Effect of carboxymethyl cellulose on dissolution kinetics of carboxymethyl cellulose-sodium carbonate two-component tablet
Changdong Li,Carlos Amador ,Yulong Ding *
1 Institute of Particle Science and Engineering,University of Leeds,Leeds,UK
2 School of Engineering,University of Warwick.,Coventry.CV4 7AL,UK
3 Procter&Gamble Newcastle-Upon-Tyne Innovation Centre,Newcastle,UK
4 School of Chemical Engineering,University of Birmingham,Birmingham,UK
1.Introduction
As one kind of popular cellulose derivative,carboxymethyl cellulose has been widely used into industries over 20 years.The molecular formula of carboxymethyl cellulose can be written as C6H7O2(OR1)(OR2)(OR3)where R1/R2/R3is-H5/-CH2COONa/-CH2COOH respectively[1].The applications on carboxymethyl cellulose is normally involved in the industry of food,pharmaceutical and household products for example carboxymethyl cellulose is used as the surfactant in detergent to form micelles and minimize the redisposition of soil[2].Meanwhile,the function of carboxymethyl cellulose is investigated and discussed through previous research.Li,Lewis and Dahn[3]implemented carboxymethyl cellulose as a potential binder in Li-ion battery and the results showed a better performance in cycling than conventional battery.He and Zhao[4]applied carboxymethyl cellulose as the stabilizer to manipulate the size and dispersibility of zero-valent iron nanoparticles.These nanoparticles showed more effective utility into soil and groundwater.Rossiet al.[5]presented carboxymethyl cellulose containing a better performance on drug release and wash ability of gels than polyacrylic acid.Although carboxymethyl cellulose has been widely implemented it is rarely investigated of its effecton dissolution properties ofmulti-compound products including its effects on dissolution mechanism and kinetics which is also scarcely quantified effectively.In this way,this paper is highly motivated to study its effect of carboxymethyl cellulose on dissolution mechanism,quantify its effect on dissolution kinetics and discuss its dissolution properties based on the dissolution experiments of two-component tablet.The study in thispaperalso provides constructive information to compensate for the lack of data-supported understanding of industrial preparation of dissolving-related products containing carboxymethyl cellulose.
The conventional studies on dissolution kinetics of multi-component products are normally achieved by the approach of either multi-particle[6]or single-particle[7]method.The dissolution process is usually carried out with a dissolution apparatus and measured with converted conductivity or refractometry values[8].Based on measured dissolution process,the dissolution rate constant is quantified by fitting the solute concentration with mathematical models.The mathematical models,such as Noyes-Whitney Equation[9]and Hixson-CrowellModel[10],are basically developed from different dissolution models including diffusion layer model,surface reaction model and inter-facial barrier model which also suggest the mechanism of dissolution process.Pillay and Fassihi[11]reviewed various dissolution theories and methodologies on drug release and introduced dissolution models briefly based on different dissolving conditions.Dash et al.[12]also compared different theoretical and semi-empirical models for dissolution kinetics quantifications.
In this paper,the electrical conductivity tracking method is implemented into the measurement of dissolution process of both kinds of tablets.Both diffusion layer model and surface reaction model are used into the quantification of dissolution kinetics of tablets tested in this work.The quantifications with both dissolution models will also evaluate the appropriate dissolution mechanism and obtain the accurate dissolution kinetics of the tablets.In this way,the effect of carboxymethyl cellulose on dissolution kinetics of tablets is properly investigated and discussed with experimental evidence of dissolution mechanism and the rate constant.Furthermore,the work in this paper is believed to provide constructive information on determining the dissolution kinetics of multi-component tablets in the future works.
2.Preparation of Tablet
In this works,sodium carbonate and carboxymethyl cellulose powders were used to prepare the experimental tablets including the reference pure sodium carbonate tablets and sodium carbonatecarboxymethyl cellulose two-component tablets.Both material powders were well mixed with the mass ratio of 97%Na2CO3:3%Carboxymethyl cellulose,95%Na2CO3:5%Carboxymethyl cellulose and 93%Na2CO3:7%Carboxymethyl cellulose respectively.Here it is worth noting that every 0.3 g mixed powders were then compressed into tablets with a D10.0mmtablet module and manual Baileigh Industrial Compression Machine at 20 kN.Therefore,all prepared tablets used in this work had the same size of10.0 mm(Diameter)×3 mm(Height).For a convenient recording,the tablets containing 3%Carboxymethyl cellulose were coded as 0.03CMC tablet,containing 5%Carboxymethyl cellulose were coded as 0.05CMC tablet and containing 7%Carboxymethyl cellulose were coded as 0.07CMC tablet.The pure sodium carbonate tablets would be used as the reference tablets to evaluate the effects of carboxymethyl cellulose on dissolution kinetics.A graphic example of prepared tablet is shown in Fig.1.
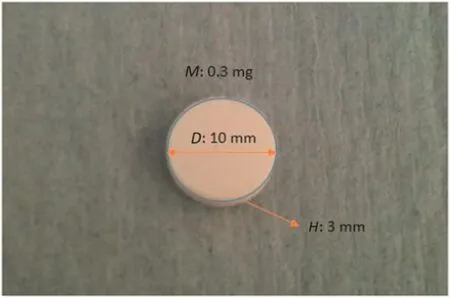
Fig.1.Graphic example of prepared tablet used for dissolution test.
3.Experimental Methodology
For a typical dissolution experiment in this work,one tablet dissolved in 100 mldeionized water stirred at300 RPM.Acopperwire weaved basket was hanging on the dissolution beaker and the tablet was placed into the basket.The position of the basket was fixed and the whole tablet was immersed in the deionized water during the whole dissolution process.On the top of the basket,a probe of Jenway Conductivity TDS Portable Meter was placed vertically.The distance from the probe sensor to the bottom of basket was fixed and the probe sensor was also con firmed to be immersed in the deionized water.In this way,the value of electrical conductivity was collected every 10 s for each dissolution experiment and then used for further analysis.An illustration of operation scheme of one typical dissolution test is shown in Fig.2.
For both two-component and pure sodium carbonate tablets,the dissolution experiments were carried out at 20 °C,30 °C,40 °C,50 °C and 60°C respectively.The dissolution beaker was placed on and controlled by Bibby Scientific's Ceramic Stirrer Digital Hot-plate.And,the stirring speed could also be set at 300 r·min-1by the magnetic stirring bar which was also placed in the beaker.The beaker was also covered by the sealing film to minimize the influence of water evaporation on the measurement of conductivity.
4.Mathematical Model
In this work,both surface reaction model and diffusion layer model[13]were used for the fitting of quantified dissolution data.For surface reaction model,the dissolution process was determined by the chemical reaction rate on the surface of tablet which is the decomposition speed of sodium carbonate into sodium and carbonate ions in this case.The mathematical model representing surface reaction model could be expressed as Eq.(1).

wherekris the dissolution rate constant of surface reaction model,andfis the fraction of dissolved reactant.
For diffusion layer model,the limiting step of dissolution process was the diffusion rate of dissolved reactant from the surface of tablet through diffusion layer into the solvent.The mathematical model representing diffusion layer theory could be shown as Eq.(2).

wherekdis the dissolution rate constant of diffusion layer model.
It was worth noting that the selectivity of dissolution model greatly determined the quantification method for dissolution kinetics and the understanding of dissolution process and mechanism of the tablets.Therefore,both surface reaction and diffusion layer model would be applied into the fittings with pre-quantified experimental dissolution data of each case.In this way,the mathematical model presenting higher squared correlation factor would be suggested to interpret the dissolution mechanism of dissolved tablets used in this work and also used for further quantification of dissolution kinetics.
