,释放出醇或水,形成特定结构的凝胶,然后经陈化、室温干燥成固凝胶。
孙长青、Wang等[20,21]分别以四乙氧基硅烷和三甲基硅烷作为前驱体采用溶胶-凝胶法制备了POMs功能化的纳米复合储能材料。Wang的课题组[22]也采用了溶胶-凝胶法制备了高分散的POMs-Cs2SO4@TiO2纳米材料:先将SiW11Co(0.05 g)溶液滴加到钛酸异丙酯(5 mL)与正丁醇(3 mL)的混合溶液中。然后将浑浊溶液加热到45 ℃保持3 h,再升温到80 ℃保持3 h形成凝胶,最后经过洗涤干燥焙烧得到POMs-Cs2SO4@TiO2。
溶胶-凝胶法制备POMs功能化纳米储能材料具有热稳定性好,不易分解,可以形成特定结构,并且能维持POMs的活性和稳定性。为POMs功能化储能材料的应用奠定基础。
1.2 电化学沉积法
法国学者Keita和Nadjo最早发现电化学沉积法方法[23]。制备过程是:先将预处理的电极放置于含有POMs的酸性溶液中。然后在一定的电位下,将POMs沉积到电极表面得到POMs功能化电极材料。杜金艳等[24]通过电化学沉积法制备了多层POMs修饰的玻碳电极。方法是先将清洁的玻碳电极放入4-氨基硫酚的乙醇溶液中。取出后,超纯水清洗去除物理吸附的物质。然后在一定电流电位下,将预修饰的电极放置于[SiCu(H2O)W11O39]6-(SiCuW11)的醋酸/醋酸钠的缓冲溶液中,得到单层POMs修饰的电极材料。重复多次得到多层POMs修饰的电极材料。杜金艳等[25,26]还采用相似的方法制备了SiZnW11和SiMnW11修饰的玻碳电极。黄正国等[27]也采用相同的方法制备了多酸SiMo11V修饰的金电极。同时,学者们[28,29]发现多酸阴离子可以与金属共同沉积到电极表面,提高电极材料性能,降低对杂质敏感程度。该方法的缺点是制备过程不可控制、电化学沉淀情况比较复杂,因此很少采用该方法。
1.3 分子层层组装法
分子层层组装法是利用不同材料之间的共价键或静电作用把材料组装起来的方法。分为单层和多层POMs功能化的储能材料。主要的连接化合物包括重氮盐、4-氨基苯甲酸、L-半胱氨酸等或相应的衍生物[17,29-34]。
董绍俊课题组[30]先用重氮盐还原法形成4-硝基苯,然后将硝基还原成氨基,质子化后可以得到带正电荷的表面,可以将SiW12修饰碳材料的表面形成的电极材料。王升富课题组[32]在酸性条件下通过半胱氨酸将PMo6W6修饰到电极表面。Kim等[33]发现还原氧化石墨烯首先与3-丙氨基三乙氧基硅烷结合,然后加入磷钨酸反应24 h得到多酸修饰的石墨烯材料。2016年,Genovese研究小组[17]用分子层层组装法制备多酸基复合碳材料,通过咪唑阳离子将GeMo12或SiMo12与碳纳米管结合。该方法结构稳定性强、操作简单,已成为制备多酸功能化储能材料最广泛的一种方法。
除此之外,还有吸附法、聚合物包埋法等方法[29,35],但存在制备过程繁琐、机械性能差等缺点,在多酸功能化储能材料的研究中比较少见。
2 POMs与基质材料的结合方式
在POMs功能化储能材料的制备过程中,POMs与其它材料的结合方式主要有共价键结合和非共价键结合。
2.1 共价键结合
功能化的POMs与其它官能团修饰的基质材料可以通过化学反应形成稳定的化学键。通过共价键结合材料具有稳定的结构,可控性强。2014年,宋宇飞课题组[36]采用氨基功能化的Keggin型多酸SiW11与氧化的多层碳纳米管制备POMs/CNTs。首先用强酸将CNTs氧化成CNTs-COOH,然后与SOCl2反应生成酰氯修饰的碳纳米管CNTs-COCl,在氮气保护下,超声将20 mg CNTs-COCl分散到60 mL乙腈中,然后将0.5 mL三乙胺滴加到溶液中,除氧、氮气保护,冷却到0 ℃,最后将1 g SiW11-NH2溶于10 mL乙腈溶液,30 min内滴加到CNTs溶液中,在0 ℃下保持2 h,然后加热到70 ℃维持24 h,分离干燥得到SiW11/CNTs。宋宇飞课题组[37]还采用氨基功能化的Anderson型MnMo6多酸与氧化的单层碳纳米管制备MnMo6/SWNT。
2.2 非共价键结合
POMs与其它材料的非共价键结合是通过分子间相互作用(静电作用或氢键等)[38-41]。例如芳香烃类有机物修饰的POMs可以与碳材料产生π-π共轭的静电作用,得到多酸功能化的碳材料[39-41]。Toma[39]发现嵌二萘修饰的多酸可与碳纳米管结合,宋宇飞的课题组[40,41]实现了嵌二萘修饰的PW11、MnMo6等多酸通过π-π共轭与碳纳米管结合。另外,Wang等[4]通过化学吸附作用成功的将PMo12嫁接到SWCNT上,并作为阴极材料提高电池性能。
3 多酸复合储能材料的应用
目前,储能材料与技术正在快速的发展。主要的储能技术包括:a)将可再生能源转化为电能的太阳能电池;b)电能转化成化学能储存,然后释放电能的化学电池(锂离子电池);c)通过氧化还原反应,可以将燃料的化学能转化成电能的方式(燃料电池);d)以电能的形式储存释放的超级电容器。开发稳定高效的储能材料是突破储能技术的关键。由于POMs具有电子存储好、化学可调性高和稳定性高等特性,还具有氧化还原的活性位点,因此POMs功能化储能材料是非常重要的研究方向[42-45]。
