Aluminum impregnated silica catalyst for Friedel-Crafts reaction:Influence of ordering mesostructure☆
Yibo He,Qinghua Zhang*,Xiaoli Zhan,Dangguo Cheng,Fengqiu Chen
College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,China
1.Introduction
Linear alkyl benzene(LAB),which is a major feedstock for synthetic detergent,is synthesizedviaFriedel-Craft alkylation of benzene with linear chain olefin(C10-C14)[1-3].The alkylation of benzene with olefins proceeds through a carbonium ion mechanism[4].LAB is produced industrially over HF or AlCl3homogenous catalyst.Yet,the homogeneous catalyst is hydrolysed to produce aluminum oxide after reaction.Moreover,large amounts of acidic gas and toxic waste water are produced in catalyst separation,which makes the process problematic from an environmental point of view.The phenyl group position of LAB has an effect on the surface-active property and biodegradability of LAS[5].Among the isomers of LAB,2-LAB exhibits the most biodegradable,making it a dominant detergent intermediate[6].
Notice that 2-LAB selectivity is a function of olefin conversion and zeolite type,but is not directly correlated with pore size.The molecular size of 2-,3-,4-,5-,6-LAB are in the range of 0.529 nm,and 2-LAB is the smallest one among the isomers.For mesoporous molecular sieve(pore size > 2 nm),“shape selective catalysis”is not as significant as all the isomers can pass through the pore channel.It can be concluded that 2-LAB selectivity is sensitive to the pore structure of zeolite[7-10].As research shown,diffusion limitation is the main influencing factors in Friedel-Craft alkylation of benzene.For molecular sieves,smaller pore size leads larger resistance of mass transport,extreme large pore may lead to decreasing of specific surface area which directly correlated with reactivity[11-13].There are some recent reports on catalytic activity over macroporous materials,they are not in such high demand for catalysis owing to the low surface area of nanoporous[14,15].Functionalized acid catalysts include AlCl3grafted MCM-41[13],mesoporous molecular sieves[16-18],mesoporous silica[19],cage type porous gallosilicate[20]and other catalysts[21-25]were reported.However,few reports have compared aluminum grafted silicate with different ordered or disordered channel network,to explore insight into mesostructure effect on reactivity and selectivity of grafted catalysts,from diffusion limitation perspective.The comparison is necessary for deeper understanding to how mesostructure affects reactivity and selectivity of catalysts.And it provided scientific basis for choosing apposite supporters for catalysts design in this reaction[26-34].
In this work,four kinds of molecular sieves were synthesized,in order to optimize synthesis conditions for LAB,and deals with the relationship between pore channel structures and their catalytic activity.Catalysts with differentchannel structures,hexagonal packing channels(SBA-15,MCM-41),disordered channel network(SiO2,SiO2-Gel),were synthesized by impregnation.These catalysts were analyzed by nitrogen sorption,ICP,and were found to be highly reactive and give nearly 100%1-dodecene conversion with considerably high 2-LAB selectivity(about50%).The results show that aluminum impregnated silica molecular sieves with order channel network gives higher catalytic activity than that with disorder channel network.Larger pore size is favorable to catalytic activity.
2.Experimental
2.1.Catalysts preparation
The support SiO2,which was purchased from QingDao Banke Separation Materials Co.,Ltd.,was dried at 400°C overnight and kept under dry argon.
SBA-15 was synthesized in a typical method,4 g of Pluoronic P123,water and HCl 0.28 mol·L-1were stirred at 35 °C for 16 h[35].The appropriate amount(8.5 g)of tetraethylorthosilicate(TEOS)was then added dropwise to the surfactant solution,followed by stirring for 24 h.the resulting gel was introduced into a Te flon lined stainless steel autoclave and heated at 100°C for two days.The products were filtered,washed with warm distilled water and finally dried at 100°C,and followed by calcination in air at 500°C,then kept under dry argon[36].
The reactants for the synthesis of MCM-41 at room temperature were TEOS,water,hexadecyltrimethylammonium bromide(CTAB)either NaOH or NH4OH can be used as a base source.Reactions were performed at room temperature,205 ml of NH4OH were mixed with 270 ml of distilled water,and 2.0 g of surfactant were added into the solution with stirring and heating.When the solution became homogenous,10 ml of TEOS was introduced,giving rise to a white filtered,washed with distilled water,dried at ambient temperature,and followed by calcination in air at 500°C,then kept under dry argon[37,38].
10 ml of TEOS and 7 ml of CH3CH2OH were added into a flask,respectively.After the formation of clean and homogeneous liquid mixture,5 ml HCl(5 mol·L-1)was added and the mixture became coagulated gradually.After aged at 60°C for 12 h,the resulted solid mixture was dried in vacuum for 3 h at 150°C and kept under dry argon[39,40].
Aluminum impregnated silica was prepared by reacting anhydrous AlCl3with the terminal Si-OH groups of silica[36].The method was as follows:5 g of silica was mixed with 200 ml CCl4and refluxed for 2 h.bubbled N2during refluxed to remove traces of moisture.About 2.0 g of anhydrous AlCl3was added to the mixture,and the mixture was refluxed for 6 h under N2.After that,the aluminum impregnated silica was filtered and washed with CCl4for several times.The resulting catalyst was dried under vacuum at 80°C and stored in N2.
