肿瘤化疗联合免疫治疗 从理论基础到临床实践
刘潇衍 综述 陈闽江 王孟昭 审校
肿瘤化疗联合免疫治疗 从理论基础到临床实践
刘潇衍 综述 陈闽江 王孟昭 审校
在过去的几十年里,化疗是肿瘤内科治疗的基石。尽管化疗疗效显著,但维持病情稳定时间不持久。随着对肿瘤免疫学研究的不断深入,近年来肿瘤免疫治疗再次成为热点。与化疗相比,免疫治疗疗效相对缓和,但更为持久。越来越多的研究表明化疗除了对肿瘤细胞的直接杀伤作用,亦可以增加肿瘤细胞的免疫原性,抑制负性免疫信号,改变肿瘤免疫微环境,从而发挥免疫增强作用,为化疗和免疫治疗的联合应用奠定了理论基础。本文根据已知的肿瘤免疫反应机理和近期发现的化疗免疫调节机制,阐述化疗联合免疫治疗的理论基础,并进一步探讨现有的关于联合治疗的基础和临床研究,从而为后续研究指明方向。
肿瘤 化疗 免疫治疗 协同作用
随着对肿瘤免疫研究的不断深入和新的免疫治疗药物不断出现,肿瘤内科治疗方法变得愈加丰富,包括化疗、内分泌治疗、靶向治疗、免疫治疗。如何将不同治疗方法合理地序贯或联合应用以发挥最佳的治疗效果,面对不同肿瘤负荷、不同肿瘤生物学行为、不同免疫状态的患者如何制定最合理的治疗策略,已成为临床工作者亟待解决的问题。近年来一系列研究探索了化疗药物对肿瘤免疫应答的影响,以期更好地指导免疫治疗药物和细胞毒性药物的合理搭配。本文结合理论基础和现有研究证据对化疗及机体肿瘤免疫应答的影响和免疫治疗联合化疗的临床应用进行综述。
1 化疗对机体肿瘤免疫应答的影响
目前研究发现化疗可以发挥正向免疫调节作用,增强机体的抗肿瘤免疫应答(图1),其具体作用机制可以分为两个方面:1)肿瘤细胞自身介导的免疫增强作用,即化疗可以诱导肿瘤细胞发生免疫原性的细胞死亡(immunogenic cell death,ICD),诱发机体的抗肿瘤免疫应答。基础及临床研究发现部分化疗药物可以诱发ICD,常见的包括蒽环类药物(多柔比星、表阿霉素、柔红霉素)、米托蒽醌、奥沙利铂、氟尿嘧啶、伊立替康、环磷酰胺、硼替佐米等[1-5]。然而,必须注意的是同一类的化疗药物,虽然化学结构相似,但是其诱发ICD的能力却不同。如铂类药物奥沙利铂可以诱导ICD,但是因为结构的差异,顺铂则不能[6];但在给予顺铂的同时加入刺激内质网应激反应的药物则可以诱导ICD[7]。所以药物任何理化性质的细小差别都可能影响其诱发ICD的能力,同一类化疗药物并非均可诱导ICD,继而联合免疫治疗,还需要结合临床前和临床研究结果,合理地搭配应用;2)化疗虽然导致淋巴细胞总数减少,但可以改变各免疫细胞亚群的比例及其功能,降低外周血和肿瘤病灶免疫微环境中抑制性免疫细胞的比例,减少抑制性免疫细胞因子的分泌,增加具有抗肿瘤作用的效应淋巴细胞,从面增强抗肿瘤免疫应答[8]。
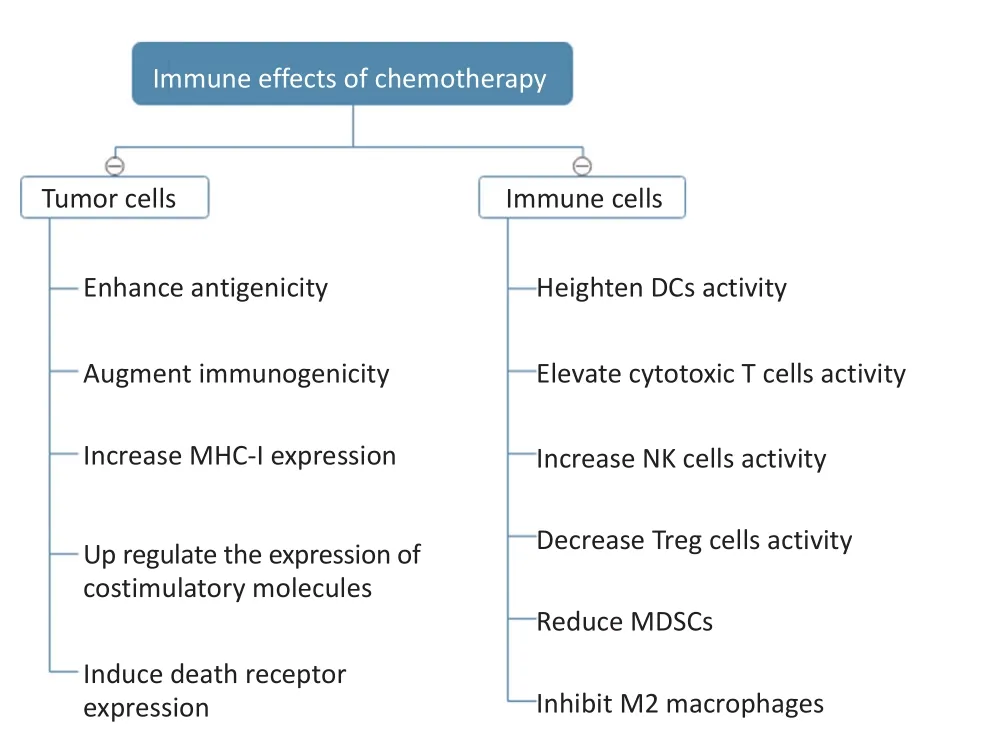
图1 化疗的免疫效应Figure 1 Immune effects of chemotherapy
1994年Matzinger提出任何细胞诱发免疫应答必须满足两个条件:免疫原性和佐剂的辅助[9]。其研究发现病毒感染宿主细胞后,病毒相关蛋白被呈递到细胞表面,与细胞表面主要组织相容性复合体(major histocompatibility complex,MHC)分子结合,细胞的免疫原性增加,但这不足以诱发有效的免疫应答;之后细胞裂解释放一系列可溶性的,或与膜结合的信号因子,最终诱导机体产生免疫反应。这些信号分子称为损伤相关分子模式(damage-associated molecular patterns,DAMPs),这些分子可以与髓系和淋系来源的免疫细胞(如巨噬细胞、自然杀伤细胞和树突细胞)表面的模式识别受体(pattern recognition recep⁃tors,PRRs)结合,继而激活免疫应答。
