2,3-2H-2-(2-(4-苯基哌嗪-1-基)喹啉-3-基)-4-苯基-1,5-苯并的合成与表征
杨 鹏, 戚璐璐, 许芯宁, 刘方明
(杭州师范大学材料与化学化工学院,浙江 杭州 310036)
杨 鹏, 戚璐璐, 许芯宁, 刘方明
(杭州师范大学材料与化学化工学院,浙江 杭州 310036)


0 引 言


1 实验部分
1.1 仪器和试剂
实验所用试剂都是从商业来源获得,使用前没有经过进一步提纯.
X-5显微熔点仪(温度计未校正);Bruker-Tensor 27红外光谱仪(KBr压片),Advance 400NMR型核磁共振仪(TMS为内标);Agilent-5975质谱仪(EI,轰击电压70 eV),日立F-4500 R-AXIS信息平台衍射仪,Perkin-Elmer 240 CHN分析仪.
1.2 化合物的合成
目标化合物的合成路线如图 1所示.
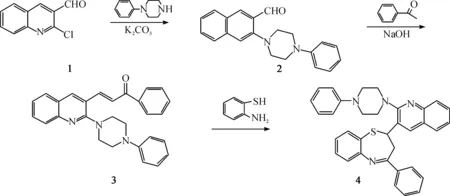
图1 目标化合物4的合成Fig. 1 Synthesis route for titled compound
1.2.1 2-氯-3-甲醛喹啉(化合物1)的制备
根据文献方法[21]制备.
1.2.2 2-(4-苯基哌嗪-1-基)-喹啉-3-甲醛(化合物2)的制备
取250 mL的三口烧瓶,将 1(10 mmol)加热溶解在20 mL DMF中,15 mmol K2CO3和11 mmol的1-苯基哌嗪依次加入反应液.溶液搅拌回流反应6~7 h,反应通过TLC跟踪监测.待反应完全后将反应混合液倒入冰水中,有大量黄色固体析出,冰醋酸调节溶液pH至中性,抽滤溶液并用水洗涤得固体,60 ℃真空干燥,产物用乙醇重结晶.
2:淡黄色固体 (96%);mp:161~162 ℃;IR(KBr)υ/cm-1:1 691(C=O),1 596 (C=N);1H NMR (CDCl3,400 MHz)δ:10.24 (s,1H,-CHO),8.54 (s,1H,quinolin H4),6.92~7.68 (m,10H,ArH),3.68 (s,4H,phenylpiperazine,H3),3.44 (s,4H,phenylpiperazine,H2);MS (EI):m/z317 (M+);Anal calcd for C20H19N3O: C,75.69, H,6.03, N,13.24, O,5.04;Found: C,75.67, H,6.05, N,13.27.
1.2.3 1-苯基-3-(2-(4-苯基哌嗪-1-基)喹啉-3-基)丙-2-烯-1-酮(化合物3)的制备
将 2(10 mmol)溶解在20 mL乙醇中,室温下搅拌.20 mmol NaOH溶于2 mL水,滴入反应液中,室温搅拌反应3~4 h,反应进程通过TLC跟踪检测.反应完全后,将反应液倒入水中,用5%HCl调节溶液至中性,抽滤溶液得固体,真空干燥.产物用乙醇/CH2Cl2重结晶.
3:黄棕色晶体 (87%);mp:155~157 ℃;IR(KBr)υ/cm-1:1 651(C=O),1 602 (C=N);1H NMR (CDCl3,400 MHz)δ:3 44 (s,4H,phenylpiperazine,H2),3.56 (s,4H,phenylpiperazine,H3),8.37 (s,1H,quinolin,H4),6.93~8.17(m,16H,ArH);MS (EI):m/z419 (M+);Anal calcd for C28H25N3O: C,80.16, H,6.01, N,10.02;Found: C,80.58, H,5.83, N,10.19.

将3(10 mmol)和10 mmol邻氨基苯硫酚在甲苯中搅拌回流反应5~6 h,反应进程通过TLC跟踪检测.将反应混合物冷却至室温,减压蒸馏浓缩,粗产物用乙醇/甲苯重结晶后得到淡黄色晶体.
