Crystallization of calcium silicate at elevated temperatures in highly alkaline system of Na2O-CaO-SiO2-H2O☆
Ganyu Zhu ,Huiquan Li,2,*,Shaopeng Li,Xinjuan Hou ,2,Xingrui Wang
1 Key Laboratory of Green Process and Engineering,National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
2 School of Chemistry and Chemical Engineering,University of Chinese Academy of Sciences,Beijing 100049,China
1.Introduction
High-alumina fly ash(HAFA)is mainly generated in Northwest of China,which is regarded as one of the most important energy base.Its annual emissions of 30 Mt and comprehensive utilization of 20%lead to serious pollutions to soil,air,and water[1].Given the high alumina content of over 45 wt%and the total resource amount of 15 billion ton,HAFA is regarded as a novel,valuable,and renewable mineral resource to substitute bauxite for alumina production[2].Soda-lime sintering is an effective method for alumina extraction,and has been used to treat HAFA in Inner Mongolia in China with an annual amount of 200 kt.In order to improve the Al/Si ratio in solid HAFA and reduce residue level,a desilication process is used to dissolve silica in HAFA(about 40 wt%)with alkali before sintering process[3-6].In order to achieve alkali recycling,silicon removed from HAFA,which exists as silicate in the highly alkaline solution after desilication,is preferred to prepare calcium silicate[7](the diagram is shown in Supplementary material A).Therefore,preparation of calcium silicate in highly alkaline solutions is critical for reduction and economic utilization of HAFA.Silicon treatment in HAFA can also contribute to environment-friendly utilization of low-grade ores and other kinds of solid wastes containing high silicon contents.
There are various amorphous and crystalline forms of calcium silicate.Previous researches mainly focused on CaO-SiO2-H2O system at different temperatures and Ca/Si(C/S)ratios,which are the most important effects on forming calcium silicate of different phases and morphologies[8-11].Some studies have also been performed in NaO-CaO-SiO2-H2O system to investigate the influence of sodium ion on composition,phase,and morphology of calcium silicate[12-14].Blakemanet al.[15]found that semi-crystalline calcium-silicate-hydrate(C-S-H),tobermorite,pectolite,and xonotlite phases could form at constant CaO:SiO2molar ratio 0.83 and varying NaOH:SiO2molar ratio 0.05-0.63.Nocuń-Wczelik[16]obtained the Ca-Na-containing phases at over 20%Na2O by weight and at SiO2content exceeding 50%by weight.In these studies,amorphous silica or quartz was used as SiO2componentin starting materials.The presentstudy aims to the utilization of desilication solution,which contains large amount of sodium and silicate ions(Na/Si molar ratio=2-12),from the HAFA treatment process.Silicate ions in the solution are quite different from solid starting materials in previous reports[17-20],and may affect the structure,phase transition,and crystal lattice of calcium silicate.In addition,influence of sodium to the optimization of calcium silicate was only considered at a relatively low concentration of sodium(Na/Si molar ratio less than 1)in previous researches[21-23].Sodium ions may be arranged into the structure to affect the C/S ratio of calcium silicate to form different phases.Therefore,the research on crystallization control to form amorphous and crystalline calcium silicate of different phases and morphologies in highly alkaline system is very necessary.
In this work,the influences of temperature,C/S ratio,and NaOH concentration,which dominantly affect phase and morphology of calcium silicate,were investigated.In the highly alkaline system,the synthesis condition regions of specific phases were obtained through X-ray diffractometry(XRD).In addition,the morphologies of different phases were determined.In order to reveal the formation mechanism of specific morphology,high resolution transmission electron microscope(HRTEM)and selected-area electron diffraction(SAED)analysis combined with calculations of surface energy were conducted.Meanwhile,the effect of temperature on crystal orientation of calcium silicate was also investigated.Through these studies,the morphology and phase can be controlled in Na2O-CaO-SiO2-H2O system with high alkalinity.