1.1 增加肿瘤细胞的抗原性
化疗药物诱导肿瘤细胞抗原性增强是通过不同机制实现的。基础研究表明吉西他滨杀伤肿瘤细胞可以增加抗原表位的种类,进而引发更强的免疫应答,这种现象称为抗原表位扩增[5]。而氟尿嘧啶和阿糖胞苷则通过增加肿瘤相关抗原(如CEA、睾丸抗原)表达数量,继而增加其抗原性[10-12]。
1.2 增加肿瘤细胞的免疫原性
既往观点认为细胞凋亡是自身程序性死亡,不会引发免疫反应。然而后续研究发现某些刺激因素诱导下的特殊类型的细胞凋亡会释放抗原分子和DAMPs,继而引发机体的免疫应答,这种凋亡称为ICD[13]。2005年Casares等[1]发现将体外经过多柔比星处理过的结直肠腺癌CT26细胞和纤维肉瘤MCA205细胞重新回输小鼠体内,可以使其产生相应抗体,再次将未经处理的相同肿瘤细胞输入小鼠体内后,肿瘤细胞无法增殖。此后诸多研究证实肿瘤细胞在化疗药物的作用下,会发生ICD。尽管ICD的形态学表现和生化代谢特征同细胞凋亡相似[14-15],但肿瘤细胞裂解后释放肿瘤抗原的同时,生成DAMPs信号分子,包括:钙网蛋白(calreticulin,CRT)、ATP、高迁移率族蛋白B1(high mobility group protein B1,HMGB1)、热休克蛋白70和90(heat shock protein,HSP70/90)等。同病毒感染后引发的免疫应答类似,上述DAMPs信号分子可以促进抗原提呈细胞(antigen-presenting cells,APCs)的摄取提呈抗原,进而激活固有和适应性免疫应答,清除肿瘤细胞[16]。
CRT是一种主要定位于细胞内质网上的伴侣蛋白,当内质网稳态破坏时,会转位到细胞膜,诱导巨噬细胞和树突状细胞吞噬凋亡细胞[17-19]。据研究报道蒽环类药物(如多柔比星、米托蒽醌等)、奥沙利铂、环磷酰胺可以诱导CRT在细胞膜表面表达,进而引发肿瘤细胞ICD[4,19]。蒽环类药物诱导CRT转位于细胞膜的机制包括:1)细胞因子如白介素-8(IL-8)的旁分泌作用;2)内质网应激时,真核细胞转录起始因子(eukaryotic initiation factor 2α,eIF2α)的磷酸化;3)细胞凋亡信号的激活[15,20]。
胞外ATP主要作用于purinergic受体,包括P2Y purinoceptor 2(P2RY2)和purinergic receptor P2X 7(P2RX7)受体。P2RY2受体介导的信号通路可以诱导前体DC细胞分化和募集,促进中性粒细胞浸润肿瘤组织[18,21];P2RX7受体则可介导IL-1b分泌,诱导细胞毒性T细胞的分化[22]。有研究发现奥沙利铂和米托蒽醌可以诱导小鼠结直肠癌肿瘤细胞自噬,并释放ATP,促使DC细胞和T细胞浸润[23]。
HMGB1则可激活PRRs,包括Toll样受体4(tolllike receptor 4,TLR4),继而增强树突状细胞的抗原提呈作用[24-25]。Yamazaki等[26]研究发现乳腺癌进展时肿瘤组织内HMGB分子表达缺失;Suzuki等[27]发现HMGB分子表达缺失与患者预后不佳相关。此外,研究发现编码P2RX7(ATP的受体)和TLR4(HMGB1的受体)基因功能缺失的乳腺癌患者,蒽环类药物治疗后疗效劣于基因正常患者[25];TLR4功能缺失的结直肠癌患者,接受含奥沙利铂方案治疗后预后较TLR4功能正常者预后差[4]。
1.3 化疗药物对肿瘤细胞的其他影响