2.2.Catalytic reaction
The reactions were carried out under an inert atmosphere due to the sensitivity of the catalyst toward moisture.All reactants and solvents were dried before use.The reactions were conducted in flask with a magnetic stirrer.Catalyst was added into the flask quantitatively,followed by benzene and 1-dodecene.Starting stirring to start the reaction.After the completing of the reaction,the organic layer containing the products and unreacted reactants was separated by decantation and centrifugation.The organic layer was washed with distilled water,dried over Na2SO4and analyzed by gas chromatography[41].
2.3.Analysis
Surface properties of catalysts were analyzed by physical adsorption of nitrogen in ASAP 2020.X-ray diffractometry(XRD)was done on a DMAX-RA.ICP test was carried out by Agilent 710 ICP.Materials and products of reaction were measured by GC.GC was equipped with HP-5 column(30 m).The initial column temperature was maintained at 110 °C for 3 min,raised at the rate of 15 °C·min-1and maintained at 300 °C for 9 min.The injection temperature was at 300 °C[42].
3.Results and Discussion
3.1.Catalyst characteristic
Five catalysts were synthesized and analyzed,three of them were mesoporous materials with ordered pore structures.Two of them were based on SBA-15,with different pore sizes.SBA-15(A)and MCM-41 were synthesized in typical method,whereas SBA-15(B)was synthesized in double template method for expanding pore size.SBA-15(A)differed from MCM-41 not only because of larger mesoporous and irregular interconnections but also because of higher hydrothermal and thermal stability caused by its thicker mesoporous pore walls[43].SBA-15 with larger pore size was synthesized by adding second template F127 for pore expanding.The shape of the colloids formed from F127 remains spherical but their size increases,compared with that of P123[44].
The XRD patterns for order channel network samples,SBA-15(A),SBA-15(B),MCM-41,before and after immobilization obtained in the present work are shown in Fig.1.It shows 3 well resolved peaks that can be indexed as(100),(110)and(200)diffraction.This is an indication of a good long-range hexagonal ordering.The XRD data shows that the mesoporous structure is preserved partly after the addition of aluminum species,indicating loss of structural order compared with untreated silica materials.The loss of structural order is a consequence of the surface modification by impregnation of aluminum species,and resulting loss of long-range order of mesoporous structure.Due to the addition of F127,the diffraction peaks declined,which is similar to SBA-16.The mesoporous materials synthesized above can be further confirmed by nitrogen sorption and TEM analysis(Table 1,Figs.2 and 3).
The results of catalysts surface properties are given in Table 1.The specific surface areas of MCM-41 and other untreated catalysts are estimated as1058 m2·g-1and in the range of300-800 m2·g-1,respectively.Enlarging of pore sizes lead to the decreasing of specific surface areas.The immobilization of aluminum species on the surface of the support is believed to be due to covalent bonding between the aluminum atoms and the Si-OH.The main change observed on the support after impregnation with aluminum species was the decrease of the specific surface area,which can be explained by the obstruction of the pore,which resulted from the carriers by the aluminum species.Besides,the pore walls thickened after impregnation with aluminum species.In general,catalyst after impregnation exhibited large specific surface area(almost larger than 300 m2·g-1),which supplied enough contact area for acidic sites and reactants.ICP investigation showed that Al capacity of catalysts were in the range of 1.43 to 1.50 mmol·g-1.
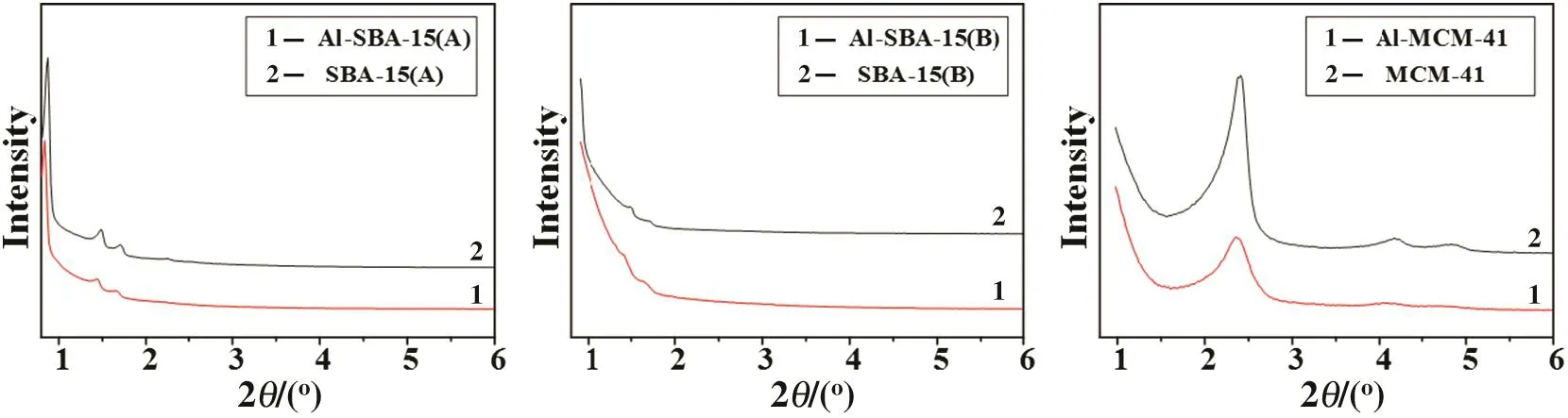
Fig.1.X-ray diffraction of order channel network samples.

Table 1Textural properties of various catalyst samples
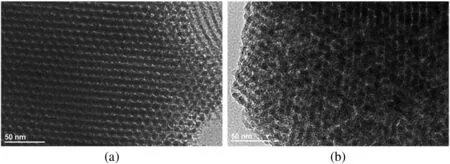
Fig.2.TEM analysis of SBA-15 catalysts before(a)and after(b)immobilization.