4:淡黄色晶体(62%);mp:204~206 ℃;IR(KBr)υ/cm-1:3 057(Ar—H),2 967(CH2CH2),1 597 (C=N);1H NMR (CDCl3,400 MHz)δ:3.45 (s,4H,phenylpiperazine,H2),3.56 (s,4H,phenylpiperazine,H4),8.32 (s,1H,quinolin,H4),6.89~8.09 (m,18H,ArH),6.95 (dd,1H,H2x,Jax=12.4 Hz,Jbx=4.2 Hz),5.61(dd,1H,H3b,Jbx=4.2 Hz,Jab=12.6 Hz),2.90(dd,1H,H3a,Jax=12.4 Hz,Jab=12.6 Hz);MS (EI):m/z526 (M+);Anal calcd for C34H30N4S: C,77.53, H,5.74, N,10.64;Found: C,77.35, H,5.42, N,10.77.
2 结果和讨论
2.1 IR,1H NMR和质谱分析
目标化合物 4 的IR吸收带出现了一些特征峰:在3 057 cm-1有苯环上的C—H伸缩振动,2 967 cm-1处有—CH2—伸缩振动;在1 597 至651 cm-1出现C=N和C—S伸缩振动;在1 255 cm-1处有C—N—C伸缩振动;在701 cm-1有芳香族单取代的振动.
目标化合物 4(溶剂CDCl3)的1H NMR数据显示喹啉H4出现在δ8.32 ppm,苯环上多重峰出现在δ8.09~6.89 ppm,在δ6.95 ppm和δ5.61,2.90 ppm出现了该硫氮杂环Hx的特征三重峰和 Ha, Hb双重峰.亚甲基(环上)相邻的N1的哌嗪在δ3.56 ppm为三重峰,亚甲基(环上)相邻的N4哌嗪在δ3.45 ppm的范围内也为三重峰.