此外,化疗药物还可以通过以下几种方式来增强免疫系统对肿瘤细胞的识别和杀伤。1)上调肿瘤细胞表面MHCⅠ类分子表达。研究表明依托泊苷、拓扑替康、长春新碱、紫杉醇还可以通过诱导肿瘤细胞分泌干扰素(interferon,IFN)-b,继而通过自分泌环的作用增加肿瘤细胞MHCⅠ分子表达[28];2)上调B7-1等共刺激分子表达[29-30],下调B7-H1/pro⁃grammed death-ligand 1(PD-L1)等负性共刺激分子表达[31];3)部分作用于细胞DNA结构的化疗药物可以诱导肿瘤细胞表达死亡受体,包括FAS受体(CD95)和TNF相关凋亡诱导配体的受体(TNF-relat⁃ed apoptosis-inducing ligand receptor,TRAIL-R)表达,介导免疫细胞分泌的FASL和TRAIL对肿瘤细胞的攻击作用[32-33]。此外,研究还表明紫杉醇、顺铂、多柔比星可以促进肿瘤细胞表面表达M6P受体(man⁃nose-6-phosphate receptor,M6PR),继而增加颗粒酶-B(granzyme-B)的细胞穿透力,增加杀伤性T细胞的功能[2]。
综上所述,化疗药物对肿瘤细胞的影响包括以下几种途径:1)增加肿瘤细胞表达的抗原种类和数量;2)增加肿瘤细胞释放DAMPs,诱导ICD;3)上调肿瘤细胞表面MHC分子、共刺激分子、死亡受体等表达,下调肿瘤细胞表面负性共刺激分子表达。
2 化疗药物对免疫系统的影响
诚然,细胞毒药物会导致粒系和淋巴系细胞减少,机体出现一过性的免疫抑制。但对于肿瘤,粒系和淋巴系的免疫细胞既有监视杀伤肿瘤的作用,亦有促进肿瘤免疫逃避的作用;而全身化疗后随着骨髓功能的恢复,免疫细胞亚群和功能发生改变,可打破机体对肿瘤的免疫耐受[34]。临床研究中亦观察到化疗联合树突状细胞疫苗或免疫检查点抑制剂治疗结直肠癌、黑色素瘤、非小细胞肺癌具有协同作用[35-37]。
尽管体外研究发现化疗药物,如紫杉醇可以通过抑制微管蛋白的解聚,直接促进单核细胞分泌细胞因子IL-1β和TNF-α,并且呈剂量依赖性[38-39]。但需要强调的是因为化疗可以诱导肿瘤细胞发生ICD,增加其抗原性和免疫原性,激活固有免疫应答和适应性免疫应答,所以化疗药物对免疫系统各细胞亚群的影响包括:1)化疗药物本身对髓系和淋巴系来源免疫细胞的直接影响;2)化疗诱导肿瘤细胞ICD,继而增强机体免疫应答,间接改变各种免疫细胞亚群的比例。
如前所述,参与肿瘤免疫微环境的细胞主要包括来源于淋系和髓系的免疫细胞。淋系来源的免疫细胞包括CD4和CD8阳性的T细胞、NK细胞、部分DC细胞。其中T淋巴细胞激活后分化为CD4和CD8阳性T淋巴细胞,在不同细胞因子调控下,CD4阳性的T淋巴细胞又分化为Th1、Th2、Th17、调节性T细胞(regulatory T cell,Treg)等不同亚型,在机体抗肿瘤免疫、肿瘤免疫逃避中发挥不同作用,Th1淋巴细胞主要介导细胞免疫,Th2淋巴细胞主要介导体液免疫,Treg细胞主要抑制抗肿瘤免疫应答,Th17细胞则具有双向调节作用。淋巴细胞亚群的比例不同,直接影响了最终肿瘤免疫应答的强度。髓系来源的免疫细胞包括单核细胞、巨噬细胞、中性粒细胞、部分DC细胞。近年来,针对肿瘤相关的髓系细胞(tumor-as⁃sociated myeloid cells,TAMCs)在抗肿瘤免疫应答中的调节作用有较为深入的研究,其主要包括:1)肿瘤相关的巨噬细胞(tumor associated macrophage,TAM),其在肿瘤组织内分化为M1型,分泌一氧化氮(NO)和活性氧(ROS)等,攻击杀伤肿瘤细胞;还分化为M2型,分泌促进肿瘤增殖转移细胞因子[40];2)髓源性抑制细胞(myeloid-derived suppressor cells,MD⁃SCs),抑制肿瘤组织内T细胞功能,促进肿瘤免疫逃避[41];3)肿瘤相关的中性粒细胞(tumor-associated neutrophils,TANs),其在肿瘤组织内亦分化为N1型(抑制肿瘤)和N2型(促进肿瘤)[42];4)肿瘤相关的树突状细胞(tumor associated dendritic cells,TADCs),主要表现为不成熟表型(immature phenotype,iDC),促进肿瘤免疫逃避[43]。