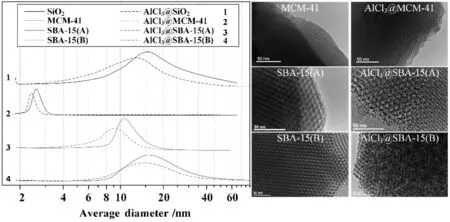
Fig.3.The pore size distribution and TEM analysis of catalyst samples.
Fig.3 gives the pore size distribution of different catalyst samples before and after immobilization.The pore size distribution of disorder channel network SiO2was really wide because of the existing microporous,mesoporous and macroporous pores.The pore size distribution of order channel network SBA-15(B)was also wide,but almost belonged to mesoporous.The reason why the pore size distribution of SBA-15(B)was wider than SBA-15(A)was that adding F127 for pore expanding.The shapes of the colloids formed from P123 and F127 were different,P123 formed an elongated shape while F127 formed spherical.The differences of colloids shape made partly pore wall collapsing,pores connecting to others.It led to the pore size distribution becoming wider and long range order becoming weaker.
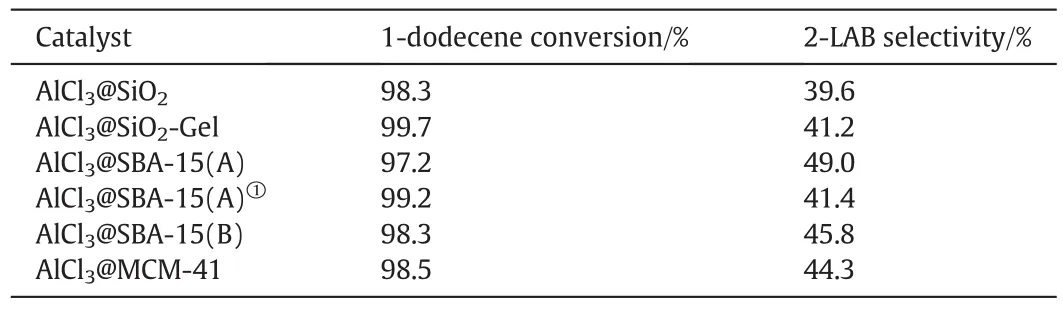
Table 2Results of catalysis via various catalysts
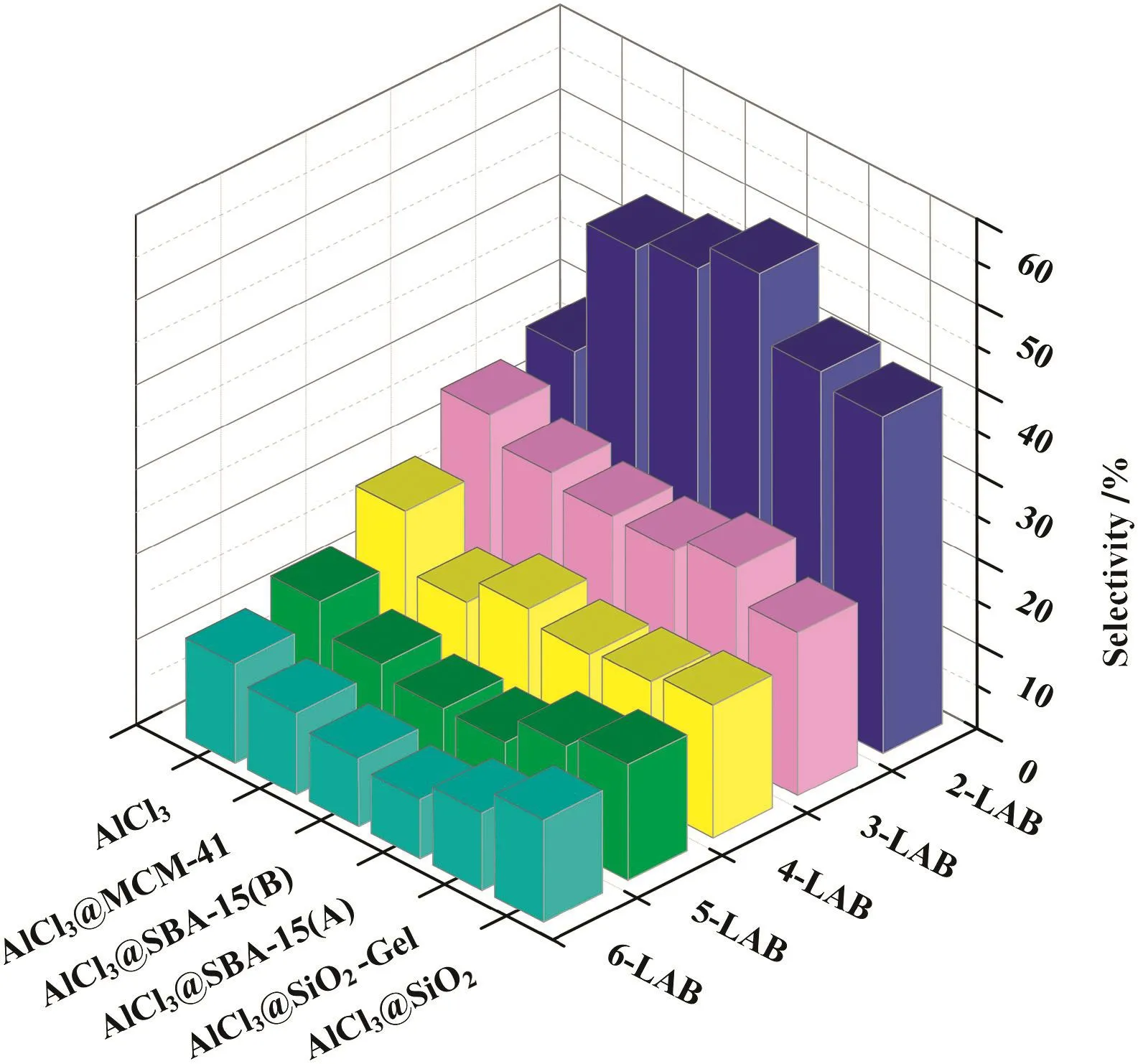
Fig.4.The product distribution of catalysis via various catalysts.