目标化合物显示出低强度的M+离子峰(图2).质谱m/z526,显示其分子的离子峰M+在182(100%),基峰和其他显著峰出现在m/z394,257,211,证明其结构的正确性.
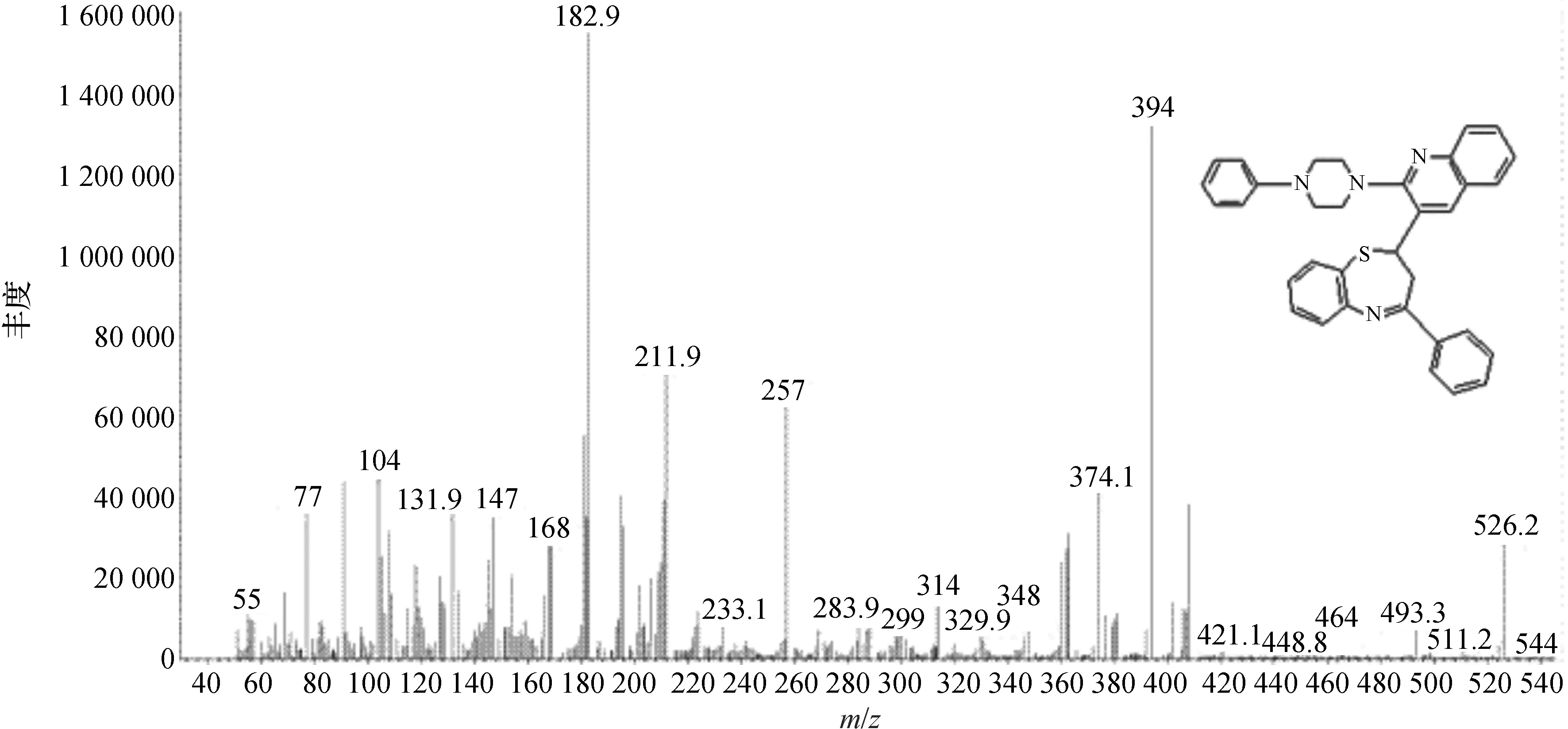
图2 目标化合物的质谱图Fig. 2 MS of titled compound
2.2 晶体结构研究
化合物 4 的晶体结构和晶体分子堆积如图3和图4所示.
Rigaku R-AXIS Spider单晶X衍射(296(2)K石墨单色MoKa辐射)显示化合物 4 的晶体尺寸大约为0.420 mm×0.380 mm×0.240 mm.使用SHELXL-97[22]和PLATON[23]软件进行单晶数据计算.通过全矩阵最小二乘对F2位置参数和热力参数精修,得到R1=0.041 7, wR2=0.115 1.晶体学数据和结构的具体信息见表1所示.
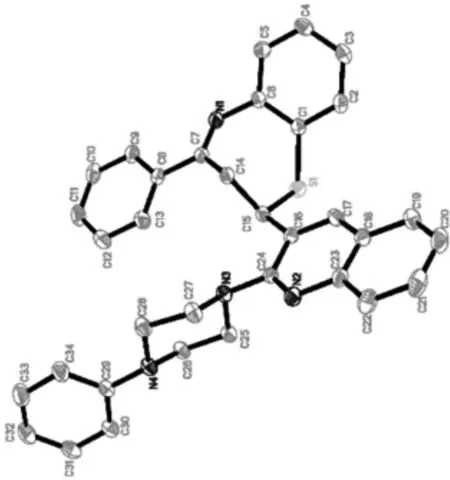
图3 化合物 4 的晶体结构Fig. 3 The structure of crystal 4
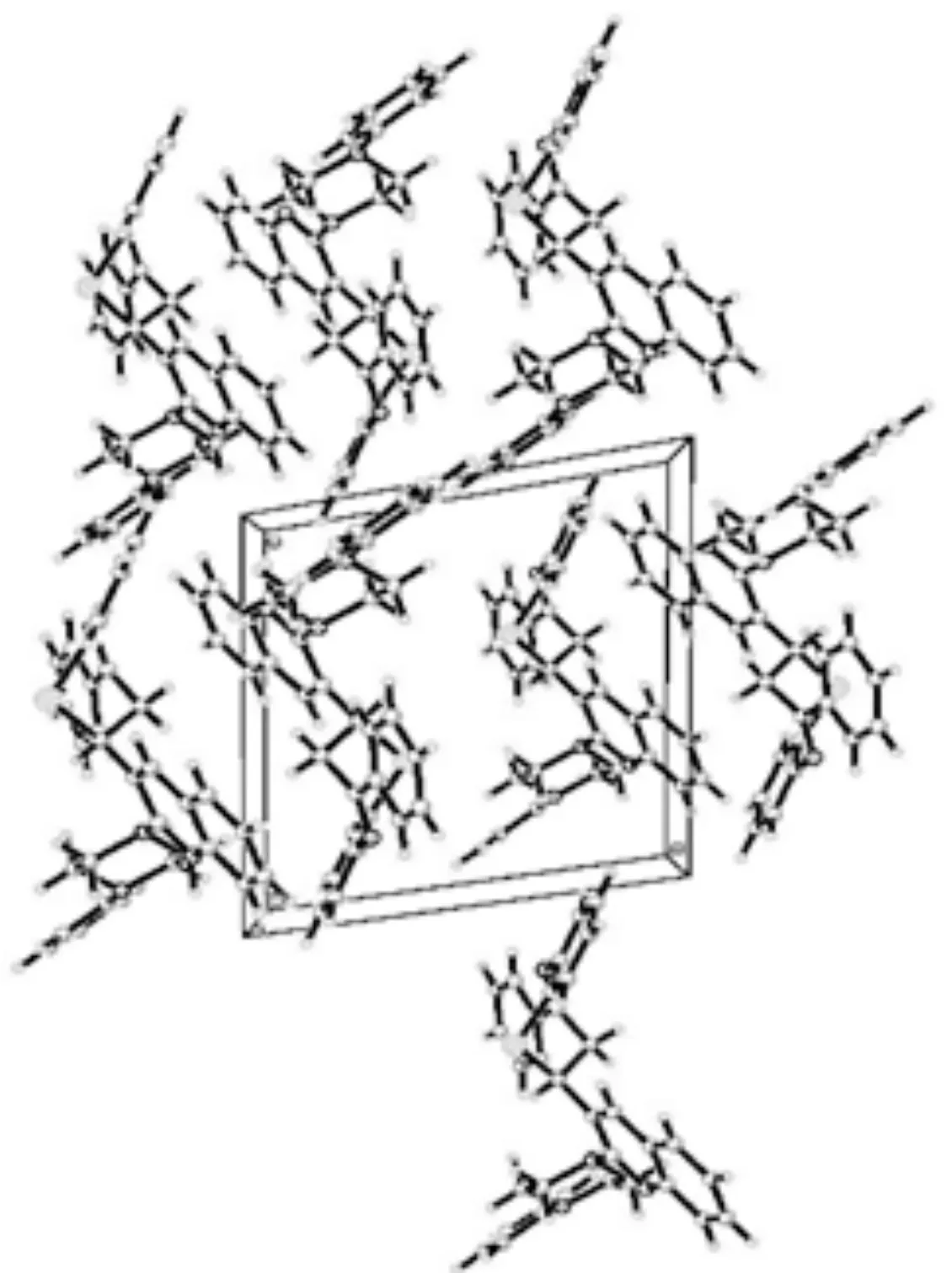
图4 化合物 4 晶体的分子堆积Fig. 4 The molecular packing of crystal 4

表1 化合物 4 晶体的相关实验数据Tab. 1 Crystal and experimental data for 4
注:CCDC-1041492包含本文晶体数据,可以从剑桥晶体数据中心(CCDC)免费获得.