5.Results and Discussions
With electrical conductivity tracking method,the electrical conductivity of each pure sodium carbonate,0.03CMC,0.05CMC and 0.07CMC tablet is recorded separately at 20-60°C respectively.Based on measured conductivity values,the concentration of dissolved sodium carbonate from each tested tablet should be converted and the fraction of dissolved tablet is then calculated.To convert measured conductivity to concentration,a series of calibration measurement are carried out to determine the conversion relationship between conductivity with amount of dissolved sodium carbonate.Specifically speaking,the value of electrical conductivity depends on the current amount of free sodium ions and carbonate ions in the solution.Therefore,when a certain amount of sodium carbonate dissolves completely in 100 mldeionized water,the conductivity of this dissolved solution should obtain a specific value.In this way,a series of sodium carbonate with specific amount,0.05 g/0.10 g/0.15 g/0.20 g/0.25 g/0.30 g,are dissolved in 100 ml deionized water respectively for 1 h and then the dissolving solution is kept stationary for another 1 h.The conductivity of each solution is measured 3 times and the values of measured conductivity are plotted against the amount of dissolved sodium carbonate which is shown in Fig.3 to determine the relationship equation.
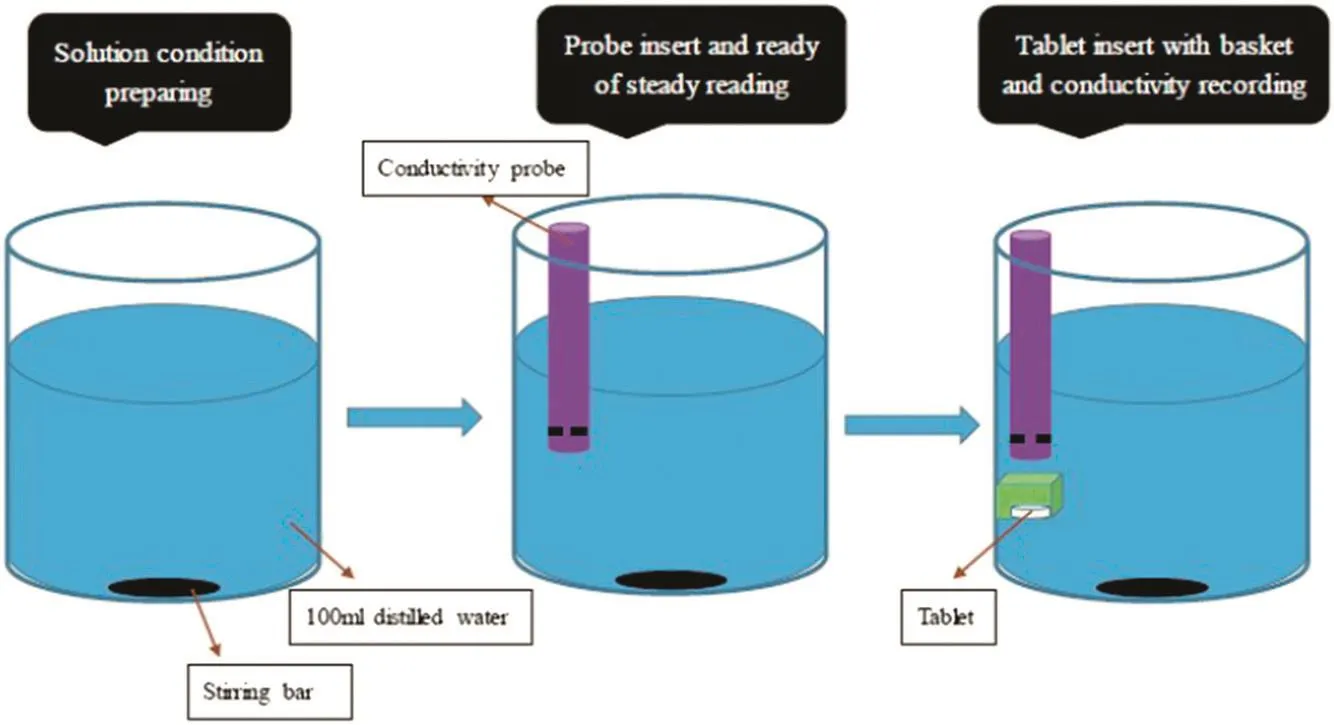
Fig.2.Operation scheme of one typical dissolution test.
According to the data shown in Fig.3,it is clearly shown a linear relationship between conductivity with mass of dissolved sodium carbonate.Therefore,this relationship can be presented as Eq.(3).
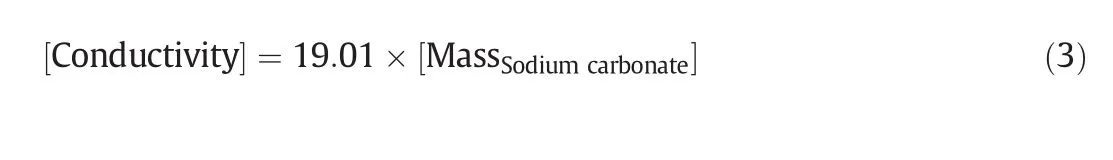
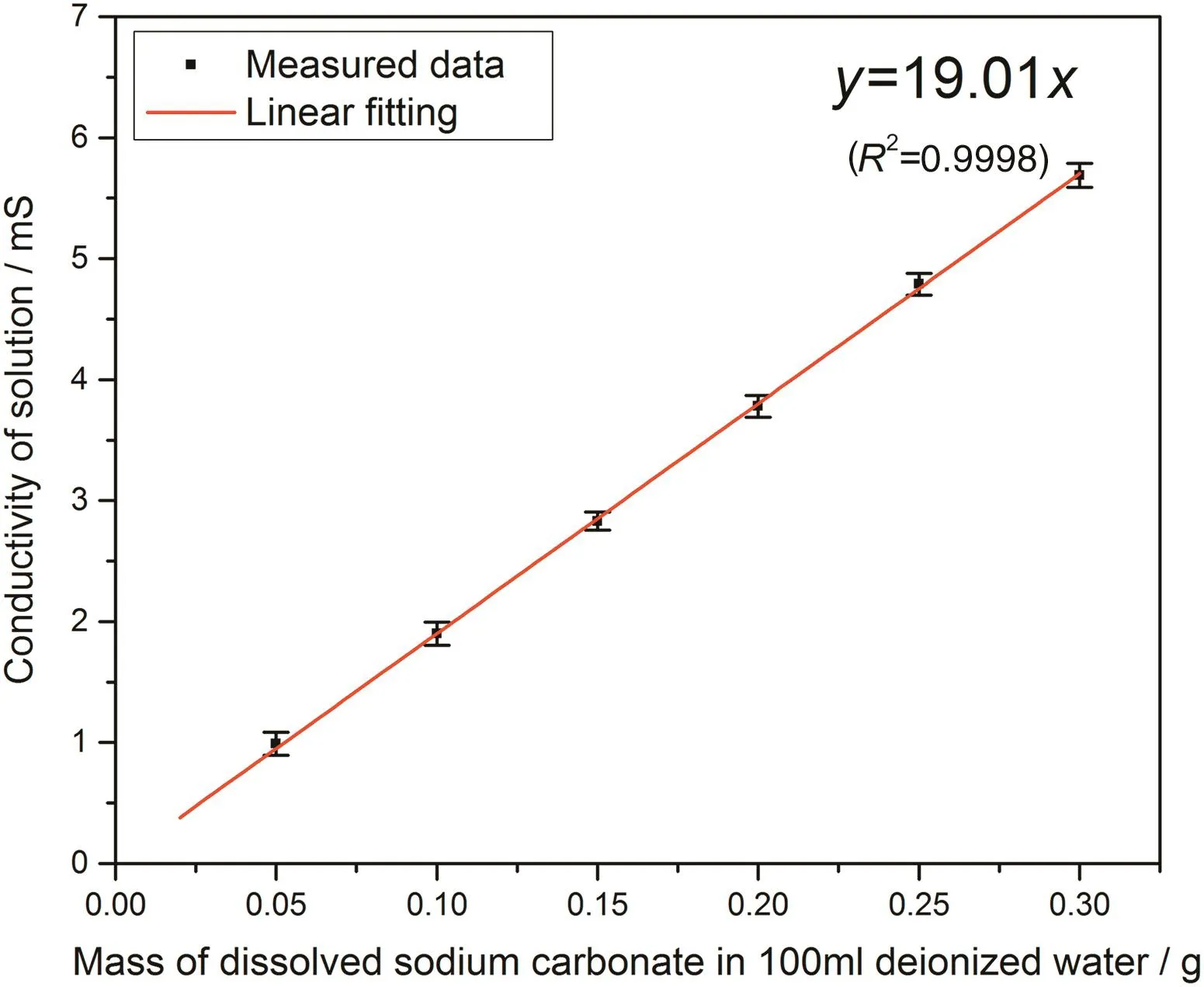
Fig.3.Linear relationship of conductivity with mass of dissolved sodium carbonate.