3.2.Friedel-Crafts reaction
The alkylation of benzene with 1-dodecene over aluminum impregnation silica molecular sieves was carried out,Table 2 and Fig.4 present the catalytic performance of the different catalysts in terms of 1-dodecene conversion,and 2-LAB selectivity.In general,2-LAB selectivity was about 40%with catalysts based on disorder channel network supports,while that were higher with catalysts based on order channel network supports as SBA-15(A),SBA-15(B)and MCM-41.It can be seen that a 49.0%conversion of 1-dodecene was realized within 12 h under relatively optimal condition over AlCl3@SBA-15(A)(as shown in Table 2).Catalysts based on different supports(AlCl3@SiO2and AlCl3@SBA-15(B))had properties of wide pore size distribution in common.Assuming that AlCl3on unit surface area was constant,catalysts with smaller pore might have more acidic sites in unit volume space due to higher specific surface area of smaller pore.It made reaction conduct faster and led to increasing of regional carbenium concentration.Isomerization was conducted faster due to diffusion limitation and high regional carbenium concentration,which led to decreasing of 2-LAB selectivity(as shown in Fig.5).Though,surface area in volume space decreased as pore size becoming larger.Larger size of pore contributed to more favorable mass transport.Small amount of large pore,even macro pore could not affect the reaction significantly.Thus,mesoporous catalysts with a narrow pore size distribution would be batter catalysts for their higher 2-LAB selectivity.
SBA-15(A)and MCM-41 with high long-range order showed narrow pore distribution,which created similar mass transport condition.It led to decreasing of regional carbenium concentration.Although pore size distribution of SBA-15(B)was wide,little,small or micro pore which led to accumulation of carbenium concentration existed.
Alkylation experiments at different temperature(35 °C and 75 °C)are compared in Table 2.As the results shown,reaction temperature increased,2-LAB selectivity decreased.The isomerization is a parallel reaction to the alkylation and reaction temperature promotes isomerization more than alkylation.Further experiments were carried outwith AlCl3@SBA-15(A)in order to study the influence of different temperature(as shown in Table 3).As expected the 1-dodecene conversion increased with temperature,while the 2-LAB selectivity decreased slightly.The isomerization tended to conduct toward producing high substituent formation product.