3.1 太阳能电池
太阳能是取之不尽用之不竭的可再生的清洁能源,是化石能源的替代能源之一。太阳能电池具有成本低廉、制作简单、光电转化效率较高和兼容性强等优点。研制太阳能电池是太阳能的有效利用方式之一,染料光敏化太阳能电池(DSSCs)是第三代太阳能电池。近年来,在DSSCs上POMs功能化材料是研究的热点[46-48]。
虽然POMs具有较小光电流密度,但可与TiO2结合用于光电阳极材料的制备[49]。2013年,Xu的课题组[50]探究了H3PW12O40(PW12)、K6P2W18O62(P2W18)与TiO2复合材料薄膜用于光电阳极材料。发现0.75%的多酸PW12的DSSCs的参数分别是η:0.13%,Jsc:0.59 mA cm-2,Voc:0.28 V,明显大于纯TiO2材料。2016年,Li等[51]制备了PW12/TiO2的复合材料,并用于DSSCs的光电阳极材料。结果表明短路电流密度提高了150%,能量转化效率提高了140%。Wang的课题组[52]通过溶胶-凝胶法合成了高分散的POMs-Cs2SO4@TiO2纳米材料。DSSCs的效率由5.9%提高到8.4%。主要原因是POMs可以加快电子转移和延缓电子的复合。Shan等[53]制备了 SiW9Co3的还原氧化石墨烯纳米材料(SiW9Co3/RGO),与TiO2结合(SiW9Co3/RGO-3@TiO2)用于DSSCs的阳极材料。DSSCs参数分别是Jsc:17.5 mA cm-2,Voc:0.705 V,η:6.88%,都明显高于TiO2太阳能电池的参数。
POMs功能化储能材料也可以用于DSSCs的阴极电极材料。Wang的团队[45]使用Sn(CH2)2COOH-Cu-GeW9-Cu-Sn(CH2)2COOH、Sn(CH2)2COOH-Co-GeW9-Co-Sn(CH2)2COOH修饰单层碳纳米管材料,用于DSSCs的阴极电极材料来测试相关电化学性能。POMs/SWNT电化学性能明显都高于SWNT材料,并且略低于贵金属Pt。因此,POMs功能化储能材料可以大大降低太阳能电池的成本。Yuan等[54]研究了[SiW11O39]8-与聚3,4-乙烯二氧噻吩复合材料用于DSSCs的阴极材料。发现POMs修饰的材料可以降低电荷转移的电阻,电池总效率高达η= 5.93%。
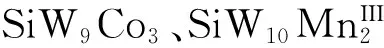
3.2 锂离子电池
在锂离子电池中,POMs功能化材料一般用于电极活性物质。主要考察POMs功能化材料的储存锂离子和放电能力。
近年来,Wang等[4]发现PMo12通过化学吸附作用嫁接到SWCNT上,用于锂离子电池的阴极材料能够提高电池性能。PMo12/SWCNT提高锂离子分散和在一维架构中电子的有效转移。30%的PMo12量时,锂离子电池的放电能力高达320 mAh·g-1。经过15次的充放电循环,放电能力300 mAh·g-1。Song课题组[36,37]制备了不同的多酸碳纳米管复合材料。将氨基功能化的SiW11、MnMo6与具有酰氯基团的碳纳米管结合,在电流密度0.5 mA·cm-2下,首次放电容量均达到1 200 mAh·g-1,而100次循环充放电以后,MnMo6/CNTs的放电容量(932 mAh·g-1)远大于SiW11/CNTs的放电容量(650 mAh·g-1),说明MnMo6/CNTs的稳定性较强。Song[39]还通过π-π共轭制备嵌二萘修饰的MnMo6与碳纳米管结合的储能材料。发现初次放电容量高达1 898.5 mAh·g-1。经过100次循环充放电以后,MnMo6/CNTs的放电容量达665.3 mAh·g-1,说明共价键与碳纳米管结合的复合材料具有更高的稳定性。最近他们[57]通过超声波处理的方法制备了一维的TBA-PMo11V/CNT复合材料,发现POM/CNT的晶体形状与超生波强度和超声时间等参数有关,还与多酸阴离子和阳离子有关。该复合材料作为锂离子电池的阳极材料,呈现出良好的电化学性能和良好的稳定性,100次循环充放电以后,放电能力达到850 mAh·g-1。
3.3 燃料电池
燃料电池(fuel cells)是一个非常有前途的清洁发电系统,并可能替代化石能源。燃料电池的商业化可以降低石油使用量和有害污染物的排放量。自从1979年,Nakamura等[58]首次发现H3PMo12O40可以用于燃料电池的固体电解质以来,多酸在燃料电池中的应用研究迅速的发展起来[59-62],相关研究见表1[63-73]。
Xu等[63]制备了H3PW12O40修饰的聚乙烯吡咯烷酮和聚醚砜树脂的质子交换膜材料,用于直接甲醇燃料电池,发现甲醇的渗透率1.65×10-6cm2/s,稳定长达130 h左右。2017年,Kim等[72]制备了H3PW12O40不同含量的Nafion膜材料(PWA-Nafion),用于直接乙醇燃料电池,发现多酸用量影响膜材料的质子导电率和乙醇分子的传递,多酸用量为15%的膜材料具有最大功率密度。