For a specific tested tablet,the content of sodium carbonate is known as a certain value.Therefore,the change of the concentration of sodium carbonate in water only comes from the dissolution of the tablet to release the ions.Because it is believed that sodium carbonate is well equally distributed in the tablet,the fraction of dissolved sodium carbonate over the whole sodium carbonate in the tablet is determined as the value of the fraction of dissolved tablet over the initial whole tablet.In this way,the change of the fraction of dissolved sodium carbonate could be used to represent the dissolution process of the tested tablet.According to Eq.(3),the concentration of dissolved sodium carbonate from each tablet is to be converted from measured conductivity data and the fraction of dissolved sodium carbonate is shown in Figs.4-7 for pure sodium carbonate tablet,0.03CMC tablet,0.05CMC tablet and 0.07CMC tablet respectively.
With converted dissolution data shown in Figs.4-7,both surface reaction model(Eq.(1))and diffusion layer model(Eq.(2))are applied into the quantification of dissolution rate constant of each kind of tablet at different temperatures respectively.With surface reaction model,1-(1-f)1/3is plotted against dissolution time and a linear is fitted to obtain the dissolution rate constant of surface reaction model(kr/s-1)for each case.With diffusion layer model,(1-)-(1-f)2/3is plotted against dissolution time and a linear is also fitted to obtain the dissolution rate constant of diffusion layer model(kd/s-1).In this way,the fittings with both models for pure sodium carbonate tablets,0.03CMC,0.05CMC and 0.07CMC tablets at different temperatures are shown in Figs.8-9,10-11,12-13 and 14-15 respectively.
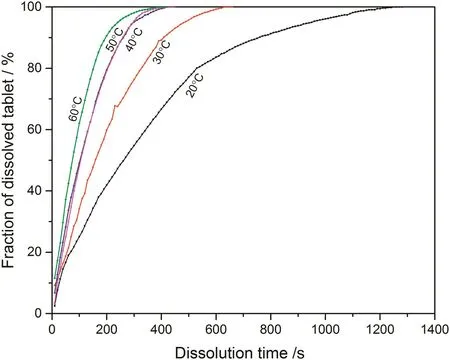
Fig.4.Fraction of dissolved sodium carbonate from pure sodium carbonate tablet at different temperatures.
According to the fitting results shown above(Fig.8-Fig.15),it is clearly shown that the fittings with surface reaction model obtain apparent higher correlation coefficient(R2)than that with diffusion layer model for all measured tablets at all tested tablets and temperatures.The correlation coefficients with surface reaction model are also high enough to suggest that the surface reaction model can be the proper dissolution model to interpret the dissolution mechanism of all kinds of two-component and pure sodium carbonate tablets.Therefore,the dissolution process of all tested tablets is controlled by the chemical reaction of sodium carbonate on the surface of the tablet,which is regarded as the decomposition of sodium carbonate into sodium ions,and carbonate ions and the dissolution speed is limited by the reaction rate of sodium carbonate in water.Furthermore,it is worth noting that the two-component tablets obtain apparent same dissolution mechanism with the reference pure sodium carbonate tablet according to the fitting results.According to the practical applications of carboxymethyl cellulose which is mostly used as the “supporting”element,its contentis always at a relative low range,for example the content of carboxymethyl cellulose in commercial detergent is normally at the level of 1 wt%-2 wt%.In this research,the investigated content of carboxymethyl cellulose within two-component tablets is up to 7%which is considered to cover the most potential industrial or commercial applications of carboxymethyl cellulose.Therefore,from the view point of practical applications,it is believed that the usage of carboxymethyl cellulose at its most common industrial or commercial content level will not change the dissolution mechanism of the tablet actively.
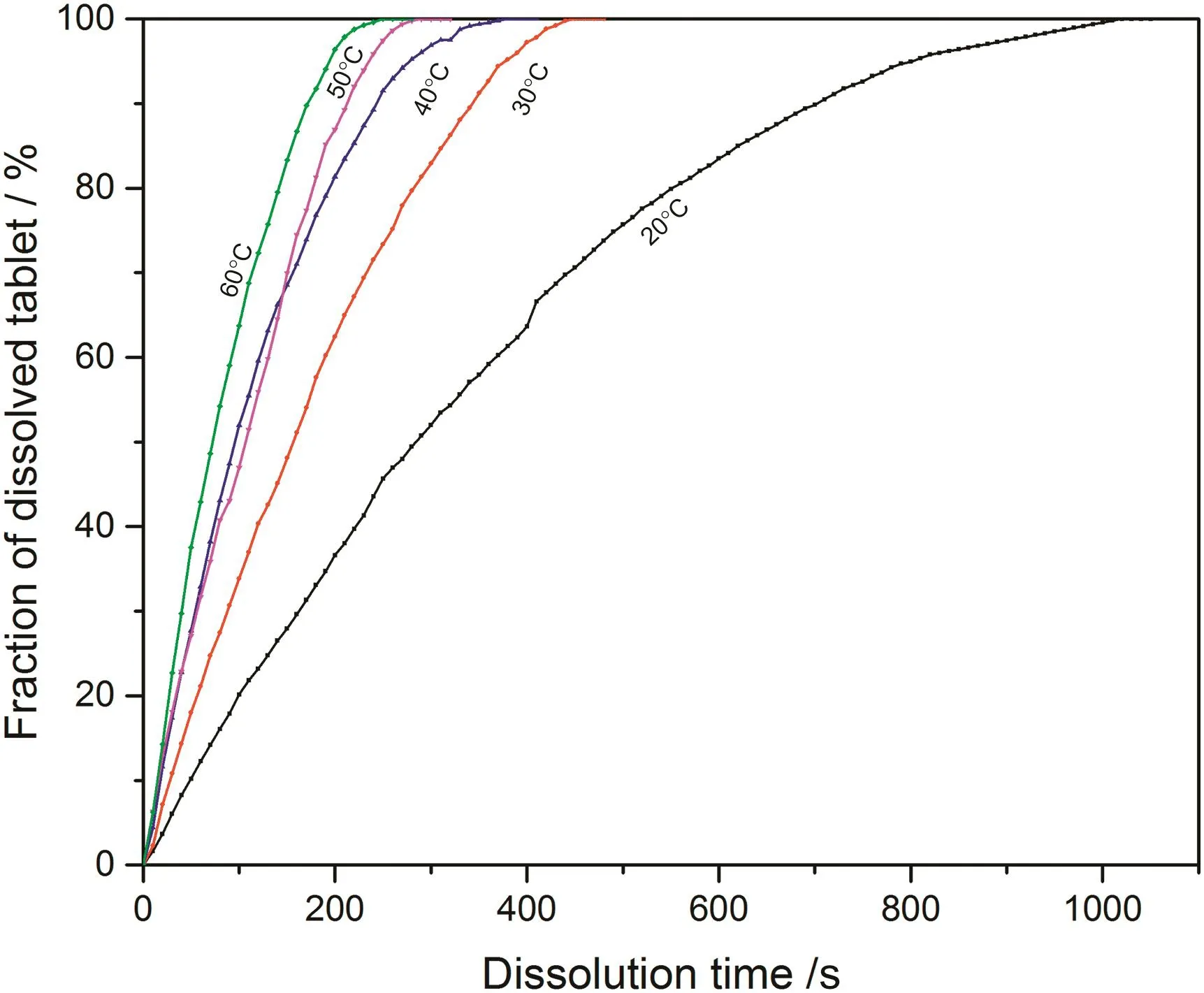
Fig.5.Fraction of dissolved sodium carbonate from 0.03CMC tablet at different temperatures.