2.Materials and Methods
Model solutions were used to obtain the fundamental rules of crystallization behavior in Na2O-CaO-SiO2-H2O system.CaO,Na2SiO3·9H2O,and NaOH used in this work,which are all supplied by Xilong Chemical Reagent Co.,were analytical grade and used without any further purification.In the experiments,mass of distilled water and crystallization water in Na2SiO3·9H2O was 150.0 g,and the total mass of CaO and calculated Na2SiO3was 10.7 g(L/S=14).C/S ratio was 0.5,0.8,1,1.5,and 2,respectively.All the reactants above were added into a rotational autoclave with the volume of 0.25 L.Then NaOH was added to make the initial concentration of NaOH ranged from 0 mol·L-1to 5 mol·L-1without consideration of sodium in Na2SiO3.Hydrothermal reaction was carried out in the closed autoclave,in which the influence of CO2can be neglected,at 453 K to 533 K under saturated pressure for 5 h,followed by cooling down to room temperature naturally.The obtained solid was filtrated,washed with distilled water,and dried at 353 K for 24 h.
Morphology and phase of the dried powders were detected by field emission scanning electron microscopy(FESEM,JEOL JSM 6700F)and XRD with CuKαdiffraction(PANalytical Empyrean),respectively.In addition,HRTEM and SAED were performed at 200 kV with FEI Tecnai G220S-TWIN to analyze the crystal structure.In order to certify the specific crystal orientation,surface energies of different crystal planes obtained by SAED were also calculated by density functional theory method.The calculation details are given in Supplementary material B.
3.Results and Discussion
In Table 1,all the experimental conditions are listed.The corresponding phases obtained at these conditions,which have been analyzed by XRD(typical figures are given in Supplementary material C),are also shown in the table.It needs to be specified that the mass of Na from Na2SiO3is not considered in the mentioned concentration below.Temperature,C/S ratio,and NaOH concentration have great influences on phases of C-S-H.Although many contributions have been made by the researchers to study the phase relation in CaO-SiO2-H2O or Na2O-CaO-SiO2-H2O system[13,14,24,25],interesting results have been obtained in our studies with the special system and a relatively short reaction time.
3.1.Phase analysis
The phases obtained at different concentrations of NaOH in Na2OCaO-SiO2-H2O system are shown in Fig.1.C-S-H,which is poorly ordered and crystallized,was obtained at 453 K due to the substitution of Na+in the structure to stabilize it against transformation to crystalline forms[23].
In Fig.1(a),the main phases are foshagite,tobermorite,pectolite,and hillebrandite.It can be found that the compositions of the phases are different at different temperatures,while the C/S ratio is 0.5 to 1.5.It keeps stable at C/S ratio of 2.Hillebrandite is stable at hightemperature and will not transform to α-C2SH at the conditions,and this is contrary with previous results[25].Foshagite was obtained at C/S ratio of 1 and temperature of over 423 K in this work,compared with xonotlite as reported in previous work under the same conditions[25].Because the actual concentrations of sodium ions in these reactions were all about 0.8 mol·L-1without the addition of NaOH,the sodium ions brought with Na2SiO3are assumed to be the main reason to affect the phase.To illustrate the influence of alkali with small quantity on the phase change,the hydrothermal reactions were conducted with the C-S-H already synthesized(C/S ratio=1), filtered and completely washed to reach pH value of 9.The experiments have been performed for 5 h at different NaOH concentrations,L/S of 14,and 513 K.In Fig.2,the tiny excursions of the peak position may be caused by the different heights of the samples in the sampling process.It can be seen that xonotlite was obtained in nearly neutral system without the addition of NaOH.With the increasing of NaOH concentration,the enhancement of(210)reflection of foshagite and weakening of(-112)reflection of xonotlite indicates the mass increase of foshagite.Then,it becomes the only phase at NaOH concentration of 0.50 mol·L-1.It means that NaOH with a relatively low concentration in the system may affect the composition and combination during its crystallization process to form different phases.