多酸修饰的电解质膜还可以应用于H2/O2质子交换膜燃料电池。Shao等[74]制备了Nafion/SiO2-PWA膜材料,并用于H2/O2质子交换膜燃料电池的性能测试,发现Nafion/SiO2-PWA膜材料的电流密度值(540 mA/cm2) 明显大于Nafion/SiO2(340 Ma/cm2)和Nafion115(95 mA/cm2),并且Nafion/SiO2-PWA膜材料的氢气交换非常小。Mehdi等[75]合成了含有Cs元素的杂多酸Cs2.5H0.5PMo12O40(CsPMo)和Cs2.5H0.5PW12O40(CsPW),并制备了Nafion/CsPMo和Nafion/CsPW膜材料用于质子交换膜燃料电池。研究表明Nafion/CsPMo膜材料的最大能量密度大于Nafion/CsPW膜材料,并远远大于Nafion膜材料。通过耐久性测试发现多酸复合膜材料的耐久性明显提高。主要原因是含Cs的杂多酸可以提高膜材料的耐水性能。Kim等[33]采用多酸功能化的还原氧化石墨烯制备的Nafion膜电解质(Nafion/PW-mGO)可应用于H2/O2质子交换膜燃料电池,随着相对湿度的增大,Nafion/PW-mGO的质子导电率增大,并大于没有多酸修饰的还原氧化石墨烯的Nafion膜电解质(Nafion/mGO),最大功率密度达841 mW/cm2(RH 20%,温度80 ℃)。

表1 近年来,多酸作为活性物质在燃料电池中的应用[63-73]
多酸除了在膜材料中广泛应用外,还可以作为修饰电极材料以及催化材料的活性中心。2016年,Renzi等[76]制备了Pt/Cs3HPMo11VO40的电极材料,并用于质子交换膜燃料电池。发现Pt/Cs3HPMo11VO40电极材料表现的电化学性质与Pt相差不大,可以减少电极Pt金属的使用量,有利于降低燃料电池的成本。Renzi等[77]进一步研究了Pt/Cs3H2PMo10V2O40电极材料的电化学性能,发现多酸可以提高Pt的分散和等同活性下降低Pt在电极材料中的用量,进而提高Pt电极材料的性能。
3.4 超级电容器
超级电容器(电化学电容器)是一种新型储能元件[78,79]。根据储能原理分为:双电层超级电容器(通过在电解液和电极活性物质表面形成的界面双电层来储存电荷的新型储能器件)和赝电容器(理想的双电层电容器是通过双电层储存电荷,充放电过程中在电极材料和电解液之间均没有法拉第氧化还原反应的发生)。具有能量密高(1-20 Wh/kg)、功率密度高(300 kW/kg~5 kW/kg)、转换效率高和循环稳定性高;除此之外,还具有充电速度快、安全系数高和使用范围广(航空航天、国防以及电动汽车等领域)的特点。碳材料或聚合物的储能材料研究较多且成熟,具有稳定性高、价格低廉、原料丰富、技术成熟的特点,但受到电荷机理的限制。据文献报道,将多酸与其它材料复合作为超级电容器储能材料还可以提高稳定性和比电容。有关多酸功能化的储能材料用于超级电容器的研究见表2[78-91]。
21世纪初,Romero等[92]发现化学-电化学沉积法(Ch-ECh method)和电化学沉积法(ECh method)制备的H3PMo12O40/carbon foil(碳箔材料)具有不同的电化学性能,电化学沉积法的循环伏安曲线明显优于化学-电化学沉积法。电化学沉积法制备的材料表面有大量的微孔结构,可以有效促进电解液在表面进行电荷转移。2007年,他们[93]用硝酸或硫酸氧化处理碳纳米管与Cs-PMo12,通过化学吸附作用制备Cs-PMo12/CNTs。结果表明Cs-PMo12是能量密度的增强剂,500次循环充放电以后,电容值达到285 F/g(200 mA/g)。Park等[38]通过化学沉积法制备了H3PMo12O40的碳材料,发现不仅比表面积和表面微孔结构对电容量有影响,而且碳原子的杂化形式也有影响。导电率可以随着碳原子SP2/SP3的比值增大而增大。

表2 POMs修饰的超级电容器电极材料[78-91]
2016年,Genovese小组[17]采用分子自组装法制备POMs功能化碳纳米管材料。通过咪唑阳离子将GeMo12与碳纳米管结合,发现GeMo12/CNT作为电极材料时电容(84 F/cm3)是CNT的4倍;GeMo12和SiMo12共同修饰的碳纳米管,电容(191 F/cm3)是CNT的9倍,表明POMs种类对电极材料具有较大影响。
多酸不仅可以用于修饰储能电极材料,还可以用于超级电容器的电解质材料。Lian等[94]采用磷钨酸(PW12)和硅钨酸(SiW12)的水溶液,作为超级电容器(石墨基和RuO2基)的电解质,通过直流电和交流电方式测试,参数见表3,表明多酸具有良好的离子导电率和稳定性,SiWA和PWA的水溶液适合作为超级电容器的电解液。

表3 0.3 M H2SO4、SiW12、和PW12溶液用于超级电容器的电解质材料[94]
4 结论和展望
综上所述,POMs功能化储能材料具有广泛的应用前景。不同的多酸与有机或无机材料复合,用于不同储能技术的不同结构材料,均表现出较好的储能性能。通过多酸分子的设计和多酸与功能材料结合的方式来提高储能材料的性能是未来研究的重点。研究和开发稳定、高效POMs功能化储能材料的研究具有重要意义。