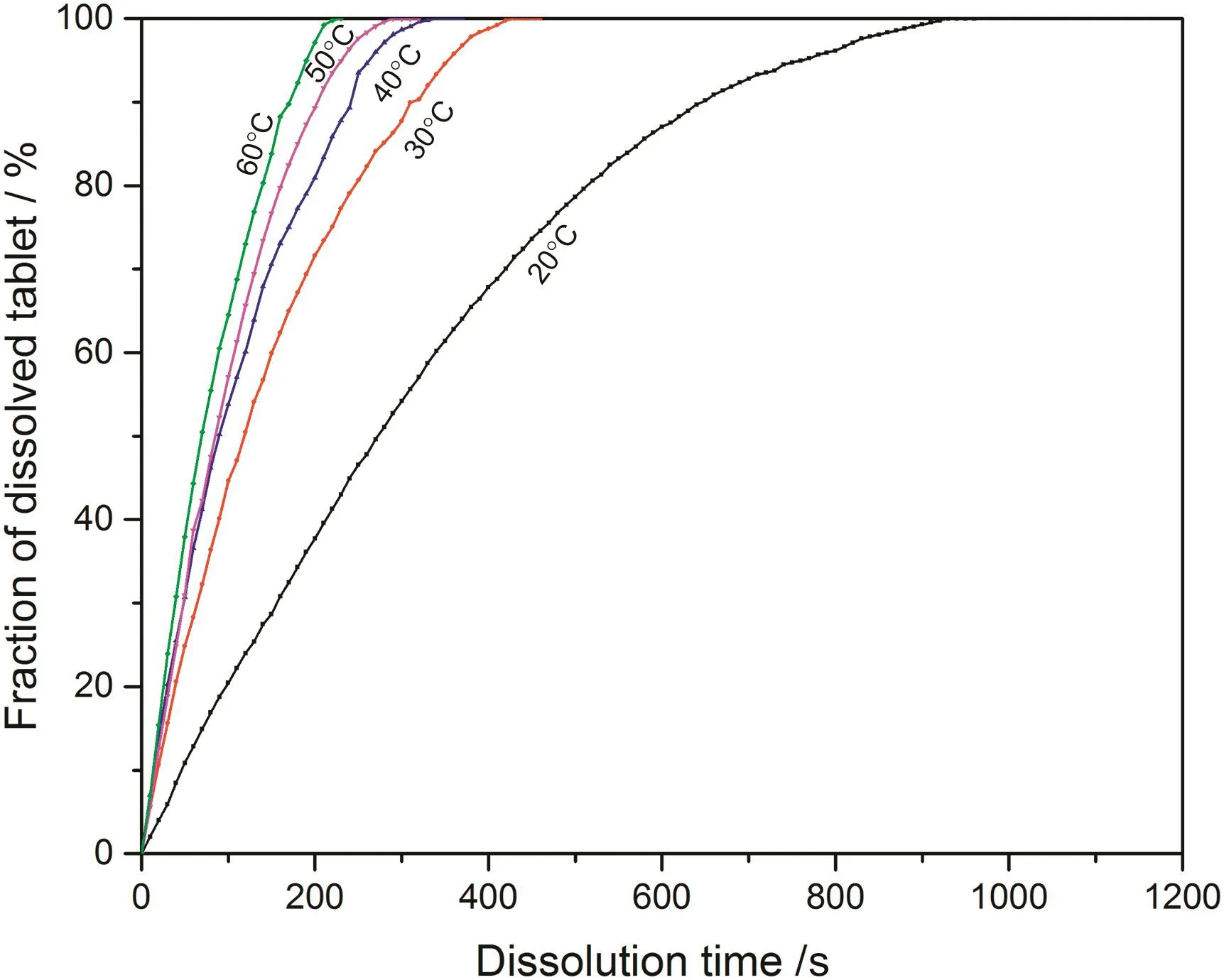
Fig.6.Fraction of dissolved sodium carbonate from 0.05CMC tablet at different temperatures.
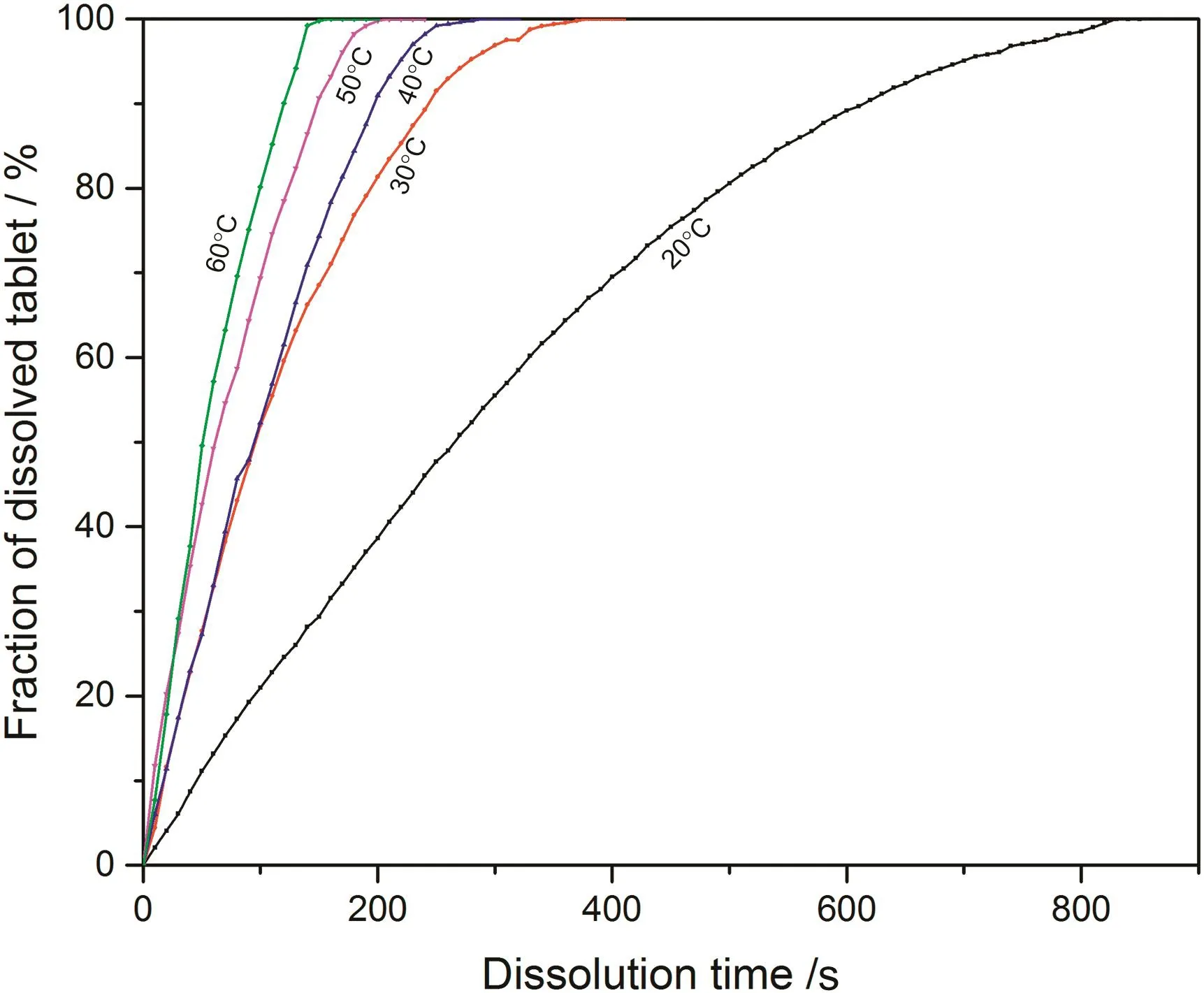
Fig.7.Fraction of dissolved sodium carbonate from 0.07CMC tablet at different temperatures.