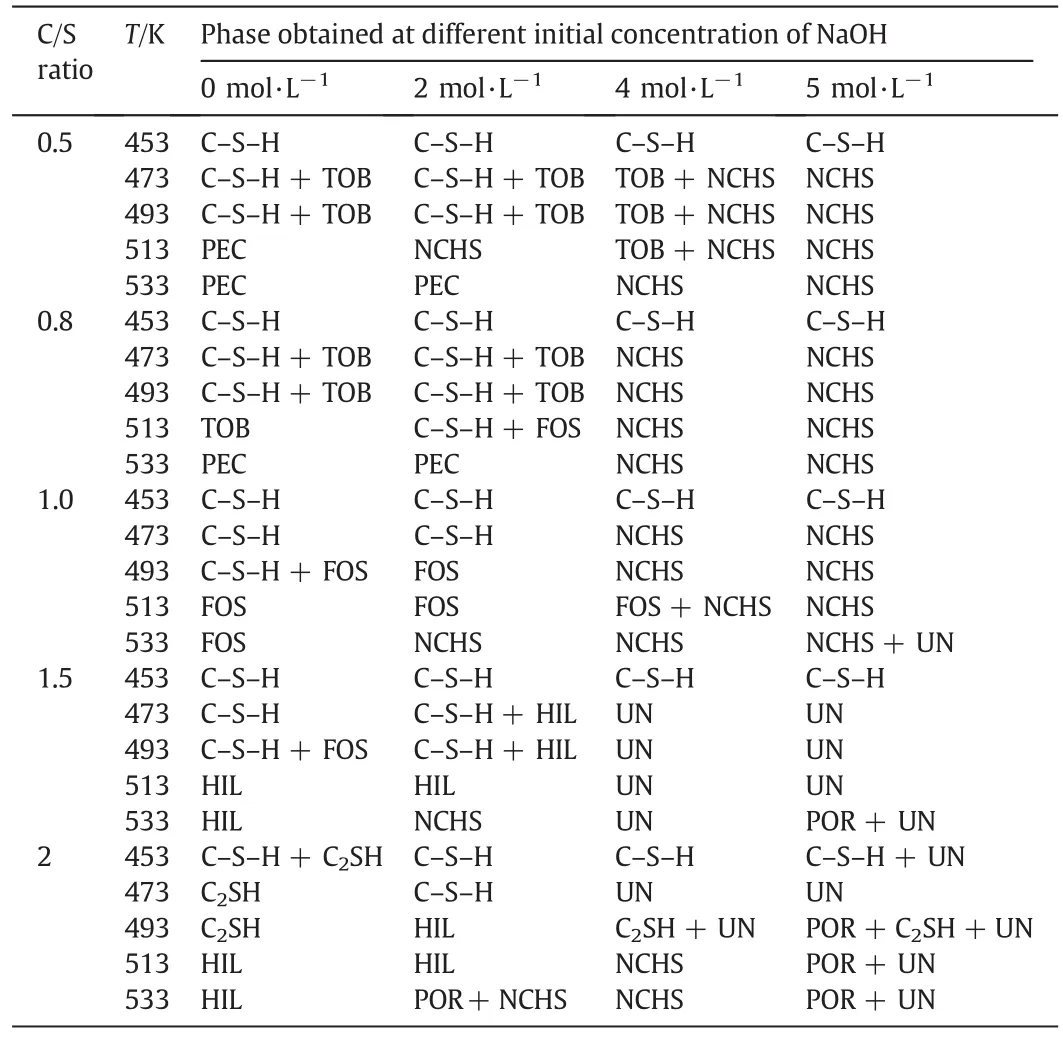
Table 1Phases obtained at different conditions
Returning to Fig.1(b),pectolite and NaCaHSiO4that comprising sodium elements become the dominant phases at 533 K while NaOH concentration increases to 2 mol·L-1.It means that higher temperature is beneficial for the arrangement of Na into the structure of the compounds[16].With the further increasing of NaOH concentration to 4 mol·L-1and 5 mol·L-1in the system(Fig.1(c)and(d)),Na is easily to be combined into the structure.At the temperature of above 473 K,the main phases are Na2Ca3H8Si2O12in high C/S ratio region and NaCaHSiO4in low C/S ratio region,respectively.Meanwhile,the content of Na in the phase increases with C/S ratio and NaOH concentration.C/S ratios decrease and(Na+Ca)/Si ratio increases in the phases obtained at high NaOH concentration comparing with which at the same conditions of low NaOH concentration.