化疗对抗肿瘤免疫应答的激活作用主要体现在以下两个方面:1)正性调节识别杀伤肿瘤的免疫细胞,包括增加抗原提呈细胞(如树突状细胞)、杀伤性T细胞、NK细胞比例或数量,恢复其功能;2)负性调节抑制肿瘤免疫反应的免疫细胞,包括减少M2型巨噬细胞、MDSCs、Treg细胞的数量或比例。
2.1 对T细胞的影响
Treg细胞分泌免疫抑制因子,在肿瘤免疫逃避中发挥重要作用。一系列动物研究发现化疗药物如环磷酰胺、紫杉醇、奥沙利铂、三氧化二砷、替莫唑胺可以减少Treg细胞数量或比例[44-45],体外研究亦观察到环磷酰胺和奥沙利铂可以直接抑制Treg细胞的分化[46]。临床研究中亦发现蒽环类药物、紫杉醇、氟达拉滨可以使乳腺癌、淋巴瘤等肿瘤患者Treg细胞数量或比例下降,同时可伴有CD8阳性杀伤性T细胞增多,以及NK细胞活性升高,这种反应与疗效相关[47-48]。但是需要强调的是,化疗药物的给药剂量、周期对于发挥其最佳的免疫调节作用非常重要,临床上观察到对于肿瘤患者环磷酰胺节拍化疗可以减少Treg细胞数量,恢复杀伤性T细胞和NK细胞活性[45,49]。
此外,体内体外研究发现环磷酰胺可以诱导Th17 T细胞的分化,外周血循环和肿瘤组织内Th17 T细胞明显增加[50]。在乳腺癌中亦观察到紫杉类药物治疗有效的患者中,治疗后血清中IFN-γ和IL-2分泌明显增加,促进T细胞向Th1表型分化[3]。
2.2 对其他参与肿瘤免疫应答细胞的影响
研究表明化疗药物多柔比星、甲氨蝶呤、丝裂霉素、紫杉醇可以上调树突状细胞表面MHCⅡ分子和共刺激分子CD80/CD86的表达,提高其抗原提呈能力[51]。胰腺癌患者在接受吉西他滨治疗后CD14+单核细胞和CD11c+树突状细胞数量增加[52]。
此外,研究发现MDSCs和M2巨噬细胞抑制免疫应答,分泌促进肿瘤生长的细胞因子[53]。多种化疗药物可以减少MDSCs和M2巨噬细胞的数量,抑制其功能,促使肿瘤免疫微环境的免疫监视和清除作用。目前发现紫杉类药物、吉西他滨、5-氟尿嘧啶(5-FU)等可以减少带瘤小鼠体内MDSCs数量,从而减少免疫抑制性细胞因子的分泌,增强杀伤性T细胞和NK细胞功能[54-56]。然而在小鼠无瘤模型中,给予环磷酰胺后外周血中MDSC数量增加[57]。在接受环磷酰胺联合多柔比星治疗的患者中,亦观察到MDSC数量增加[58],这提示不同化疗药物对MDSC的影响可能不同,亟需进一步深入的研究。
3 化疗联合免疫治疗的临床研究
目前临床研究中,与化疗联合应用的免疫治疗方法主要包括:过继细胞治疗、肿瘤疫苗、免疫检查点抑制剂等。化疗的给药剂量、周期亦不尽相同,初步可以分为免疫治疗联合标准剂量化疗、高剂量化疗、和低剂量化疗。
3.1 标准剂量化疗联合免疫治疗
标准剂量的化疗可以导致骨髓抑制,淋巴细胞减少;此外部分化疗药物前的预处理还会应用糖皮质激素,这些都会引起机体的免疫抑制状态。但标准剂量化疗药物同样可以诱导肿瘤细胞发生ICD,增强肿瘤免疫原性,增强免疫治疗效果。
早期临床研究发现标准剂量的化疗药物可以抑制机体的免疫应答,一项给予表达CEA的实体瘤患者疫苗治疗的研究中发现,疫苗治疗前接受的化疗周期数越多或距离末次化疗结束时间越近,疫苗诱导产生的针对CEA的T细胞数量越少[59];在另一项关于表达间皮素并分泌GM-CSF的Ⅱ/Ⅲ期胰腺癌细胞疫苗的临床研究中,给予入组患者疫苗治疗,术后放化疗后予以疫苗治疗3次后产生的特异性T细胞数量相当于放化疗前给药一次时的数量,说明放化疗后患者存在免疫抑制状态[60]。但后续越来越多的临床研究提示标准剂量化疗联合免疫治疗的疗效优于单用化疗或免疫治疗。