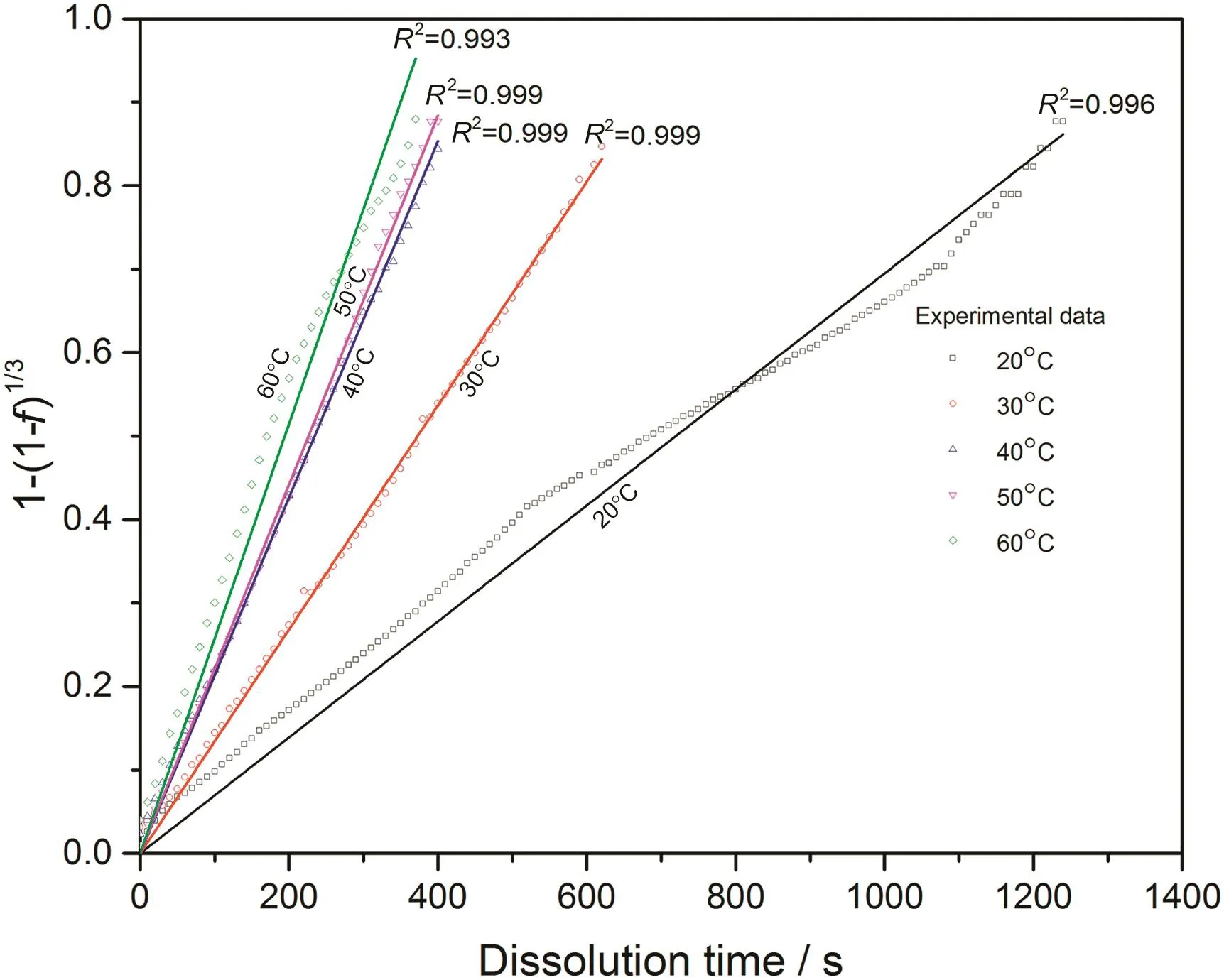
Fig.8.Linear fitting with surface reaction model for pure sodium carbonate tablets at different temperatures.
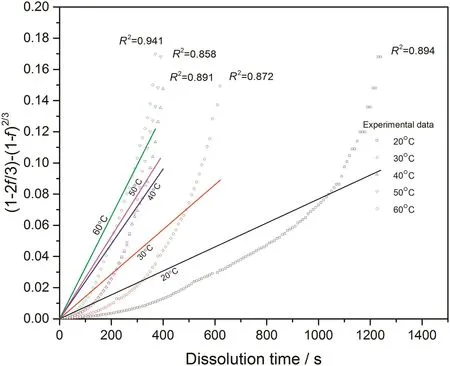
Fig.9.Linear fitting with diffusion layer model for pure sodium carbonate tablets at different temperatures.
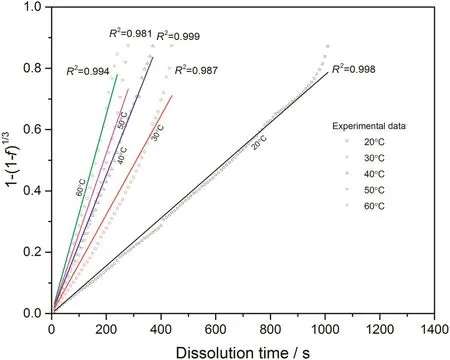
Fig.10.Linear fitting with surface reaction model for 0.03CMC tablets at different temperatures.