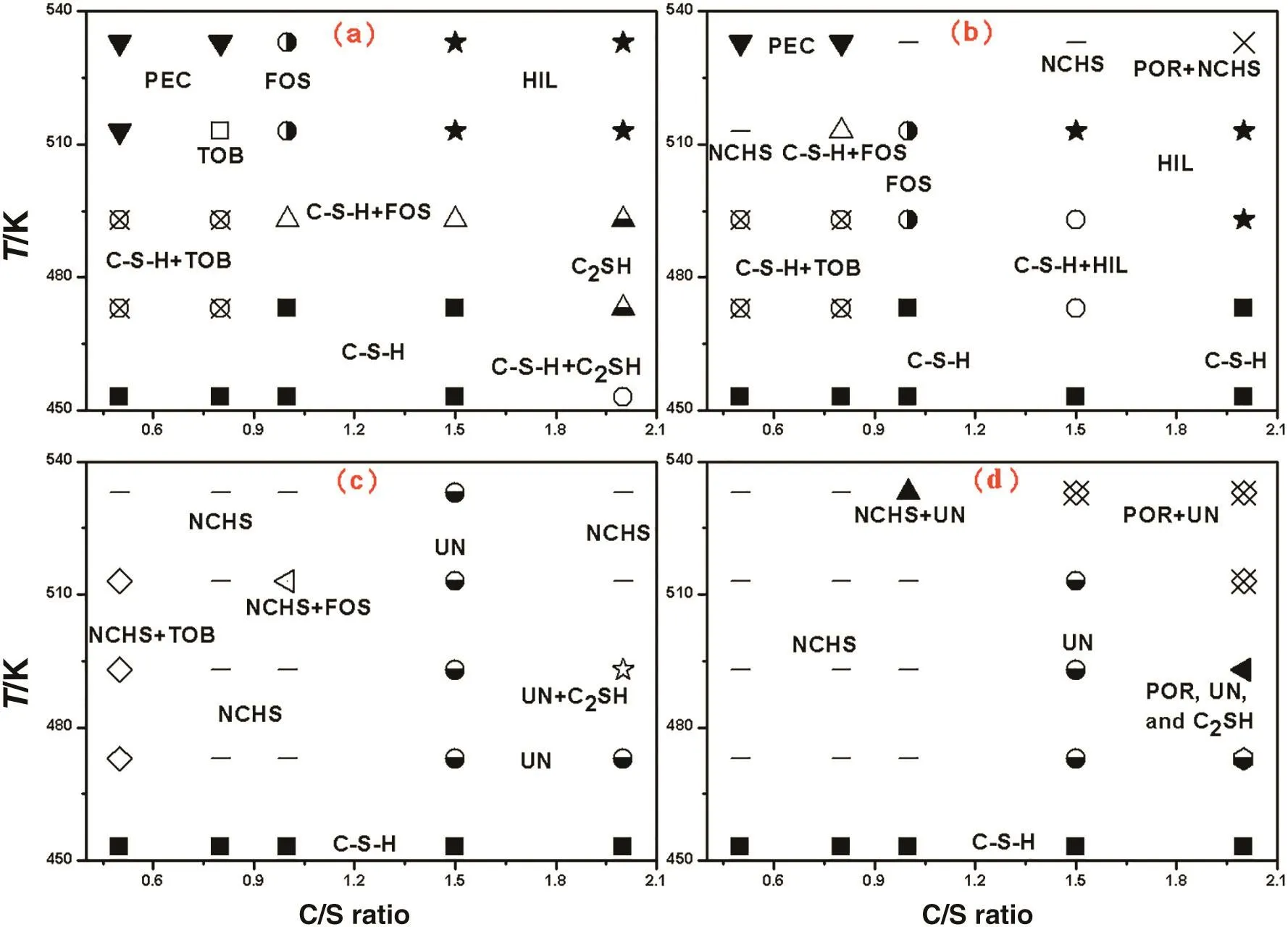
Fig.1.Phases obtained at different conditions in Na2O-CaO-SiO2-H2Osystem.Extra concentration of NaO His(a)0 mol·L-1,(b)2 mol·L-1,(c)4 mol·L-1,and(d)5 mol·L-1,respectively.
3.2.Morphology of different phases
Through the investigations of the phase and morphology under different conditions in Na2O-CaO-SiO2-H2O system,the specific relations between phase and morphology are shown in Fig.3.Tobermorite obtained in this work is stacking and amorphous because of its poor crystallinity as reported in alkaline system[18].NaCaHSiO4is mainly cubic and well dispersed,and Na2Ca3H8Si2O12is plate-like.Only the morphologies of phases in wollastonite group[12],such as pectolite,foshagite,and hillebrandite are nanofibers.However,pectolite is the thinnest and flexible,which leads to the wrapping of the nanofibers.Foshagite is straighter,and mostly keeps separate from each other.Hillebrandite is the thickest in diameter,and the nanofibers grow together to form an aggregate.