[1] N.S.Lewis,D.G.Nocera.Powering the planet:Chemical challenges in solar energy utilization[J].Proc.Natl.Acad.Sci.,2006,103(43):15729-15735.
[2] M.Kourasi,R.G.A.Wills,A.A.Shah,et al.Heteropolyacids for fuel cell applications[J].Electrochimica Acta,2014,127(5):454-466.
[3] Meng Lu,Baohan Xie,Jeonghee Kang,et al.Synthesis of main-chain polyoxometalate-containing hybrid polymers and their applications in photovoltaic cells[J].Chem.Mater.,2005,17(2):402-408.
[4] N.Kawasaki,Heng Wang,R.Nakanishi,et al.Nanohybridization of polyoxometalate clusters and single-wallcarbon nanotubes:Applications in molecular cluster batteries[J].Angew.Chem.,2011,50(15):3471-3474.
[5] M.T.Pope..杂多和同多金属氧酸盐[M].王恩波,等,译.长春:吉林大学出版社,1991.
[6] 王恩波,胡长文,许林.多酸化学导论[M].北京:化学工业出版社,1998.
[7] 王恩波,李阳光,鹿颖,等.多酸化学概论[M].长春:东北师范大学出版社,2009.
[8] J.F.Keggin.Structure of the molecle 12-phosphotungstic acid[J].Nature,1933,131(3321):908-909.
[9] M.T.Pope.Heteropoly and is poly oxometalates[M].Berlin:Springer-Verlag,1983:1-10.
[10] D.E Katsoulis.A survey of applications of polyoxometalates[J].Chem.Rev.,1998,98(1):359-387.
[11] S.Omwoma,C.T.Gore,Yuanchun Ji,et al.Environmentally benign polyoxometalate materials[J].Coordination Chemistry Reviews,2015,286(286):17-29.
[12] S.Herrmann,C.Ritchie,C.Streb,et al.Polyoxometalate-conductive polymer composites for energy conversion,energy storage and nanostructured sensors[J].Dalton Trans.,2015,44(16):7092-7104.
[13] Yuanchun Ji,Lujiang Huang,Jun Hu,et al.Polyoxometalate-functionalized nanocarbon materials for energy conversion,energy storage and sensor systems[J].Energy Environ.Sci.,2015,8(3):776-789.
[14] J.T.Rhule,C.L.Hill,D.A.Judd,et al.Polyoxometalates in medicine[J].Chem.Rev.,1998,98(1):327-357.
[15] 张延林,王德鑫,于海辉.新型PW杂多酸合成及处理甲基橙废液的应用研究[J].东北电力大学学报,2015,35(5):33-37.
[16] S.Li,X.Yu,G.Zhang,et al.Green synthesis of a Pt nanoparticle/polyoxometalate/carbon nanotube tri-component hybrid and its activity in the electrocatalysis of methanol oxidation[J].Carbon,2011,49(6):1906-1911.
[17] G.Matthew,L Keryn.Ionic liquid-derived imidazolium cation linkers for the layer-by-layer assembly of polyoxometalate-MWCNT composite electrodes with high power capability[J].ACS Appl.Mater.Interfaces,2016,8(29):19100-19109.