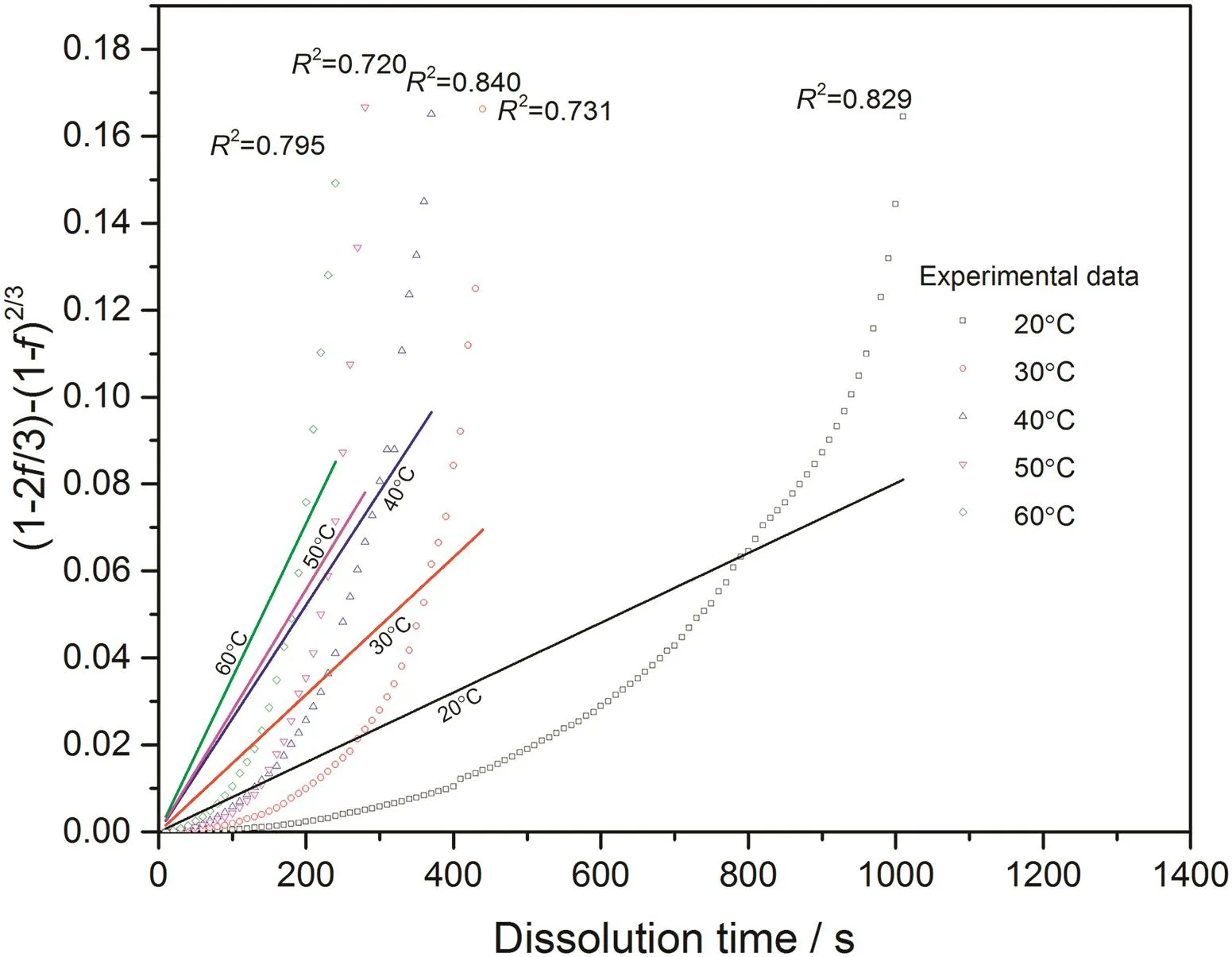
Fig.11.Linear fitting with diffusion layer model for 0.03CMC tablets at different temperatures.
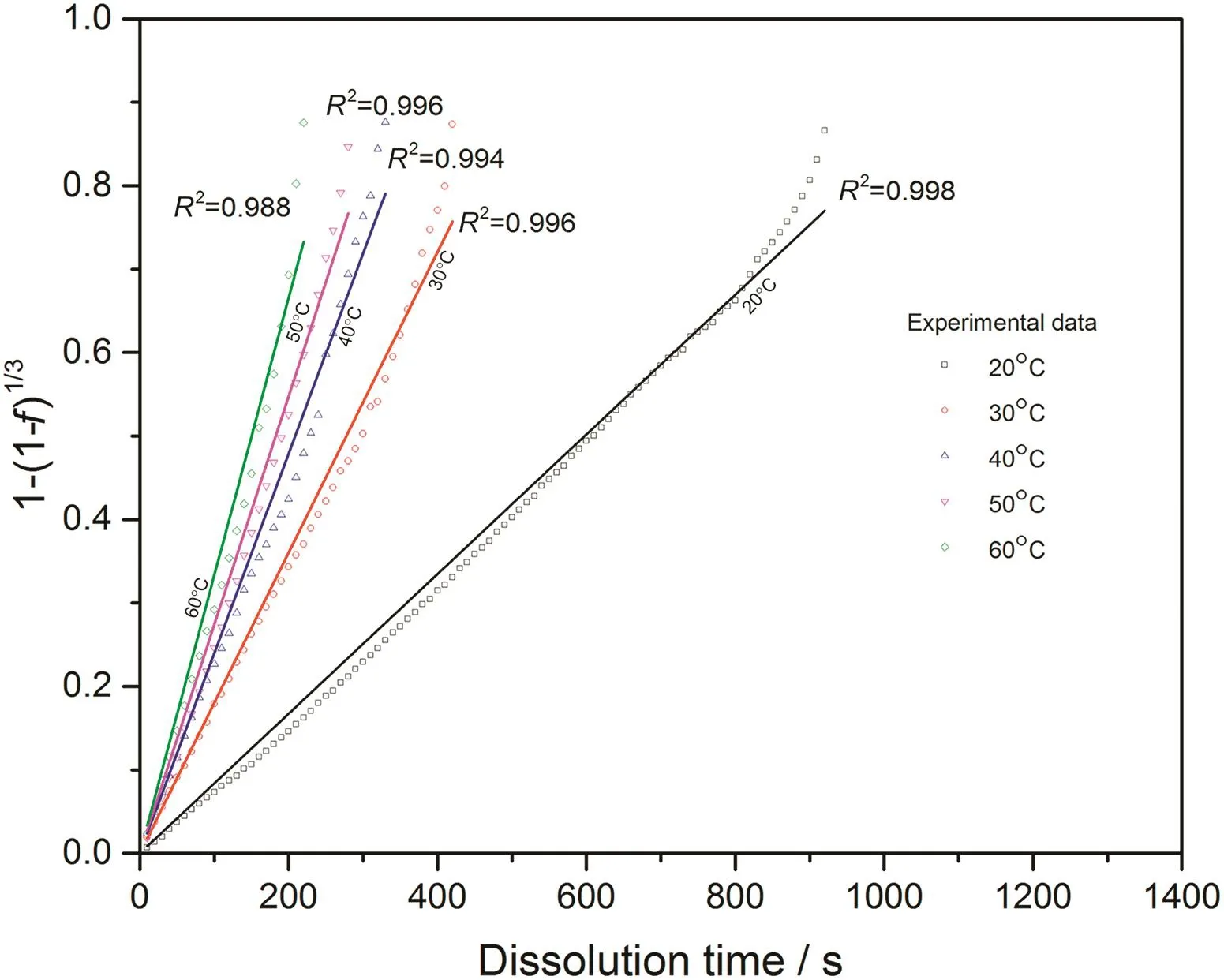
Fig.12.Linear fitting with surface reaction model for 0.05CMC tablets at different temperatures.
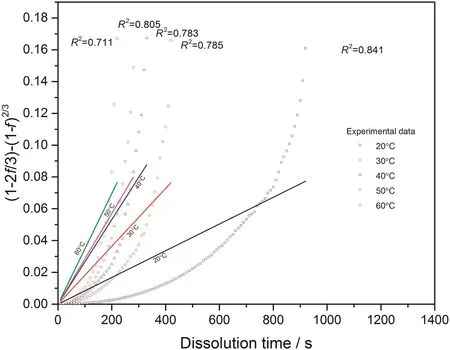
Fig.13.Linear fitting with diffusion layer model for 0.05CMC tablets at different temperatures.