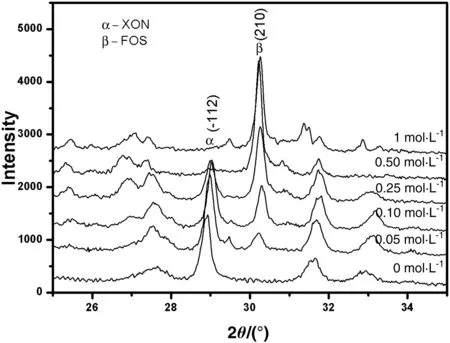
Fig.2.XRD patterns of calcium silicate hydrate at different NaOH concentrations and the temperature of 513 K.
3.3.Growth analysis of nanofiber
As the structure similarity of the inosilicates between the nanofibers,foshagite was taken as an instance to investigate the crystal growth mechanism.HRTEM analysis was studied as shown in Fig.4,which is a typical image of foshagite and has been characterized by XRD.It is obvious that the axial direction is the growth orientation of foshagite.Through the calculation of SAED patterns of foshagite,crystal planes of axial and radial direction are determined as(314)and(311)respectively.The surface energies were predicated by theoretical calculations for(14)and(311)surfaces of foshagite,which are 0.057×104eV·nm-3and 0.027× 104eV·nm-3,respectively.The higher surface energy of(14)indicates that the stability of(314)is weaker than that of(311)surfaces,which means that the growth speed of nanofiber along(314)direction is much faster than that along(311)direction.Therefore,the phase of foshagite has the morphology of nanofiber.
It is noticeable that the morphology of the nanofibers shows different variations with temperature.Morphologies of foshagite and pectolite keep stable at 513 K and 533 K,and only morphology of hillerbrandite changes(Fig.5).The nanofibers of hillerbrandite are well crystallized and have aggregates of radiolitic texture at the temperature of513 K.When the temperature increases to 533 K,the aggregates have been damaged and the alignment of nanofibers becomes parallel,and then they grow together to be larger in width.

Fig.3.Morphologies of different phases.(a)Tobermorite,C/S=0.8,C NaOH=0 mol·L-1,513 K,(b)NaCaHSiO4,C/S=0.5,C NaOH=5 mol·L-1,533 K,(c)Na2Ca3H8Si2O12,C/S=2,C NaOH=5 mol·L-1,473 K,(d)pectolite,C/S=0.5,C NaOH=0 mol·L-1,533 K,(e)foshagite,C/S=1,C NaOH=2 mol·L-1,533 K,and(f)hillebrandite,C/S=1.5,C NaOH=2 mol·L-1,513 K.
Therefore,a further study of XRD patterns of hillerbrandite obtained at different temperatures has been shown in Fig.6.While the temperature varies from 513 K to 533 K,maxima of XRD patterns of hillerbrandite moves from 30.45°to 18.43°,and the peak at 31.49°is also strengthened.According to JCPDS no.00-042-0538,the intensity of the peak at 31.49°is usually maximum.The enhancement of(202)and(403)reflections and the weakening of(311)reflection indicate that the crystal orientation has changed with the increase of the temperature,while the temperature varies from 513 K to 533 K.The change of the growth orientation with the conditions can also be seen in previous reports about the crystal growth of other materials[26,27].
To correlate the morphology change and growth orientation,HRTEM study of hillebrandite obtained at 533 K is shown in Fig.7.In the image,it can be seen that the samples were severely damaged because of fast electron-irradiation[28].Through the calculation of the d-spacing in SAED patterns,the crystal plane reflections of different diffraction patterns and the orientations of different planes were determined.To hillebrandite,crystal planes of axial and radial direction are(310)and(604)respectively.Meanwhile,the plane of(403),which reflection is enhanced as shown in Fig.6,is nearly parallel with(604)of radial direction.The enhancement of(403)reflections means the optimal orientation,which leads the larger in width at the temperature of 533 K.