一项纳入36例Ⅱ~Ⅳ期黑色素瘤的临床研究,一组患者第1日给予800mg/m2达卡巴嗪,后给予melan-A/MART-1/gp-100多肽疫苗;另一组患者单纯予多肽疫苗治疗,化疗联合免疫治疗与单独免疫治疗相比,可以增加免疫应答相关基因的表达、增加体内特异性T细胞数量、促进记忆性T细胞应答,患者生存期有延长的趋势[61]。此外亦有数项研究探索了标准剂量化疗联合免疫检查点抑制剂。一项转移性黑色素瘤的研究发现达卡巴嗪联合伊匹单抗治疗可以延长总生存期(overall survival,OS)[62]。在另外两项关于非小细胞肺癌和小细胞肺癌患者的随机对照临床研究中,标准剂量卡铂加用紫杉醇化疗后序贯应用伊匹单抗较化疗同时给予伊匹单抗,可以延长免疫相关的PFS(immune-related progression-free survival,irPFS)[37,63]。一项关于转移性乳腺癌的研究中,标准剂量紫杉醇化疗后给予IMP321(LAG-3抑制剂),与既往数据比较,提高了有效率;此外患者外周血中NK细胞和效应记忆CD8 T细胞明显增多[64]。
综上所述,多数研究支持标准剂量化疗序贯免疫治疗可以起到协同作用,但值得注意的是亦有部分研究首先给予免疫治疗后序贯化疗,可以提高化疗疗效。
3.2 高剂量化疗联合免疫治疗
目前临床研究中较少应用高剂量化疗药物联合免疫治疗。一项研究在白血病患者接受自体造血干细胞移植复发后,给予针对次要组织相容性抗原的过继T细胞治疗,7例患者中有5例获得完全缓解(complete remission,CR),但后期再次复发[65]。另外2项研究是高剂量化疗联合自体干细胞移植后给予分泌GM-CSF自体失活肿瘤疫苗,疫苗治疗后可以成功诱导出机体免疫反应,患者长期获得CR[66-67]。还有一项针对晚期黑色素瘤患者的研究,给予大剂量环磷酰胺+氟达拉滨化疗联合全身放疗(total body irra⁃diation,TBI),彻底清除患者体内的免疫细胞后将体外扩增的肿瘤浸润淋巴细胞TIL回输。这种方法可以最大程度促进回输的TIL增殖,而且早期研究显示放化疗后淋巴细胞减少比例越大,TIL回输后治疗效果越好;该研究结果显示,化疗+TIL治疗组的43例黑色素瘤患者有效率为49%,化疗+2 Gy TBI+过继治疗组的25例患者有效率为52%,化疗+12 Gy TBI+过继治疗组的25例患者有效率为72%[68]。
3.3 低剂量化疗联合免疫治疗
低剂量化疗对机体主要产生免疫调节作用,而不是对肿瘤细胞的直接杀伤作用。早期多项研究证实低剂量环磷酰胺可以减少抑制性T细胞,解除免疫抑制;后续在胰腺癌、非小细胞肺癌、乳腺癌、卵巢癌、肾癌等实体瘤研究中发现予环磷酰胺250 mg/m2或300 mg/m2治疗后序贯免疫治疗,相对于直接单纯免疫治疗组,可以提高机体免疫应答,增强免疫治疗效果,带来生存获益[69-70]。
4 总结与展望
综上所述,化疗联合免疫治疗具有广泛应用前景,但是临床上仍有一系列问题亟需解决。1)评估控制化疗联合免疫治疗的不良反应;2)如何设计最优的化疗剂量(大剂量、中等计量、小剂量)和化疗给药顺序(免疫治疗前/中/后、间断大剂量、持续小剂量等),以期最大程度发挥化疗的免疫激活作用;3)因为完整的免疫应答涉及到抗原释放、摄取、提呈,T细胞激活、分化、迁移、浸润,识别和杀伤肿瘤细胞多个环节,所以免疫治疗没有找到明确的单一疗效预测分子,如何筛选合适的患者接受化疗联合免疫治疗也是临床医生面临的重要问题。
[1] Casares N,Pequignot MO,Tesniere A,et al.Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death[J].J Exp Med,2005,202(12):1691-1701.