Table 3Results of catalysis at different reaction temperature
Experiments with different usage of the catalyst were carried out in order to study the effect of the concentration of catalyst on the 1-dodecene conversion and 2-LAB selectivity.As the result shown in Table 4,the 1-dodecene conversion increased significantly when the catalyst usage increased from 1 wt%to 5 wt%,while 2-LAB decreased slightly.Going on adding the catalysts usage,the 1-dodecene conversion and 2-LAB were not influenced obviously.The reaction rate of alkylation was promoted,by increasing the acidic sites in the system,which resulted from increasing of the catalyst usage.However,increasing of the carbenium producing rate led to promoting of isomerization of carbenium,which resulted in the decreasing of 2-LAB selectivity.
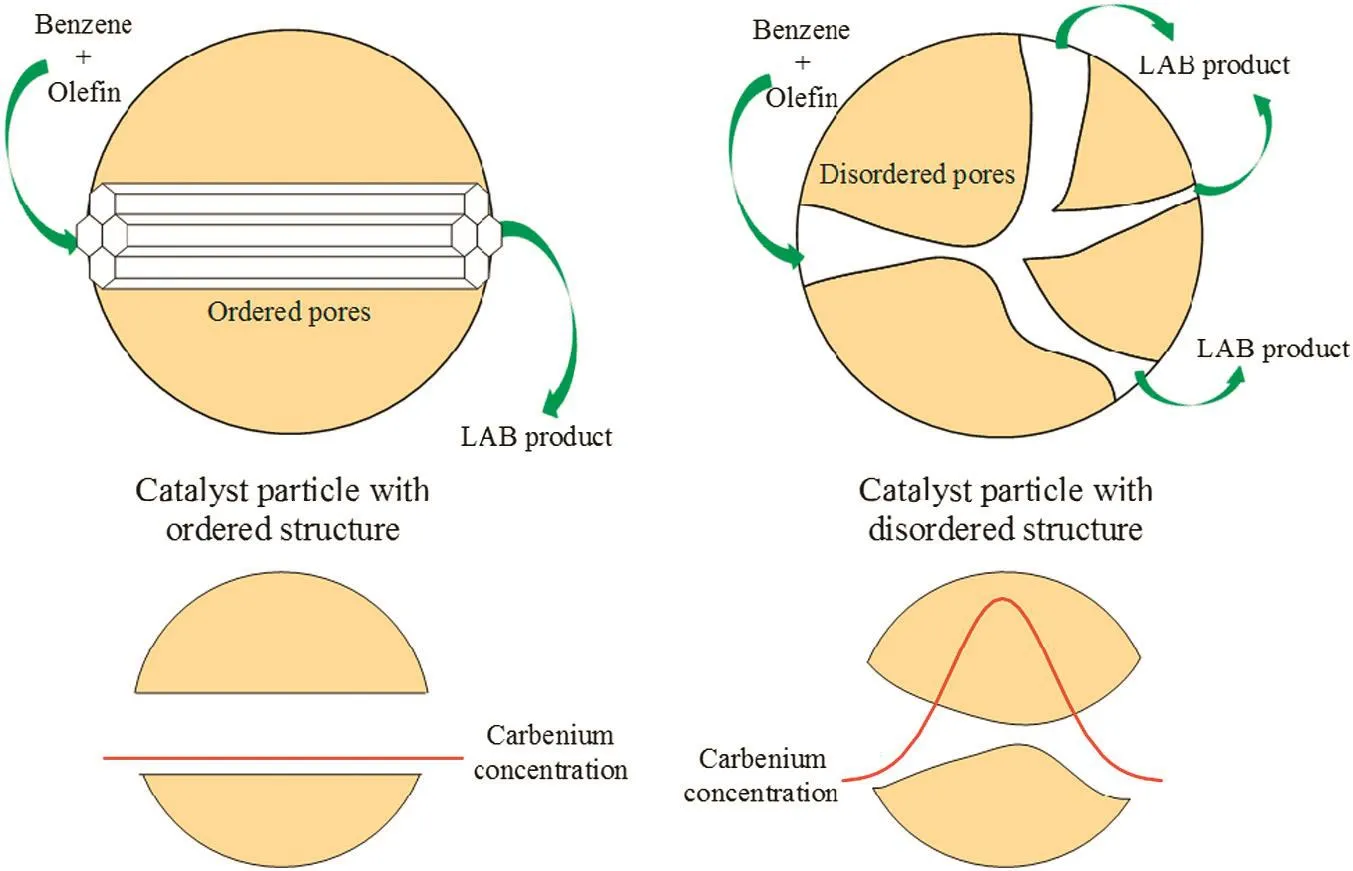
Fig.5.Schematic of the carbenium concentration profile(disregarding molecular adsorption).
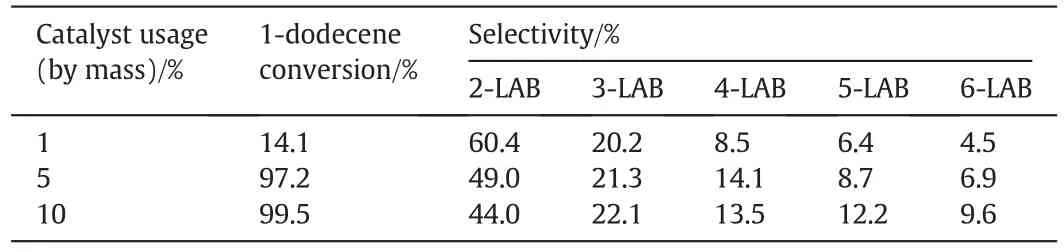
Table 4Results of catalysis at different catalyst amount
The influences on LAB selectivity of benzene/1-dodecene(molar ratio)for the alkylation reaction were studied,results are showed in Fig.6.As the ratio ofbenzene/1-dodecene increased,2-LAB selectivity increased from~38%to 60%,besides that,3-,4-,5-and 6-LAB selectivity decreased at different degrees.Increasing of benzene/1-dodecene molar ratio indicated that the dilution of carbenium ion concentration,which inhibited the hydrogen shift reactions and isomerization significantly,resulting in increasing of 2-LAB selectivity and decreasing of high substituent formation selectivity.In comparison,benzene/1-dodecene ratio is as high as 30 in industrial“Detal™”process[45,46].However,higerbenzene/1-dodecene molar ratio causes highercostand harder operation,which is resulted from surplus benzene separation and recycle.Reducing benzene/1-dodecene ratio is an essential requirement in the development of catalysts for LAB synthesis.