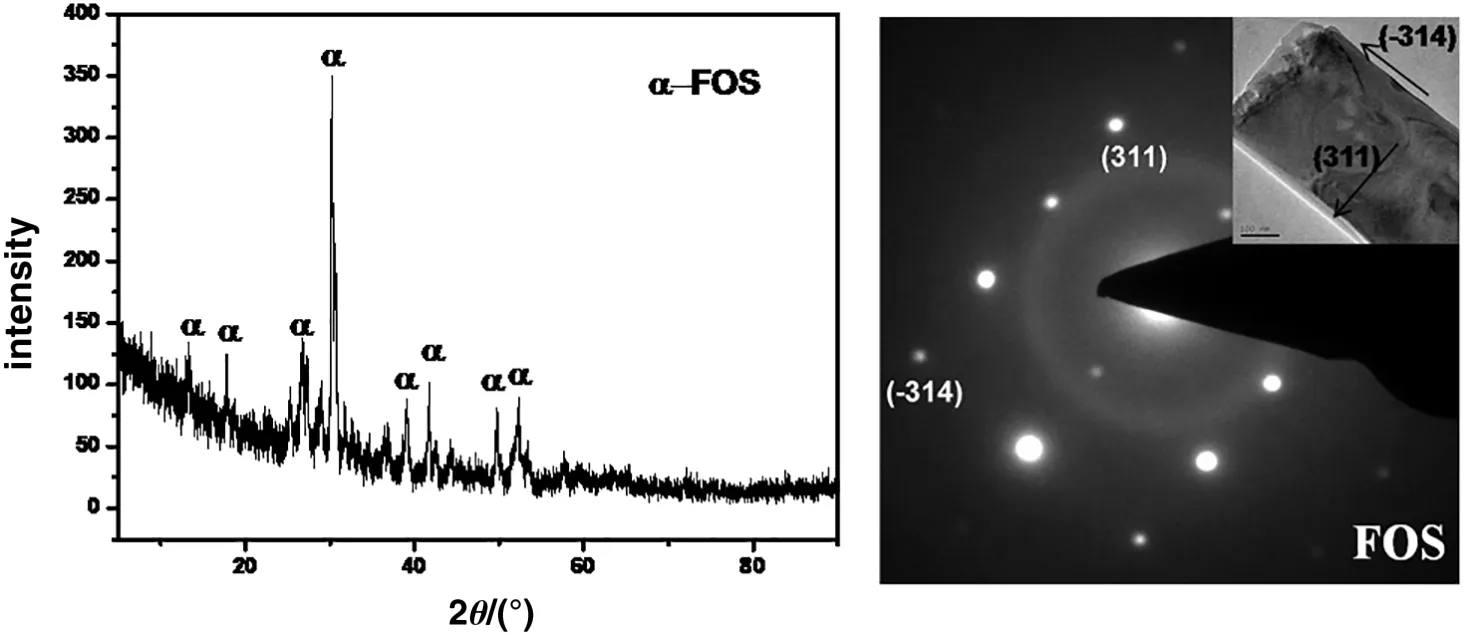
Fig.4.XRD patterns,TEM image,and SAED patterns of foshagite.
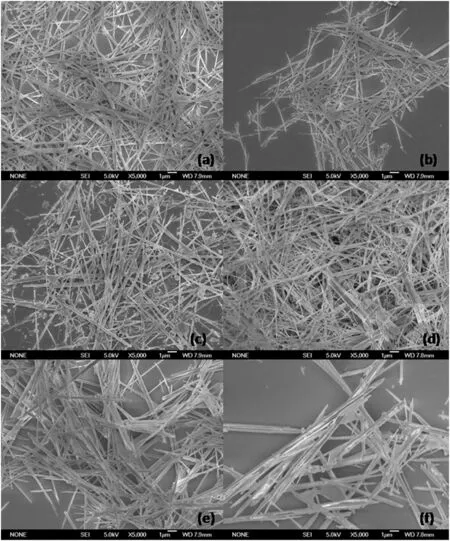
Fig.5.Morphologies of pectolite,foshagite,and hillerbrandite obtained without extra NaOH.(a)Pectolite,513 K,(b)pectolite,533 K,(c)foshagite,513 K,(d)foshagite,533 K,(e)hillebrandite,513 K,and(f)hillebrandite,533 K.
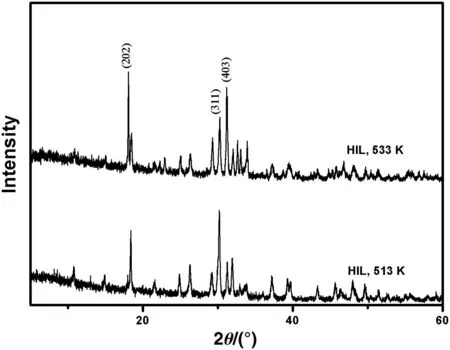
Fig.6.XRD patterns of hillerbrandite obtained without extra NaOH.