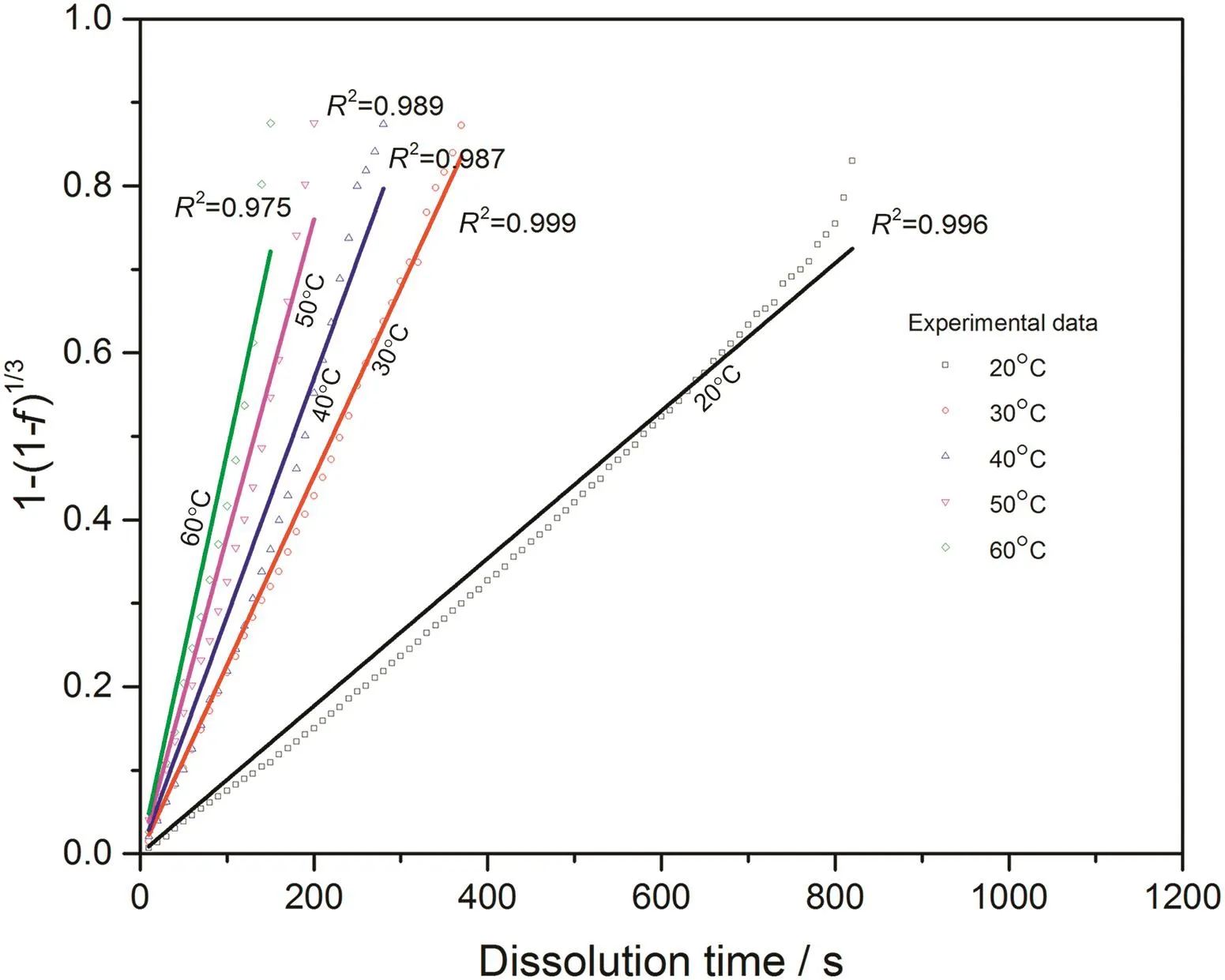
Fig.14.Linear fitting with surface reaction model for 0.07CMC tablets at different temperatures.
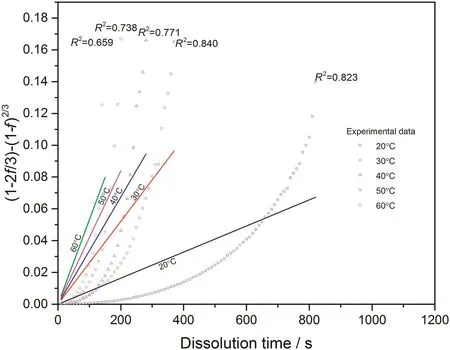
Fig.15.Linear fitting with diffusion layer model for 0.07CMC tablets at different temperatures.
Based on the fitting results,the dissolution rate constants of all tested tablets should be obtained from the fitting with surface reaction model and the results of dissolution rate constant for all measured tablets at all tested temperatures are shown in Fig.16.
According to the results shown in Fig.16,it is clearly shown that the sodium carbonate-carboxymethyl cellulose two-component tablet shows significant higher dissolution rate constant than the reference pure sodium carbonate tablet at all tested temperatures.It is thus confirmed that carboxymethyl cellulose is a positive component to improve the dissolution rate constant of tablet and higher proportion of carboxymethyl cellulose leads to a greater dissolution rate constant.Combining the results of dissolution mechanism of two-component tablet,it is understood that carboxymethyl cellulose is one kind of polymer that will significantly accelerate the dissolution process of a tablet without the side-effect of changing on dissolution mechanism of the tablet.
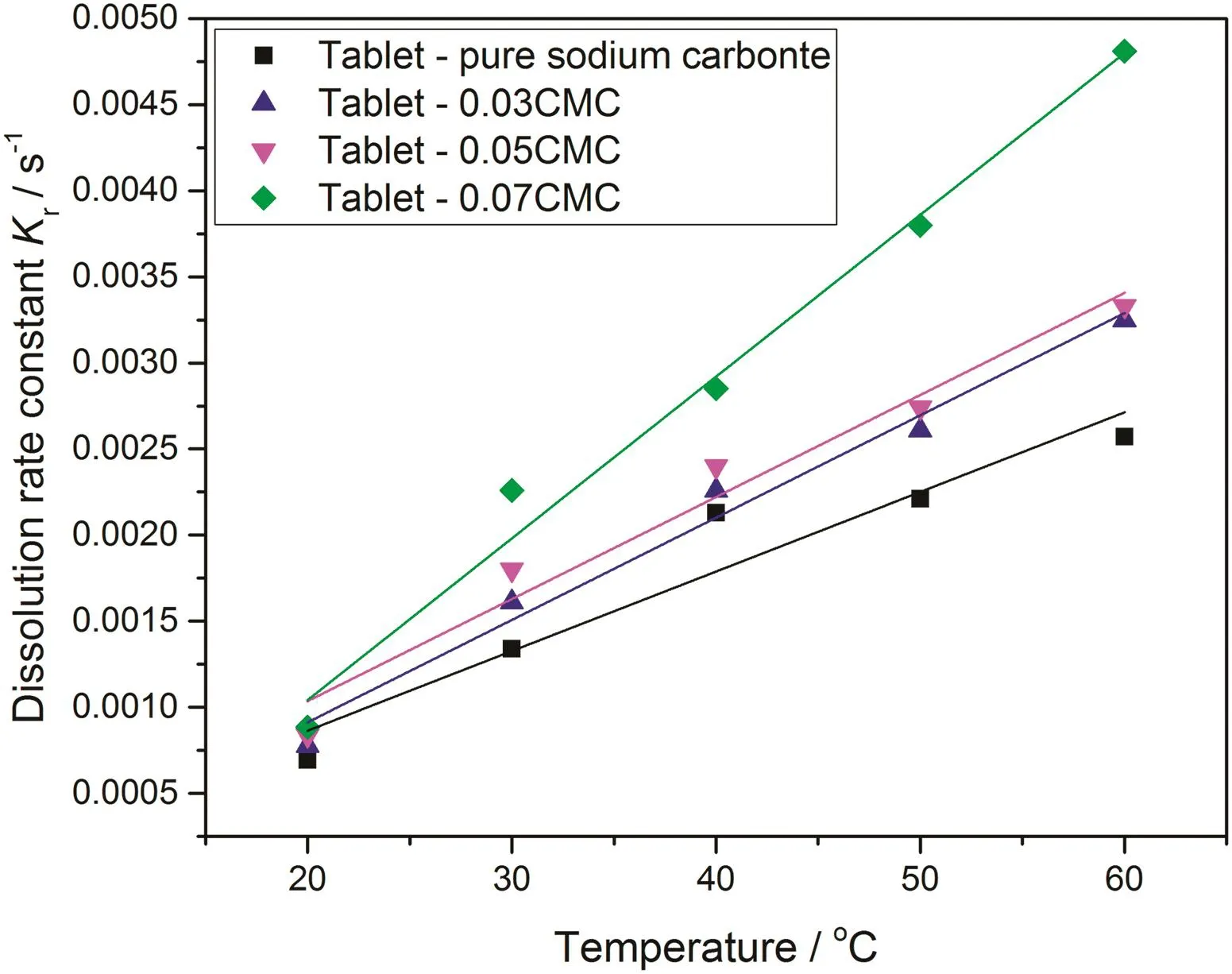
Fig.16.Dissolution rate constant of surface reaction model k r of sodium carbonate/0.03CMC/0.05CMC/0.07CMC tablets at different temperatures.