[18] Kai Yua,Baibin Zhou,Yang Yu,et al.Self-assembly of four one-dimensional molybdenum(V) phosphates linked by strontium and transition metal[J].Inorganica Chimica Acta,2012,384:8-17.
[19] 宋芳源,丁勇,赵崇超.多金属氧酸盐催化的水氧化研究进展[J].化学学报,2014,72(7):133-144.
[20] 李丽东,李文江,孙长青.基于溶胶-凝胶技术的磷钨杂多酸化学修饰电极的组装及其电催化[J].高等学校化学学报,2000,21(6):865-869.
[21] X.L.Wang,Z.H.Kang,E.B.Wang,et al.Inorganic-organic hybrid polyoxometalate nanoparticle modified wax impregnated graphite electrode:preparation,electrochemistry and electrocatalysis[J].J.electroanal.Chem.,2002,523(1/2):142-149.
[22] Liang Li,YuLin Yang,RuiQing Fan,et al.Photocurrent enhanced dye-sensitized solar cells based on TiO2 loaded K6SiW11O39Co(II)(H2O)·xH2O photoanode materials[J].Dalton Trans.,2014,43(4):1577-1582.
[23] B.Keita,L.Nadjo.Electrocatalysis by electrodeposited heteropolyanions and isopolyanions[J].J Electroanal Chem.,1987,227(1):265-270.
[24] 杜金艳,吕桂琴,胡长文.[SiCu(H2O)W11O39]6-在玻碳电极上的多层组装及电催化性能[J].无机化学学报,2005,21(5):685-689.
[25] 杜金艳,吕桂琴,郑传明.[SiZn(H2O)W11O39]6-在玻碳电极上的多层组装及电催化性能[J].分析试验室.2006,25(2):51-54.
[26] 杜金艳,黄玉成,吕桂琴,等.[SiMn(H2O)W11O39]6-在玻碳电极上的多层组装膜修饰电极及电化学行为[J].分析测试学报.2008,27(3):293-295.
[27] 黄正国,程龙,孙勤枢,等.杂多酸-聚(二烯丙基二甲基氯化铵)多层膜在4-氨基硫酚修饰金电极上的组装和电化学行为[J].分析化学,2000,28(11);1331-1335.
[28] O.Savadogo,F.Carrier.The hydrogen evolution reaction in basic medium on iron electrodeposited with heteropolyacids[J].J Electroanal Chem.1992,22(5):437-442.
[29] 王秀丽,赵岷,等.多酸电化学导论[M].北京:中国环境科学出版社,2006.
[30] P.Allongue,M.Delamar,B.Desbat,et al.Covalent modification of carbon surfaces by aryl radicals generated from the electrochemical reduction of diazonium salts[J].J.Am.Chem.Soc.,1997,119(1):201-207.
[31] J.Y.Liu,L.Cheng,B.F.Liu,et al.Covalent modification of a glassy carbon surface by 4-aminobenzoic acid and its application in fabrication of a polyoxometalates-consisting monolayer and multilayer films[J].Langmuir,2000,16(19):7471-7476.
[32] 王升富,杜丹,皱其超.磷钼钨杂多酸-L-半胱氨酸自组装膜电极的电化学性质[J].物理化学学报,2001,17(12):1102-1106.
[33] Yong Kim,Kriangsak Ketpang,Shayapat Jaritphun,et al.A polyoxometalate coupled graphene oxide-Nafion composite membrane for fuel cells operating at low relative humidity[J].J.Mater.Chem.A,2015,3(15):8148-8155.
[34] Jianyun Liu,Long Cheng,Baifeng Liu,et al.Multilayer assemblies of tungstodiphosphate anions on 1,7-diaminoheptane modified glassy carbon electrode and the electrocatalytic reduction to iodate[J].Electroanalysis,2001,13(12):993-998.
[35] T.McCormac,D.Farrell,D.Drennan,et al.Immobilization of a series of dawson type heteropolyanions[J].Electroanalysis,2001,13(10):836-842.
[36] Wei Chen,Lujiang Huang,Jun Hu,et al.Connecting carbon nanotubes to polyoxometalate clusters for engineering high-performance anode materials[J].Phys.Chem.Chem.Phys.,2014,16(36):19668-19673.
[37] Yuanchun Ji,Jun Hu,Lujiang Huang,et al.Covalent attachment of anderson-type polyoxometalates to single-walled carbon nanotubes gives enhanced performance electrodes for lithium ion batteries[J].Chem.Eur.J.,2015,21(17):6469-6474.
[38] S.Park,K.Lian,Y.Gogotsi.Pseudocapacitive behavior of carbon nanoparticles modified by phosphomolybdic acid[J].Journal of the Electrochemical Society,2009,156 (156):A921-A926.
[39] Lujiang Huang,Jun Hu,Yuanchun Ji,et al.Pyrene-anderson-modified CNTs as anode materials for lithium-ion batteries[J].Chem.Eur.J.2015,21(51):18799-18804.
[40] Yuanchun Ji,Tengfei Li,Yu-Fei Song.Adsorption of human serum albumin (HSA) by SWNTs/Py-PW11nanocomposite[J].Ind.Eng.Chem.Res.2014,53(28):11566-11570.