化合物 4 的五环体系有喹啉环Cg(1)(C24N2—C23—C18—C19),1,5-苯并硫氮杂环Cg(2)(C15—S1—C1—C6—N1—C7—14),苯基环Cg(3)(C8—C11),哌嗪环Cg(4)(N—C27—N4—C26),苯基环Cg(5)(C29—C32),苯环Cg(6)(C1—C4).其中心七元环Cg(2)通过S(1)原子、C(14)原子和C(15)原子形成了一个最小二乘平面结构,C(1)原子和C(6)原子在平面上方,C(7)原子和N(1)原子在平面下方.晶体数据中扭转角的度数可以说明七元环的扭船式构象[24].Cg(1)和Cg(2)之间的二面角为48.67°,Cg(1)和Cg(2)之间的中心距离为5.388 Å.Cg(1)和Cg(4)之间的二面角为61.98°,中心距离为5.111 Å.Cg(2)和Cg(3)的二面角为40.78°,中心距离为2.601 Å.Cg(2)和Cg(6)的二面角为35.03°,中心距离为4.248 Å.Cg(4)和Cg(5)的二面角和中心距离分别为33.92°和4.212 Å.苯基的Cg(5)几乎垂直于喹啉环Cg(1),二者之间的二面角为85.79°.因N3和N4原子分别在C25/C26/C27/C28平面形成的角度是0.638(2) Å和0.687(2) Å,有较小的偏差,可见哌嗪环Cg(4)采用典型的椅式结构.N(3)—C(24) (1.426 8(18) Å),N(4)—C(29) (1.419 4(19) Å)和N(1)—C(6) (1.405 7(19) Å)的键长比典型的碳—氮单键的N(3)—C(25) (1.472 7(18) Å)或N(4)—C(26) (1.464 8(19) Å)的键长要短,原子的共价半径Csp2小于 Csp3.具有代表性的化合物4的键长、键角、扭转角如表2所示.