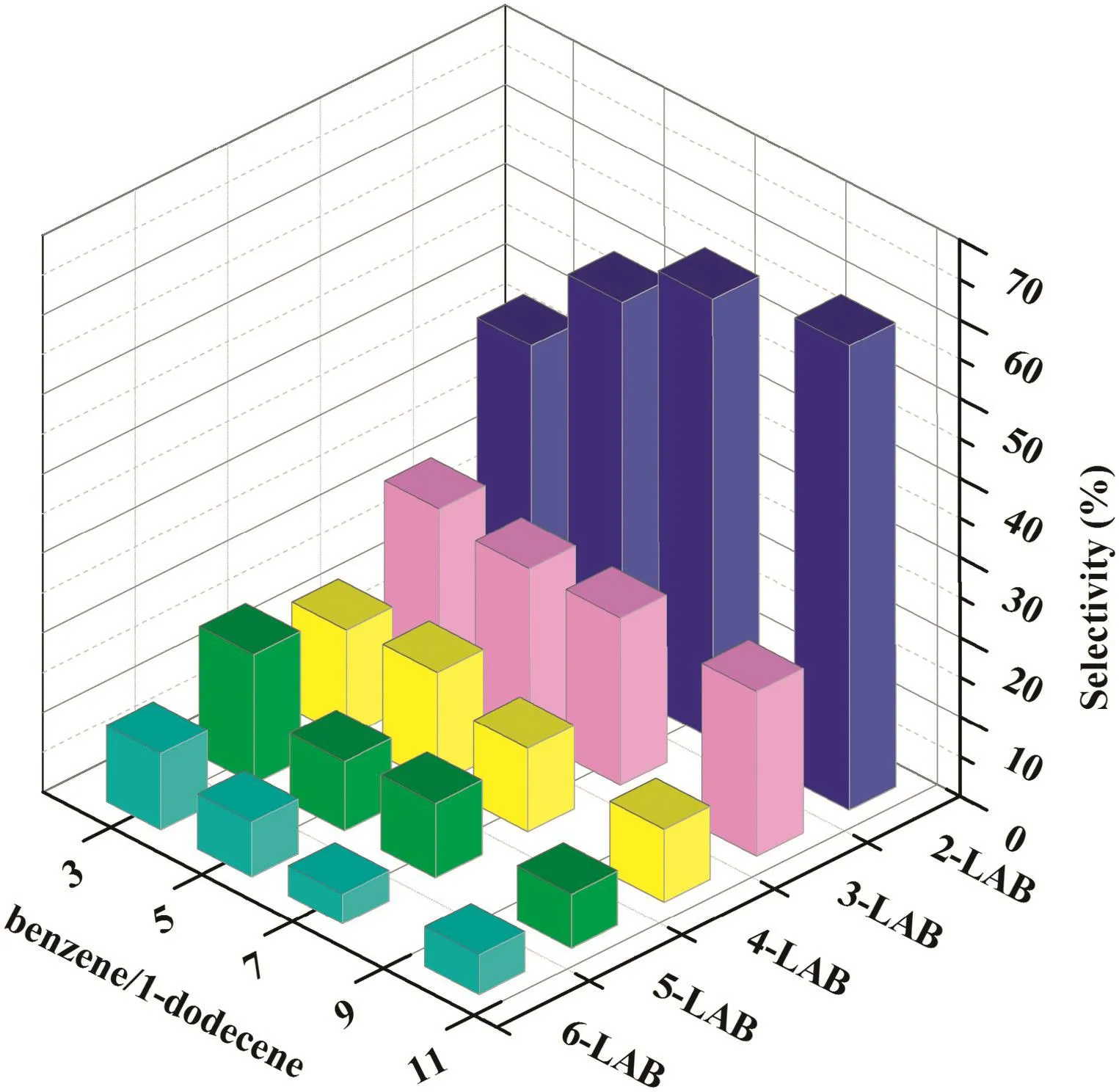
Fig.6.The product distribution of different benzene/1-dodecene molar ratio.
4.Conclusions
Aluminum impregnated silica molecular sieves proved to be active as catalysts for Friedel-Crafts alkylation of benzene with linear alkyl benzene.Results featured good conversion and high selectivity to 2-LAB.In this work,different kinds of catalysts with different channel structures,hexagonal packing channels(SBA-15,MCM-41),disordered channel network(SiO2,SiO2-Gel),were synthesized by impregnation.XRD and N2adsorption-desorption isotherms confirmed that the specific mesoporous structure was maintained for order channel network catalyst after impregnation.The pore size distribution of disorder channel network catalyst was proved to be wide due to the existing microporous,mesoporous and macroporous pores.Microporous pore may enforce the resistance of mass transport.In contract,catalyst with high order channel network did favor of mass transport.The results showed that aluminum impregnated catalysts with order channel network gave higher catalytic activity and selectivity than that with disorder channel network because diffusion limitation was an important influencing factor in the alkylation and isomerization.In this work,the influences of temperature,catalyst usage and benzene/1-dodecene molar ratio were investigated as well.The isomerization is a parallel reaction to the alkylation and reaction temperature promotes isomerization more than alkylation.The conversion increased when increasing the catalyst usage while 2-LAB selectivity decreased,it can be explained by carbenium concentration.Higher benzene/1-dodecene molar ratio is important for the product distribution.Higher benzene/1-dodecene resulted in higher 2-LAB selectivity.This study led to the development of LAB synthesis catalysts with deeper understanding of relationship between catalyst channel order and catalyst activity.
[1]A.Aitani,J.Wang,I.Wang,S.Al-Khattaf,T.Tsai,Environmental benign catalysis for linear alkylbenzene synthesis:A review,Catal.Surv.Jpn.18(1)(2014)1-12.
[2]G.D.Yadav,M.I.N.I.Siddiqui,UDCaT-5:a novel mesoporous superacid catalyst in the selective synthesis of linear phenyldodecanes by the alkylation of benzene with 1-dodecene,Ind.Eng.Chem.Res.48(24)(2009)10803-10809.
[3]D.P.Fogliatti,S.A.Kemppainen,T.N.Kalnes,J.Fan,D.R.Shonnard,Life cycle carbon footprint of linear alkylbenzenesulfonate from coconut oil,palm kernel oil,and petroleum-based paraffins,ACS Sustain.Chem.Eng.2(7)(2014)1828-1834.
[4]B.Wang,C.W.Lee,T.X.Cai,S.E.Park,Benzene alkylation with 1-dodecene over Y zeolite,Bull.Kor.Chem.Soc.22(9)(2001)1056-1058.
[5]R.J.Larson,T.M.Rothgeb,R.J.Shimp,T.E.Ward,R.M.Ventullo,Kinetics and practical significance of biodegradation of linear alkylbenzene sulfonate in the environment,J.Am.Oil Chem.Soc.70(7)(1993)645-657.