[41] Dui Ma,Liying Liang,Wei Chen,et al.Covalently tethered polyoxometalate-pyrene hybrids for noncovalent sidewall functionalization of single-walled carbon nanotubes as high-performance anode material[J].Adv.Funct.Mater.,2013,23(48):6100-6105.
[42] U.Meinhardt,F.Lodermeyer,T A.Schaub,et al.N-Heterotriangulene chromophores with 4-pyridyl anchors for dye-sensitized solar cells[J].RSC.Adv.,2016,6(71):67372-67377.
[43] Zicheng Xiao,Kun Chen,Baolin Wu,et al.An easy way to construct polyoxovanadate-based organic-inorganic hybrids by stepwise functionalization[J].European Journal of Inorganic Chemistry,2016,2016(6):808-811.
[44] S.Vanhaecht,J.Jacobs,L.V.Meervelt,et al.A versatile and highly efficient post-functionalization method for grafting organic molecules onto Anderson-type polyoxometalates[J].Dalton Trans.,2015,44(44):19059-19062.
[45] Xiaojing Sang,Jiansheng Li,LanCui Zhang,et al.Two carboxyethyltin functionalized polyoxometalates for assembly on carbon nanotubes as efficient counter electrode materials in dye-sensitized solar cells[J].Chem.Commun.,2014,50(93):14678-14681.
[46] Fei Wu,Haitao Liu,Linna Zhu,et al.New organic dyes based on biarylmethylene-bridged triphenylamine for dye sensitized solar cell[J].Chin.J.Chem.,2015,33(8):925-933.
[47] Liqian Liu,Guichuan Zhang,Baitian He,et al.Polymer solar cells based on the copolymers of naphtho[1,2-c:5,6-c] bis(1,2,5-thiadiazole) and alkoxylphenyl substituted benzodithiophene with high open-circuit voltages[J].Chin.J.Chem.,2015,33(8):902-908.
[48] Tengling Ye,Junhai Wang,Guohua Dong,et al.Recent progress in the application of polyoxometalates for dye-sensitized/organic solar cells[J].Chin.J.Chem.2016,34(8):747-756.
[49] J.J.Walsh,A.M.Bond,R.J.Forster,et al.Hybrid polyoxometalate materials for photo(electro-)chemical applications[J].Coordination Chemistry Reviews,2016,306:217-234.
[50] Zhixia Sun,Fengyan Li,Mingliang Zhao,et al.A comparative study on photoelectrochemical performance of TiO2photoanodes enhanced by different polyoxometalates[J].Electrochemistry Communications,2013,30(3):38-41.
[51] Na Li,ZhixiaSun,RanLiu,et al.Enhanced power conversion efficiency in phthalocyanine-sensitized solar cells by modifying TiO2photoanode with polyoxometalate[J].Solar Energy Materials & Solar Cells,2016,157:853-860.
[52] Lifei He,Li Chen,Yue Zhao,et al.TiO2film decorated with highly dispersed polyoxometalate nanoparticles synthesized by micelle directed method for the efficiency enhancement of dye-sensitized solar cells[J].Journal of Power Sources,2016,328:1-7.
[53] Chunhui Shan,Hongzhang,Weilin Chen,et al.Pure inorganic D-A type polyoxometalate/reduced graphene oxide nanocomposite for the photoanode of dye-sensitized solar cells[J].J.Mater.Chem.A,2016,4(9):3297-3303.
[54] Chunchen Yuan,Shuangshuang Guo,Enbo Wang,et al.Electropolymerization polyoxometalate (POM)-doped PEDOT film electrodes with mastoid microstructure and its application in dye-sensitized solar cells(DSSCs)[J].Ind.Eng.Chem.Res.2013,52(20):6694-6703.
[55] Xiangwei Guo,Jiansheng Li,Xiaojing Sang,et al.Three keggin-type transition metal-substituted polyoxometalates as pure inorganic photosensitizers for p-type dye-sensitized solar cells[J].Chem.Eur.J.2016,47(22):3234-3238.
[56] Jiansheng Li,Xiaojing Sang,Enbo Wang,et al.The application of ZnO nanoparticles containing polyoxometalates in dye-sensitized solar cells[J].Eur.J.Inorg.Chem.,2013(10/11):1951-1959.
[57] Jun Hu,Yuanchun Ji,Wei Chen,et al.“Wiring” redox-active polyoxometalates to carbon nanotubes using a sonication-driven periodic functionalization strategy[J].Energy Environ.Sci.,2016,9(3):1095-1101.
[58] O.Nakamura,T.Kodama,I.Ogino,Y.Miyake.High-conductivity solid proton conductors:dodecamolybdophosphoric acid and dodecatungstophosphoric acid crystals [J].Chem.Lett.1979,1(1):17-18.
[59] H.Tian,O.Savadogo.Silicotungstic acid Nafion composite membrane for proton-exchange membrane fuel cell operation at high temperature[J].Journal of New Materials for Electrochemical Systems,2006,9(1):61-71.