表2 具有代表性的化合物 4 的键长、键角、扭转角Tab. 2 Selected bond lengths, bond angles and torsion angles for compound 4
此外,从化合物4的晶体结构可以发现其分子间没有经典的氢键,仅由分子间较弱的C(15)-H(15)…N(3) 和C(17)—H(17)…S(1)相互作用.在Cg(1)与其他3个环Cg(2)、Cg(3)、Cg(4)之间存在弱C—H—P叠加的相互作用.这种弱C—H—P堆积的存在,有助于晶体堆积的稳定.表3列举了化合物 4 分子间的一些氢键、距离和角度.
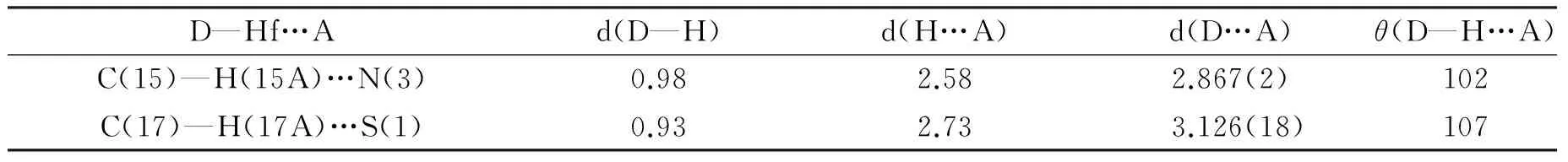
表3 化合物 4 分子间氢键,距离和角度Tab. 3 Intermolecular interactions in the compound 4
i2-x,1-y,1-z.
3 结 论

[1] PNAT S, SHARMA P, SHARMA A, et al. Syntheses and antimicrobial studies of 8-substituted-2,5-dihydro-2-(4-methoxyphenyl-3,4-dimethoxyphenyl)-4-(2-thienyl)-1,5-benzothiazepines[J]. J Indian Chem Soc,2008,85:406-411.
[2] SANJEEVA REDDY C, PURNACHANDRA REDDY G, NAGARAJ A. Synthesis and in vitro study of novel methylenebis (phenyl-1,5-benzothiazepine)s and methylenebis(benzofuryl-1,5-ben zothiazepine)s as antimicrobial agents[J]. Chin J Chem,2009,27(7):1345-1352.
[3] DI SANTO R, COSTI R. 2H-Pyrrolo[3,4-b][1,5]benzothiazepine derivatives as potential inhibitors of HIV-1, reverse transcriptase[J]. II Farmaco,2005,60(5):385-392.
[4] MC GEE M, GEMMA S, BUTINI S, et al. Pyrrolo[1,5]benzoxa(thia)zepines as a new class of potent apoptotic agents. Biological studies and identification of an intracellular location of their drug target[J]. J Med Chem,2005,48(13):4367-4377.
[5] SHARMA A K, SINGH G, YADAV A K, et al. Improved method for the synthesis of new 1,5-benzothiazepine derivatives as analogues of anticancer drugs[J]. Molecules,1997,2(9):129-134.
[6] KANEKO N, MATSUDA R, HATA Y, et al. Pharmacological characteristics and clinical applications of K201[J]. Curr Clin Pharmacol,2009,4(2):126-131.
[7] DE SARRO G, CHIMIRRI A, DE SARRO A, et al. 5H-[1,2,4]Oxadiazolo[5,4-d][1,5]benzothiazepines as anticonvulsant agents in DBA/2 mice[J]. Eur J Med Chem,1995,30(12):925-929.
[8] INOUE H, KONDA M, HASHIYAMA M, et al. Synthesis of halogen-substituted 1,5-benzo thiazepine derivatives and their vasodilating and hypotensive activities[J]. J Med Chem,1991,34(2):675-687.
[9] BARIWAL J B, UPADHYAY K D, MANVAR A T, et al. 1,5-Benzothiazepine, a rersatile pharmacophore: a review[J]. Eur J Med Chem,2008,43(11):2279-2290.
[10] MUNGRA D C, PATEL M P, PATEL R G. Microwave-assisted synthesis of some new tetrazolo[1,5-a]quinoline-based benzimidazoles catalyzed byp-TsOH and investigation of their antimicrobial activity[J]. Med Chem Res,2011,20(6):782-789.