[2] Ramakrishnan R,Assudani D,Nagaraj S,et al.Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice[J].J Clin Invest,2010,120(4):1111-1124.
[3] Tsavaris N,Kosmas C,Vadiaka M,et al.Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes[J].Br J Cancer,2002,87(1):21-27.
[4] Tesniere A,Schlemmer F,Boige V,et al.Immunogenic death of colon cancer cells treated with oxaliplatin[J].Oncogene,2010,29(4): 482-491.
[5] Jackaman C,Majewski D,Fox SA,et al.Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+)T cells in vivo [J].Cancer Immunol Immunother,2012,61(12):2343-2356.
[6] Martins I,Kepp O,Schlemmer F,et al.Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress[J].Oncogene,2011,30(10):1147-1158.
[7] Aranda F,Bloy N,Pesquet J,et al.Immune-dependent antineoplastic effects of cisplatin plus pyridoxine in non-small-cell lung cancer [J].Oncogene,2015,34(23):3053-3062.
[8] Bracci L,Schiavoni G,Sistigu A,et al.Immune-based mechanisms of cytotoxic chemotherapy:implications for the design of novel and rationale-based combined treatments against cancer[J].Cell Death Differ,2014,21(1):15-25.
[9] Matzinger P.Tolerance,danger,and the extended family[J].Annu Rev Immunol,1994,(12):991-1045.
[10]Correale P,Aquino A,Giuliani A,et al.Treatment of colon and breast carcinoma cells with 5-fluorouracil enhances expression of carcinoembryonic antigen and susceptibility to HLA-A(*)02.01 restricted,CEA-peptide-specific cytotoxic T cells in vitro[J].Int J Cancer,2003,104(4):437-445.
[11]Adair SJ,Hogan KT.Treatment of ovarian cancer cell lines with 5-aza-2'-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules[J].Cancer Immunol Immunother,2009,58(4):589-601.
[12]Fonsatti E,Nicolay HJ,Sigalotti L,et al.Functional up-regulation of human leukocyte antigen classⅠantigens expression by 5-aza-2'-deoxycytidine in cutaneous melanoma:immunotherapeutic implications[J].Clin Cancer Res,2007,13(11):3333-3338.
[13]Kroemer G,Galluzzi L,Kepp O,et al.Immunogenic cell death in cancer therapy[J].Annu Rev Immunol,2013,(31):51-72.
[14]Kroemer G,Galluzzi L,Vandenabeele P,et al.Classification of cell death:recommendations of the nomenclature committee on cell death 2009[J].Cell Death Differ,2009,16(1):3-11.
[15]Panaretakis T,Kepp O,Brockmeier U,et al.Mechanisms of preapoptotic calreticulin exposure in immunogenic cell death[J].EMBO J,2009,28(5):578-590.
[16]Krysko DV,Garg AD,Kaczmarek A,et al.Immunogenic cell death and DAMPs in cancer therapy[J].Nat Rev Cancer,2012,12(12):860-875.
[17]Gardai SJ,McPhillips KA,Frasch SC,et al.Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte[J].Cell,2005,123(2):321-334.
[18]Ma Y,Adjemian S,Mattarollo SR,et al.Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells[J].Immunity,2013,38(4):729-741.
[19]Obeid M,Tesniere A,Ghiringhelli F,et al.Calreticulin exposure dictates the immunogenicity of cancer cell death[J].Nat Med,2007, 13(1):54-61.
[20]Sukkurwala AQ,Martins I,Wang Y,et al.Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8[J].Cell Death Differ,2014,21 (1):59-68.
[21]Elliott MR,Chekeni FB,Trampont PC,et al.Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance[J].Nature,2009,461(7261):282-286.
[22]Ghiringhelli F,Apetoh L,Tesniere A,et al.Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors[J].Nat Med,2009,15(10):1170-1178.
[23]Michaud M,Martins I,Sukkurwala AQ,et al.Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice[J].Science,2011,334(6062):1573-1577.
[24]Bianchi ME.HMGB1 loves company[J].J Leukoc Biol,2009,86(3): 573-576.
[25]Apetoh L,Ghiringhelli F,Tesniere A,et al.Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy[J].Nat Med,2007,13(9):1050-1059.
[26]Yamazaki T,Hannani D,Poirier-Colame V,et al.Defective immunogenic cell death of HMGB1-deficient tumors:compensatory therapy with TLR4 agonists[J].Cell Death Differ,2014,21(1):69-78.
[27]Suzuki Y,Mimura K,Yoshimoto Y,et al.Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma[J].Cancer Res,2012,72(16):3967-3976.
[28]Wan S,Pestka S,Jubin RG,et al.Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells[J].PLoS One,2012,7(3):e32542.
[29]Sojka DK,Donepudi M,Bluestone JA,et al.Melphalan and other anticancer modalities up-regulate B7-1 gene expression in tumor cells [J].J Immunol,2000,164(12):6230-6236.