[60] S.Y.Oh,T.Yoshid,G.Kawamur,et al.Inorganic-organic composite electrolytes consisting of polybenzimidazole and Cs-substituted heteropoly acids and their application for medium temperature fuel cells[J].Journal of Materials Chemistry,2010,20(30):6359-6366.
[61] M.Helen,B.Viswanathan,S.Srinivasa Murthy.Poly(vinylalcohol)-polyacrylamide blends with cesium salts of heteropolyacid as a polymer electrolyte for direct methanol fuel cell applications[J].Journal of Applied Polymer Science,2010,116(116):3437-3447.
[62] Chengji Zhao,Haidan Lin,Hui Na.Layer-by-layer self-assembly of polyaniline on sulfonated poly(arylene ether ketone) membrane with high proton conductivity and low methanol crossover[J].International Journal of Hydrogen Energy,2010,35(19):10482-10488.
[63] Xinxu,Hainingwang,Shanfu Lu,et al.A phosphotungstic acid self-anchored hybrid proton exchange membrane for direct methanol fuel cells[J].RSC.Adv.,2016,6(49):43049-43055.
[64] Hongjun Kim,Sunghwan Lee,Suran Kim,et al.Membrane crystallinity and fuel crossover in direct ethanol fuel cells with Nafion composite membranes containing phosphotungstic acid[J].J.Mater.Sci.,2017,52(5):2400-2412.
[65] Zhiming Cui,Wei Xing,Changpeng Liu,et al.Chitosan/heteropolyacid composite membranes for direct methanol fuel cell[J].Journal of Power Sources,2009,188(1):24-29.
[66] M.Santamaria,C.M.Pecoraro,F.Di Quarto,et al.Chitosanephosphotungstic acid complex as membranes for low temperature H2-O2fuel cell[J].Journal of Power Sources,2015,276(1):189-194.
[67] C.M.Pecoraro,M.Santamaria a,P.Bocchetta,et al.Influence of synthesis conditions on the performance of chitosane heteropolyacid complexes as membranes for low temperature H2-O2fuel cell[J].International Journal of Hydrogen Energy,2015,40(42):14616-14626.
[68] M.Santamaria,C.M.Pecoraro,F.D.Franco,et al.Heteropolyacids-chitosan membranes for H2/O2low temperature fuel cells[J].ECS.Transactions,2016,75(14):597-605.
[69] Suzhen Ren,Meiling Xu,Ying Yang,et al.Effects of microstructural functional polyaniline layers on SPEEK/HPW proton exchange membranes[J].J.appl.polym.sci.,2014,131(21):41033-41040.
[70] M.J.Martínez-Morlanes,A.M.Martos,A.Várez,et al.Synthesis and characterization of novel hybrid polysulfone/silica membranes doped with phosphomolybdic acid for fuel cell applications[J].Journal of Membrane Science,2015,492:371-379.
[71] E.Gerasimova,E.Safronova,A.Ukshe,et al.Electrocatalytic and transport properties of hybrid Nafion membranes doped with silica and cesium acid salt of phosphotungstic acid in hydrogen fuel cells[J].Chemical Engineering Journal,2015,305 :121-128.
[72] T.Inoue,T.Uma,M.Nogami.Performance of H2/O2fuel cell using membrane electrolyte of phosphotungstic acid-modified 3-glycidoxypropyl-trimethoxysilanes[J].Journal of Membrane Science 2008,323 (1):148-152.
[73] Zhigang Shao,Hongfeng Xu,Mingqiang Li,et al.Hybrid Nafion-inorganic oxides membrane doped with heteropolyacids for high temperature operation of proton exchange membrane fuel cell[J].Solid State Ionics,2006,177(7):779-785.
[74] Zhigang Shao,P.Joghee,I.M.Hsing.Preparation and characterization of hybrid Nafion-silica membrane doped with phosphotungstic acid for high temperature operation of proton exchange membrane fuel cells[J].Journal of Membrane Science,2004,229(1/2):43-51.
[75] M.Amirinejad,S.S.Madaeni,E.Rafiee,et al.Cesium hydrogen salt of heteropolyacids/Nafion nanocomposite membranes for proton exchange membrane fuel cells[J].Journal of Membrane Science,2011,377(1):89-98.
[76] M.Renzi,P.Mignini,G.Giuli,et al.Rotating disk electrode study of Pt/Cs3HPMo11VO40composite catalysts for performing and durable PEM fuel cells[J].International Journal of Hydrogen Energy,2016,41(26):11163-11173.
[77] M.Renzi,R.Marassi,F.Nobili,et al.Low platinum loading cathode modified with Cs3H2PMo10V2O40for polymer electrolyte membrane fuel cells[J].Journal of Power Sources,2016,327:11-20.
[78] A.M.White,R.C.T.Slade.Polymer electrodes doped with heteropolymetallates and their use within solid-state supercapacitors[J].Synthetic Metals,2003,139(1):123-131.