[11] GARUDACHARI B, SATYANARAYANA M N, THIPPESWAMY B, et al. Synthesis, characterization and antimicrobial studies of some new quinoline incorporated benzimidazole derivatives[J]. Eur J Med Chem,2012,54(11):900-906.
[12] PERIN N, UZELAC L, PIANTANIDA I, et al. Novel biologically active nitro and amino substituted benzimidazo[1,2-a]quinolines[J]. Bioorg Med Chem,2011,19(21):6329-6339.
[13] SZKARADEK N, RAPACZ A, PYTKA K, et al. Synthesis and preliminary evaluation of pharmacological properties of some piperazine derivatives of xanthone[J]. Bioorg Med Chem,2013,21(2):514-522.
[14] CECCHETTI V, FRAVOLINI A, SCHIAFFELLA F, et al. o-Chlorobenzenesulfonamidic derivatives of (aryloxy) propanolamines as β-blocking/diuretic agents[J]. J Med Chem,1993,36(1):157-161.
[15] WALSH D A, CHEN Y H, GREEN J B, et al. The synthesis and antiallergy activity of 1-(aryloxy)-4-(4-arylpiperazinyl)-2-butanol derivatives[J]. J Med Chem,1990,33(6):1823-1827.
[16 ] SEO H J, PARK E J, KIM M J, et al. Design and synthesis of novel arylpiperazine derivatives containing the imidazole core targeting 5-HT2Areceptor and 5-HT transporter[J]. J Med Chem,2011,54(18):6305-6318.
[17] CLARKSON C, MUSONDA C C, CHIBALE K, et al. Synthesis of totarol amino alcohol derivatives and their antiplasmodial activity and cytotoxicity[J]. Bioorg Med Chem,2003,11(20):4417-4422.
[18] BERARDI F, ABATE C, FERORELLI S, et al. Novel 4-(4-aryl)cyclohexyl-1-(2-pyridyl)piperazines as Δ8-Δ7sterol isomerase (emopamil binding protein) selective ligands with antiproliferative activity[J]. J Med Chem,2008,51(23):7523-7531.
[19] DONG Z Q, LIU F M, XU F, et al. Synthesis of 1,5-benzothiazepine derivatives bearing 2-phenoxy-quinoline moiety via 1,3-dipolar cycloaddition reaction[J]. Mol Divers,2011,15:963-970.
[20] HUANG L, LIU F M, DONG Z Q. Synthesis of novel tricyclic 1,5-benzothiazepine derivatives bearing quinoline moiety via [2+2] cycloaddition reaction[J]. J Heterocyclic Chem,2014,51(5):1516-1521.
[21] DEVI I, BARUAH B, BHUYAN P J. α-Cyclization of tertiary amines: synthesis of some novel annelated quinolines via a three-component reaction under solvent-free conditions[J]. J Synlett,2006,16:2593-2596.
[22] SHELDRICK G M, SCHNEIDER R. SHELXL: high-resolution refinement[J]. Methods in Enzymology,1997,277:319-343.
[23] SPEK A L. Single-crystal structure validation with the program PLATON[J]. J Appl Cryst,2003,36:7-13.
[24] HENDRICKSON J B. Molecular geometry. I. Machine computation of the common rings[J]. J Am Chem Soc,1961,83(22):4537-4547.
Synthesis and Characterization of 2,3-Dihydro-2-(2-(4-Phenylpiperazin-1-yl)quinolin-3-yl)-4-Phenyl-1,5-Benzothiazepine
YANG Peng, QI Lulu, XU Xinning, LIU Fangming
(College of Material, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou 310036, China)
C34H30N4S with quinoline and piperazine was synthesized. Its crystal structure was determined as triclinic system by elemental analysis, IR,1H NMR, MS and X-ray crystallographic analysis. The space group isP-1. The seven membered heterocyclic ring of the thiazepino moiety adopts a twist-boat conformation and the piperazino moiety adopts a chair conformation. The crystal structure is stabilized because of the interactions of C—H…N and C—H…S.
benzothiazepine; quinoline; 4-phenylpiperazine; crystal structure
10.3969/j.issn.1674-232X.2017.03.002
2016-07-13
国家自然科学基金项目(29702007,20162004);浙江省自然科学基金项目(B02021901).
刘方明(1966—),男,教授,博士,主要从事新型杂环化合物的设计与合成研究.E-mail:fmliu859@sohu.com
O626.32+3
A
1674-232X(2017)03-0230-06