[30]Donepudi M,Raychaudhuri P,Bluestone JA,et al.Mechanism of melphalan-induced B7-1 gene expression in P815 tumor cells[J].J Immunol,2001,166(11):6491-6499.
[31]Ghebeh H,Lehe C,Barhoush E,et al.Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells:role of B7-H1 as an anti-apoptotic molecule[J].Breast Cancer Res,2010,12(4):R48.
[32]Ashkenazi A,Dixit VM.Death receptors:signaling and modulation [J].Science,1998,281(5381):1305-1308.
[33]Hellwig CT,Rehm M.TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies[J].Mol Cancer Ther,2012,11 (1):3-13.
[34]Finn OJ.Immuno-oncology:understanding the function and dysfunction of the immune system in cancer[J].Ann Oncol,2012,23 (Suppl 8):i6-9.
[35]Correale P,Cusi MG,Del VM,et al.Dendritic cell-mediated crosspresentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin,5-fluorouracil,and leucovorin,elicits a powerful human antigen-specific CTL response with antitumor activity in vitro[J].J Immunol,2005,175(2):820-828.
[36]Lesterhuis WJ,de Vries IJ,Aarntzen EA,et al.A pilot study on the immunogenicity of dendritic cell vaccination during adjuvant oxaliplatin/capecitabine chemotherapy in colon cancer patients[J].Br J Cancer,2010,103(9):1415-1421.
[37]Lynch TJ,Bondarenko I,Luft A,et al.Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stageⅢB/Ⅳnon-small-cell lung cancer:results from a randomized,doubleblind,multicenter phaseⅡstudy[J].J Clin Oncol,2012,30(17):2046-2054.
[38]Bogdan C,Ding A.Taxol,a microtubule-stabilizing antineoplastic agent,induces expression of tumor necrosis factor alpha and interleukin-1 in macrophages[J].J Leukoc Biol,1992,52(1):119-121.
[39]Oda K,Ikehara Y.Taxol,a potent promoter of microtubule assembly, inhibits secretion of plasma proteins in cultured rat hepatocytes[J]. Biochem Biophys Res Commun,1982,107(2):561-567.
[40]Mantovani A,Locati M.Tumor-associated macrophages as a paradigm of macrophage plasticity,diversity,and polarization:lessons and open questions[J].Arterioscler Thromb Vasc Biol,2013,33(7): 1478-1483.
[41]Gabrilovich DI,Ostrand-Rosenberg S,Bronte V.Coordinated regulation of myeloid cells by tumours[J].Nat Rev Immunol,2012,12(4): 253-268.
[42]Fridlender ZG,Albelda SM.Tumor-associated neutrophils:friend or foe[J]?Carcinogenesis,2012,33(5):949-955.
[43]Kusmartsev S,Gabrilovich DI.Role of immature myeloid cells in mechanisms of immune evasion in cancer[J].Cancer Immunol Immunother,2006,55(3):237-245.
[44]Gonzalez-Aparicio M,Alzuguren P,Mauleon I,et al.Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice[J].Gut,2011,60(3): 341-349.
[45]Le DT,Jaffee EM.Regulatory T-cell modulation using cyclophosphamide in vaccine approaches:a current perspective[J].Cancer Res, 2012,72(14):3439-3444.
[46]Kan S,Hazama S,Maeda K,et al.Suppressive effects of cyclophosphamide and gemcitabine on regulatory T-cell induction in vitro[J]. Anticancer Res,2012,32(12):5363-5369.
[47]Ladoire S,Mignot G,Dabakuyo S,et al.In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival [J].J Pathol,2011,224(3):389-400.
[48]Senovilla L,Vitale I,Martins I,et al.An anticancer therapy-elicited immunosurveillance system that eliminates tetraploid cells[J].Oncoimmun,2013,2(1):e22409.
[49]Ghiringhelli F,Menard C,Puig PE,et al.Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+regulatory T cells and restores T and NK effector functions in end stage cancer patients[J].Cancer Immunol Immunother,2007,56(5):641-648.
[50]Viaud S,Flament C,Zoubir M,et al.Cyclophosphamide induces differentiation of Th17 cells in cancer patients[J].Cancer Res,2011,71 (3):661-665.
[51]Shurin GV,Tourkova IL,Kaneno R,et al.Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism[J].J Immunol, 2009,183(1):137-144.
[52]Soeda A,Morita-Hoshi Y,Makiyama H,et al.Regular dose of gemcitabine induces an increase in CD14+monocytes and CD11c+dendritic cells in patients with advanced pancreatic cancer[J].Jpn J Clin Oncol,2009,39(12):797-806.
[53]Coussens LM,Zitvogel L,Palucka AK.Neutralizing tumor-promoting chronic inflammation:a magic bullet[J]?Science,2013,339(6117): 286-291.
[54]Suzuki E,Kapoor V,Jassar AS,et al.Gemcitabine selectively eliminates splenic Gr-1+/CD11b+myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity[J].Clin Cancer Res,2005,11(18):6713-6721.