[79] G.M.Suppes,B.A.Deore,M.S.Freund.Porous conducting polymer/heteropolyoxometalate hybrid haterial for electrochemical supercapacitor applications[J].Langmuir,2008,24(3):1064-1069.
[80] A.M.White,R.C.T.Slade.Investigation of vapour-grown conductive polymer/heteropolyacid electrodes[J].Electrochimica Acta,2003,48(18):2583-2588.
[81] A.M.White.,R.C.T.Slade.Electrochemically and vapour grown electrode coatings of poly(3,4-ethylenedioxythiophene) doped with heteropolyacids[J].Electrochimica Acta,2004,49(6):861-865.
[82] A.K.C.Gallegos,M.L.Cantu,N.Casan-Pastor,et al.Nanocomposite hybrid molecular materials for application in solid-state electrochemical supercapacitors[J].Adv.Funct.Mater,2005,15(15):1125-1133.
[83] J.Vaillant,M.L.Cantu,K.C.Gallegos,et al.Chemical synthesis of hybrid materials based on PAni and PEDOT with polyoxometalates for electrochemical supercapacitors[J].Progress in Solid State Chemistry,2006,34(2/3/4):147-159.
[84] G.M.Suppes,C.G.Cameron,M.S.Freund.A polypyrrole/phosphomolybdic acid||poly (3,4-ethylenedioxythiophene)/phosphotungstic acid asymmetric supercapacitor[J].Journal of the Electrochemical Society,2010,157(9):1030-1034.
[85] T.Akter,Kaiwen Hu,K.Lian.Investigations of multilayer polyoxometalates-modified carbon nanotubes for electrochemical capacitors[J].Electrochimica Acta,2011,56:4966-4971.
[86] S.Marta,G.K.Monika,S.N.Magdalena,et al.Hybrid materials utilizing polyelectrolyte-derivatized carbon nanotubes and vanadium-mixed addenda heteropolytungstate for efficient electrochemical charging and electrocatalysis[J].J Solid State Electrochem,2013,17(6):1631-1640.
[87] G.Bajwa.Nanocarbon/polyoxometalate composite electrodes for electrochemical capacitors[D].Toronto:University of Toronto,2012.
[88] S.Magdalena,C.Malgorzata,A.Iwona,et al.Improved capacitance characteristics during electrochemical charging of carbon nanotubes modified with polyoxometallate monolayers[J].Electrochimica Acta,2008,53(11):3862-3869.
[89] J.S.Guevara,V.Ruiz,P.G.Romero.Hybrid energy storage:high voltage aqueous supercapacitors based on activated carbon-phosphotungstate hybrid material[J].J.Mater.Chem.A,2014,2(4):1014-1021.
[90] V.Ruiz,J.S.Guevara,P.G.Romero.Hybrid electrodes based on polyoxometalate-carbon materials for electrochemical supercapacitors[J].Electrochemistry Communications,2012,24(1):35-38.
[91] J.S.Guevara,V.Ruiz,P.G.Romero.Stable graphene-polyoxometalate nanomaterials for application in hybrid supercapacitors[J].Phys.Chem.Chem.Phys.,2014,16(38):20411-20414.
[92] P.G.Romero,C.Malgorzata,K.C.Gallegos,et al.Hybrid organic-inorganic nanocomposite materials for application in solid state electrochemical supercapacitors[J].Electrochemistry Communications,2003,5(2):149-153.
[93] A.K.C.Gallegos,P.G.Romero,et al.Electrochemical supercapacitors based on novel hybrid materials made of carbon nanotubes and polyoxometalates[J].Electrochemistry Communications,2007,9(8) :2088-2092.
[94] K.Lian,Changming Li.Heteropoly acid electrolytes for double-layer capacitors and pseudocapacitors[J].Electrochemical and Solid-State Letters,2008,11(9):158-162.
The Research Progress of Polyoxometalate-functionalized Energy Storage Materials
Zhao Guangzhen,Jiang Tianyao,Shi Junyou
(School of Chemical Engineering,Northeast Electric Power University,Jilin Jilin 132012)
Compared with non-renewable energy (coal,oil,et al),renewable energy (solar,wind and so on) could not storage directly,and have to complete energy storage through energy transformation.Energy storage technology is the key technology to solve unsteady characteristics for generating electricity by renewable energy.It is one of the effective ways to breakthrough energy storage technology bottleneck through development of stable and efficient energy storage materials.Polyoxometalates have the strong ability of electron and proton transfer and storage,so the study of polyoxometalate-functionalized energy storage materials has raised more concerns in recent years.The paper is about the literature review of polyoxometalate-functionalized energy storage materials.
Polyoxometalates;Solar Cell;Lithium Ion Battery;Fuel cell;Supercapacitor
2016-12-11
国家公益行业重大专项(201504502)
赵广震 (1989-),男,在读博士研究生,主要研究方向:生物质转化利用技术.
1005-2992(2017)03-0073-10
TM912.9
A
电子邮箱: zhaogzgold@126.com(赵广震);240244369@qq.com(姜天尧);bhsjy64@163.com(时君友)