[6]A.M.Nielsen,L.N.Britton,C.E.Beall,T.P.McCormick,G.L.Russell,Biodegradation of coproducts of commercial linear alkylbenzene sulfonate,Environ.Sci.Technol.31(12)(1997)3397-3404.
[7]J.Lin,J.Wang,J.Wang,I.Wang,R.J.Balasamy,A.Aitani,S.Al-Khattaf,T.Tsai,Catalysis of alkaline-modified Mordenite for benzene alkylation of diolefincontaining dodecene for linear alkylbenzene synthesis,J.Catal.300(2013)81-90.
[8]R.Kumar,A.Kumar,A.Khanna,Synthesis,characterization and kinetics of AlCl3supported on silica superacid catalysts for the formation of linear alkylbenzenes,React.Kinet.Mech.Catal.106(1)(2012)141-155.
[9]J.Kang,Y.Rao,M.Trudeau,D.Antonelli,Sulfated mesoporous tantalum oxides in the shape selective synthesis of linear alkyl benzene,Angew.Chem.Int.Ed.47(26)(2008)4896-4899.
[10]N.Pal,A.Bhaumik,Mesoporous materials:Versatile supports in heterogeneous catalysis for liquid phase catalytic transformations,RSC Adv.5(31)(2015)24363-24391.
[11]M.S.Holm,E.Taarning,K.Egeblad,C.H.Christensen,Catalysis with hierarchical zeolites,Catal.Today168(1)(2011)3-16.
[12]M.Horňáček,P.Hudec,K.Velebná,P.Lovás,Positive effect of secondary structure creation in mordenites on alkylation of benzene with 1-tetradecene,Catal.Commun.64(2015)1-5.
[13]X.Hu,M.L.Foo,G.K.Chuah,S.Jaenicke,Pore size engineering on MCM-41:Selectivity tuning of heterogenized AlCl3for the synthesis of linear alkyl benzenes,J.Catal.195(2)(2000)412-415.
[14]D.Sengupta,J.Saha,G.De,B.Basu,Pd/Cu bimetallic nanoparticles embedded in macroporous ion-exchange resins:An excellent heterogeneous catalyst for the Sonogashira reaction,J.Mater.Chem.A2(11)(2014)3986.
[15]C.M.A.Parlett,K.Wilson,A.F.Lee,Hierarchical porous materials:Catalytic applications,Chem.Soc.Rev.42(9)(2013)3876-3893.
[16]D.Dubé,S.Royer,D.Trong On,F.Béland,S.Kaliaguine,Aluminum chloride grafted mesoporous molecular sieves as alkylation catalysts,Microporous Mesoporous Mater.79(1-3)(2005)137-144.
[17]A.Bordoloi,B.M.Devassy,P.S.Niphadkar,P.N.Joshi,S.B.Halligudi,Shape selective synthesis of long-chain linear alkyl benzene(LAB)with AlMCM-41/beta zeolite composite catalyst,J.Mol.Catal.A Chem.253(1-2)(2006)239-244.
[18]Y.Cao,R.Kessas,C.Naccache,Y.Ben Taarit,Alkylation of benzene with dodecene.The activity and selectivity of zeolite type catalysts as a function of the porous structure,Appl.Catal.A Gen.184(2)(1999)231-238.
[19]J.Wang,H.O.Zhu,Alkylation of1-dodecene with benzene over H3PW12O40supported on mesoporous silica SBA-15,Catal.Lett.93(3-4)(2004)209-212.
[20]C.Anand,B.Sathyaseelan,L.Samie,A.Beitollahi,R.Pradeep Kumar,M.Palanichamy,V.Murugesan,E.Kenawy,S.S.Al-Deyab,A.Vinu,Friedel-Crafts benzylation of benzene and other aromatics using 3D mesoporous gallosilicate with cage type porous structure,Microporous Mesoporous Mater.134(1-3)(2010)87-92.
[21]S.Selvakumar,A.P.Singh,Benzoylation of anisole over silicotungstic acid modified mesoporous alumina,Catal.Lett.128(3-4)(2009)363-372.
[22]J.Dou,H.C.Zeng,Preparation of Mo-embedded mesoporous carbon microspheres for Friedel-Crafts alkylation,J.Phys.Chem.C116(14)(2012)7767-7775.
[23]Y.Rao,M.Trudeau,D.Antonelli,Sulfated and phosphated mesoporous Nb oxide in the benzylation of anisole and toluene by benzyl alcohol,J.Am.Chem.Soc.128(43)(2006)13996-13997.
[24]X.Zhang,T.Lin,R.Li,T.Bai,G.Zhang,Properties and reactivity of Fe-P-O catalysts prepared by different methods for benzylation of benzene,Ind.Eng.Chem.Res.51(9)(2012)3541-3549.
[25]B.Wang,C.W.Lee,T.Cai,S.Park,Benzene alkylation with 1-dodecene over H-mordenite zeolite,Catal.Lett.76(1)(2001)99-103.
[26]Y.Sugi,H.Tamada,A.Kuriki,K.Komura,Y.Kubota,S.Joseph,A.Chokkalingam,M.E.Newehy,S.S.Al-Deyab,H.Jang,J.Kim,G.Seo,A.Vinu,Alkaline earth metal modified H-Mordenites.Their catalytic properties in the isopropylation of biphenyl,Ind.Eng.Chem.Res.54(49)(2015)12283-12292.
[27]S.Huang,S.Zhang,L.Yu,Z.Liu,W.Xin,S.Xie,L.Xu,Transalkylation of phenol with cumene on zeolite catalysts,Ind.Eng.Chem.Res.52(32)(2013)10996-11000.