For the effect of acceleration of carboxymethyl cellulose on dissolution process,it starts from the approach of carboxymethyl cellulose contacting with water.A common understanding on dissolution process of carboxymethyl cellulose in water is the process of dilation and then conglutination to colloid.When the dissolution starts,carboxymethyl cellulose dilates immediately within the tablet.This phenomenon of dilation leads to the expansion of effective surface area of the tablet contacting with water,and thus more decomposition reactions of sodium carbonate into ions happen at the expanded surface of tablet.With expanded surface area,carboxymethyl cellulose-sodium carbonate two-component tablet therefore obtains effectively higher dissolution rate constant than pure sodium carbonate tablet consequentially.Also because this dilation of carboxymethyl cellulose manifests one kind of physical transforming process,higher proportion of carboxymethyl cellulose presumes greater expansion of effective surface area and even faster dissolution process of the tablet.This deduction of the effect of carboxymethyl cellulose on accelerating dissolution process is also proved with the results of the enhancement of dissolution rate constant of two-component tablet shown in Fig.16.
Although the effect of carboxymethyl cellulose on accelerating dissolution process can attribute its behavior of dilation in water,it still has the potential possibility to disintegrate the tablet during the dissolution process on the other hand.Disintegration is an important precipitating factor to make the dissolution behavior of the tablet change absolutely during the dissolution process.However,it is known that carboxymethyl cellulose particles will mutually bond into colloid-like products which can conglutinate surrounding sodium carbonate particles at the same time.It is still difficult to distinguish its physical dissolution performance into clear separated steps.According to the observation of dissolution process of all tested two-component tablets,there has no phenomenon of disintegration happening during the test period.In this way,it is more inclined to speculate the effect of carboxymethyl cellulose coming from its dynamic physical transforming process in water.
Furthermore,it is also reasonably speculated that there may have a critical point to break the dynamic balance of carboxymethyl cellulose's effect on accelerating dissolution process and also keeping dissolution mechanism unchanged.Especially when the proportion of carboxymethyl cellulose within the tablet is high than a certain value,the possibility of the disintegration rises aggressively and the dissolution process of tablet changes absolutely.At the same time,too heavy colloid-like patterns are formed to wreathe surrounding sodium carbonate particles and then decrease the contacting surface area of the tablet effectively.Therefore,the proportion of carboxymethyl cellulose within two-component tablet used in this research is apparently and extremely the most important factor to determine its effect on dissolution properties of two-component tablets.However,according to the results shown in Fig.16,it can be confirmed that for the usage of carboxymethyl cellulose in most practical applications at a relative low-level,the effect of carboxymethyl cellulose is positive for two component tablet to enhance the dissolution process,improve dissolution rate constant and remain dissolution mechanism unchanged.
6.Conclusions
Electrical conductivity tracking method is effective,cheap and reliable to measure dissolution process of tablets containing conductible ions.With a calibrated equation of conductivity converting to concentration,the dissolution process of the tablet can be quantified accurately.As the potential dissolution model,surface reaction model is the appropriate mathematical model to interpret the dissolution mechanism of the two component tablet and the pure sodium carbonate tablets according to the fitting results.For surface reaction model,the dissolution process of all kinds of tested tablets is relying on the chemical reaction of sodium carbonate at the tablet surface which is presented as the decomposition of sodium carbonate molecule into sodium and carbonate ions.In this way,the dissolution process and the rate is regarded to be controlled and limited by the chemical reaction rate of sodium carbonate into separate ions.With this model,the dissolution rate constant of both two component and pure sodium carbonate tablets is quantified at all tested temperatures.According to the results of dissolution rate constant,carboxymethyl cellulose-sodium carbonate two-component tablets present significant higher dissolution rate constant than pure sodium carbonate tablets and higher proportion of carboxymethyl cellulose within twocomponent tablet leads to even higher dissolution rate constant.Therefore,it is confirmed that at low-level content of carboxymethyl cellulose,the effect of carboxymethyl cellulose is positive for two-component tablet to enhance the dissolution process,improve dissolution rate constantand remain dissolution mechanism unchanged.This effect of carboxymethyl cellulose is speculated coming from its dynamic physical transforming process in water including dilation and conglutination.Furthermore,it is believed that the proportion of carboxymethyl cellulose within twocomponent tablet is apparently and extremely the most important factor to determine its effect on dissolution properties of two-component tablets.
Acknowledgments
I would like to thank the Institute of Particle and Science Engineering,University of Leeds and Procter&Gamble Newcastle Innovation Centre(UK)for partially funding the project.
[1]D.Bellini,A.Pavesio and M.Terrassan.U.S.Patent Application.11/989.224.(2006).
[2]A.D.McNaught,Compendium of chemical terminology,Blackwell Science,Oxford,1997.
[3]J.Li,R.B.Lewis,J.R.Dahn,Sodium carboxymethyl cellulose:A potential binder for Si negative electrodes for Li-ion batteries,Electrochem.Solid-State Lett.10(2)(2007)A17-A20.
[4]F.He,D.Zhao,Manipulating the size and dispersibility of zerovalentiron nanoparticles by use of carboxymethyl cellulose stabilizers,Environ.Sci.Res.41(17)(2007)6216-6221.
[5]S.Rossi,M.C.Bonferoni,F.Ferrari,C.Caramella,Drug release and wash ability of mucoadhesive gels based on sodiu carboxymethyl cellulose and polyacrylic acid,Pharm.Dev.Technol.4(1)(1999)55-63.
[6]T.P.Kravtchenko,J.Renoir,A novel method for determining the dissolution kinetics of hydrocolloid powders,Food Hydrocoll.13(1999)219-225.
[7]A.Marabi,G.Mayor,Assessing dissolution kinetics of powders by a single particle approach,Chem.Eng.J.139(2008)118-127.
[8]M.Dali,Determination of mass transfer dissolution rate constants from critical time of dissolution of a powder sample,Pharm.Dev.Technol.4(1)(1999)1-8.
[9]A.A.Noyes,W.R.Whitney,The rate of solution of solid substances in their own solutions,J.Am.Ceram.Soc.19(1897)930-934.
[10]A.W.Hixson,J.H.Crowell,Dependence of reaction velocity upon surface and agitation,Ind.Eng.Chem.Res.23(1931)923-931.
[11]V.Pillay,R.Fassihi,Unconventional dissolution methodologies,J.Pharm.Sci.88(1999)843-851.
[12]S.Dash,P.N.Murthy,L.Nath,P.Chowdhury,Kinetics modeling on drug release from controlled drug delivery systems,Acta Pol.Pharm.67(3)(2010)217-223.
[13]B.Dousova,L.Fuitova,D.Kolousek,M.Lhotka,T.M.Grygar,P.Spurna,Stability of iron in clays under different leaching conditions,Clay Clay Miner.62(2)(2014)145-152.
 Chinese Journal of Chemical Engineering2017年10期
Chinese Journal of Chemical Engineering2017年10期
- Chinese Journal of Chemical Engineering的其它文章
- Crystallization of calcium silicate at elevated temperatures in highly alkaline system of Na2O-CaO-SiO2-H2O☆
- Aluminum impregnated silica catalyst for Friedel-Crafts reaction:Influence of ordering mesostructure☆
- Microencapsulation of stearic acid with polymethylmethacrylate using iron(III)chloride as photo-initiator for thermal energy storage☆
- Novel efficient procedure for biodiesel synthesis from waste oils with high acid value using 1-sulfobutyl-3-methylimidazolium hydrosulfate ionic liquid as the catalyst☆
- Toluene degradation by a water/silicone oil mixture for the design of two phase partitioning bioreactors
- Effects of anaerobic composting on tetracycline degradation in swine manure☆