[55]Kodumudi KN,Woan K,Gilvary DL,et al.A novel chemoimmunomodulating property of docetaxel:suppression of myeloid-derived suppressor cells in tumor bearers[J].Clin Cancer Res,2010,16(18): 4583-4594.
[56]Vincent J,Mignot G,Chalmin F,et al.5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity[J].Cancer Res,2010, 70(8):3052-3061.
[57]Salem ML,Al-Khami AA,El-Naggar SA,et al.Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells[J].J Immunol,2010,184(4):1737-1747.
[58]Diaz-Montero CM,Salem ML,Nishimura MI,et al.Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage,metastatic tumor burden,and doxorubicin-cyclophosphamide chemotherapy[J].Cancer Immunol Immunother,2009,58 (1):49-59.
[59]von Mehren M,Arlen P,Gulley J,et al.The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine(ALVAC-CEA B7.1)in patients with metastatic carcinoma[J].Clin Cancer Res,2001,7(5):1181-1191.
[60]Lutz E,Yeo CJ,Lillemoe KD,et al.A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma.a PhaseⅡtrial of safety,efficacy,and immune activation[J].Ann Surg,2011,253(2):328-335.
[61]Nistico P,Capone I,Palermo B,et al.Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients[J].Int J Cancer,2009,124(1):130-139.
[62]Robert C,Thomas L,Bondarenko I,et al.Ipilimumab plus dacarbazine for previously untreated metastatic melanoma[J].N Engl J Med,2011,364(26):2517-2526.
[63]Reck M,Bondarenko I,Luft A,et al.Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-diseasesmall-cell lung cancer:results from a randomized,double-blind, multicenter phase 2 trial[J].Ann Oncol,2013,24(1):75-83.
[64]Brignone C,Gutierrez M,Mefti F,et al.First-line chemoimmunotherapy in metastatic breast carcinoma:combination of paclitaxel and IMP321(LAG-3Ig)enhances immune responses and antitumor activity[J].J Transl Med,2010,8(1):71.
[65]Warren EH,Fujii N,Akatsuka Y,et al.Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens[J].Blood,2010,115(19): 3869-3878.
[66]Ho VT,Vanneman M,Kim H,et al.Biologic activity of irradiated,autologous,GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation[J].Proc Natl Acad Sci U S A,2009, 106(37):15825-15830.
[67]Borrello IM,Levitsky HI,Stock W,et al.Granulocyte-macrophage colony-stimulating factor(GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation(ASCT)as postremission therapy for acute myeloid leukemia(AML)[J]. Blood,2009,114(9):1736-1745.
[68]Dudley ME,Yang JC,Sherry R,et al.Adoptive cell therapy for patients with metastatic melanoma:evaluation of intensive myeloablative chemoradiation preparative regimens[J].J Clin Oncol,2008, 26(32):5233-5239.
[69]Chu CS,Boyer J,Schullery DS,et al.PhaseⅠ/Ⅱrandomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission[J].Cancer Immunol Immunother,2012,61(5):629-641.
[70]Walter S,Weinschenk T,Stenzl A,et al.Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival[J].Nat Med,2012,18 (8):1254-1261.

(2017-02-15收稿)
(2017-04-05修回)
(编辑:孙喜佳 校对:杨红欣)
Combining chemotherapy with immunotherapy:from bench to bedside
Xiaoyan LIU,Minjiang CHEN,Mengzhao WANG
Mengzhao WANG;E-mail:mengzhaowang@sina.com Department of Pulmonary Medicine,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences,Peking Union Medical College,Beijing 100730,China
Chemotherapy has been the cornerstone of cancer treatment for decades.However,albeit remarkable,the response to chemotherapy is generally short-lived.With deepened understanding of cancer immunology,resurgence was recently witnessed in the field of cancer immunotherapy.Unlike chemotherapy,immunotherapy induces a relatively mild but stable response.Accumulating evidence reveals that apart from chemotherapy's direct cytotoxic activity,chemotherapy exerts immune-potentiating effects by increasing the immunogenicity of tumor cells or by disrupting the tumor-induced immunosuppression and altering the immune microenvironment.The latter mechanism serves as the basis for the combined use of chemotherapy and immunotherapy.In this review,we examine the rationale for combinatorial therapy in accordance with the current understanding on tumor immunity and newly discovered immune-based chemotherapeutic mechanisms.We further discuss the available preclinical and clinical studies on the combination of chemotherapy and immunotherapy for cancer treatment.We aim to provide a framework for further research.
cancer,chemotherapy,immunotherapy,synergistic activity
10.3969/j.issn.1000-8179.2017.09.177
中国医学科学院,北京协和医学院,北京协和医院呼吸科(北京市100730)
王孟昭 mengzhaowang@sina.com
刘潇衍 专业方向为呼吸系统肿瘤的诊断及治疗。
E-mail:dr.liu-pumc@qq.com