[28]M.Osman,M.M.Hossain,S.Al-Khattaf,Kinetics study of ethylbenzene alkylation with ethanol over medium and large pore zeolites,Ind.Eng.Chem.Res.52(38)(2013)13613-13621.
[29]K.Raveendranath Reddy,D.Venkanna,M.Lakshmi Kantam,S.K.Bhargava,P.Srinivasu,SnO2-SiO2mesoporous composite:A very active catalyst for regioselective synthesis of aromatic ketones with unusual catalytic behavior,Ind.Eng.Chem.Res.54(28)(2015)7005-7013.
[30]P.V.Naumkin,T.N.Nesterova,I.A.Nesterov,A.M.Toikka,V.A.Shakun,Theory and practice of alkyl aromatic hydrocarbon synthesis.1.Branched alkylbenzenes,Ind.Eng.Chem.Res.54(35)(2015)8629-8639.
[31]S.Liu,F.Chen,S.Xie,P.Zeng,X.Du,L.Xu,Highly selective ethylbenzene production through alkylation of dilute ethylene with gas phase-liquid phase benzene and transalkylation feed,J.Nat.Gas Chem.18(1)(2009)21-24.
[32]H.Wu,M.Liu,W.Tan,K.Hou,A.Zhang,Y.Wang,X.Guo,Effect of ZSM-5 zeolite morphology on the catalytic performance of the alkylation of toluene with methanol,J.Energy Chem.23(4)(2014)491-497.
[33]H.Liu,H.Wei,W.Xin,C.Song,S.Xie,Z.Liu,S.Liu,L.Xu,Differences between Zn/HZSM-5 and Zn/HZSM-11 zeolite catalysts in alkylation of benzene with dimethyl ether,J.Energy Chem.23(5)(2014)617-624.
[34]Bokade,Deshpande,Patil,Jain,Yadav,Toluene alkylation with methanol top-xylene over heteropoly acids supported by clay,J.Nat.Gas Chem.16(1)(2007)42-45.
[35]D.Y.Zhao,J.L.Feng,Q.S.Huo,N.Melosh,G.H.Fredrickson,B.F.Chmelka,G.D.Stucky,Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores,Science279(5350)(1998)548-552.
[36]V.R.Choudhary,K.Mantri,AlCl3-grafted Si-MCM-41:Influence of thermal treatment conditions on surface properties and incorporation of Al in the structure of MCM-41,J.Catal.205(1)(2002)221-225.
[37]D.Kim,D.Lim,D.Cho,J.Koh,D.Park,Production of dimethyl carbonate from ethylene carbonate and methanol using immobilized ionic liquids on MCM-41,Catal.Today164(1)(2011)556-560.
[38]W.Chen,S.Huang,Q.Zhao,H.Lin,C.Mou,S.Liu,On the confinement effect during catalytic reaction over Al-MCM-41,Top.Catal.52(1-2)(2009)2-11.
[39]D.Li,F.Shi,S.Guo,Y.Deng,One-pot synthesis of silica gel confined functional ionic liquids:Effective catalysts for deoximation under mild conditions,Tetrahedron Lett.45(2)(2004)265-268.
[40]D.Li,F.Shi,Y.Deng,One-step CN,CO bonds cleavage and CO,CN bonds formation over supported ionic liquid in water,Tetrahedron Lett.45(36)(2004)6791-6794.
[41]H.Xin,Q.Wu,M.Han,D.Wang,Y.Jin,Alkylation ofbenzene with 1-dodecene in ionic liquids[Rmim]+Al2Cl6X-(R=butyl,octyland dodecyl;X=chlorine,bromine and iodine),Appl.Catal.A Gen.292(2005)354-361.
[42]G.Qi,F.Jiang,X.Sun,S.Zhao,Alkylation mechanism of benzene with 1-dodecene catalyzed by Et3NHCl-AlCl3,Sci.China Chem.53(5)(2010)1102-1107.
[43]R.M.Grudzien,B.E.Grabicka,M.Jaroniec,Adsorption and structural properties of channel-like and cage-like organosilicas,Adsorption12(5-6)(2006)293-308.
[44]T.Kim,R.Ryoo,M.Kruk,K.P.Gierszal,M.Jaroniec,S.Kamiya,O.Terasaki,Tailoring the pore structure of SBA-16 silica molecular sieve through the use of copolymer blends and control of synthesis temperature and time,J.Phys.Chem.B108(31)(2004)11480-11489.
[45]G.Peterson,UOP Technology and More Newsletter,UOP LLC,Des Plaines,sept 2011.
[46]J.Carrazza,M.Cleveland,B.Vora,Presented at the Middle East Petrotech,May 2012.
 Chinese Journal of Chemical Engineering2017年10期
Chinese Journal of Chemical Engineering2017年10期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of carboxymethyl cellulose on dissolution kinetics of carboxymethyl cellulose-sodium carbonate two-component tablet
- Crystallization of calcium silicate at elevated temperatures in highly alkaline system of Na2O-CaO-SiO2-H2O☆
- Microencapsulation of stearic acid with polymethylmethacrylate using iron(III)chloride as photo-initiator for thermal energy storage☆
- Novel efficient procedure for biodiesel synthesis from waste oils with high acid value using 1-sulfobutyl-3-methylimidazolium hydrosulfate ionic liquid as the catalyst☆
- Toluene degradation by a water/silicone oil mixture for the design of two phase partitioning bioreactors
- Effects of anaerobic composting on tetracycline degradation in swine manure☆