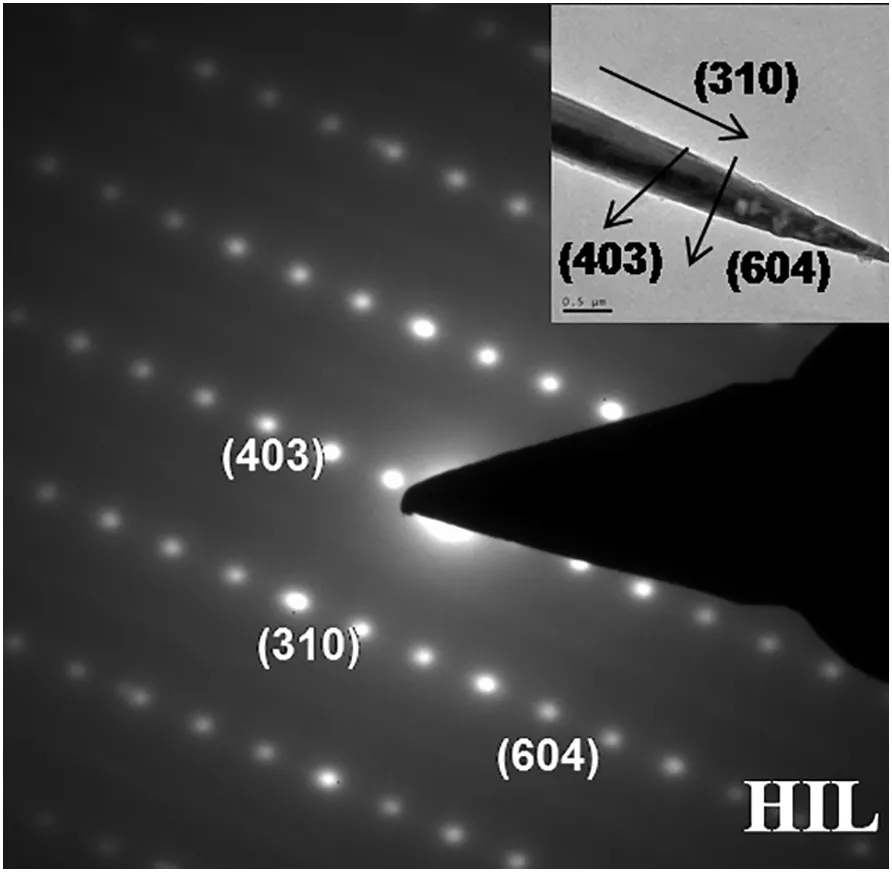
Fig.7.TEM image and SAED patterns of hillebrandite.
4.Conclusions
In this work,systematic investigation of C-S-H morphology and phase was conducted with the range of 453 K to 533 K,initial NaOH concentration of 0 mol·L-1to 5 mol·L-1,and C/S ratio of 0.5 to 2 in Na2O-CaO-SiO2-H2O system for the utilization of silicon in HAFA.The experimental results show that crystal growth is along radial direction at higher temperature.Tobermorite and nanofibers of wollastonite group are the main products at relatively low concentration of NaOH in the system,and Na is rearranged into the structure to form NaCaHSiO4and Na2Ca3H8Si2O12with different C/S ratio at high concentration of NaOH.Only the phases in wollastonite group such as pectolite,foshagite,and hillebrandite,have the morphology of nanofiber.In addition,the formation of morphology as nanofiber is due to the difference of surface energies between axial and radial direction with HRTEM analysis and surface energy calculations.Through these works,C-S-H of specific morphologies and phases can be controlled synthesized.The results can provide the guidance for the preparation of different C-S-H phases and morphologies in the solutions with high alkalinity,which is critical for the utilization of silicon resources in HAFA and other low-grade ore with high silicon content.
Acknowledgments
The theoretical calculation results described in this paper are obtained on the Deepcomp 7000 of Supercomputing Center in the Computer Network Information Center of the Chinese Academy of Sciences.
Supplementary Material
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2017.02.012.
[1]S.Dai,L.Zhao,S.Peng,C.L.Chou,X.Wang,Y.Zhang,D.Li,Y.Sun,Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant,Inner Mongolia,China,Int.J.Coal Geol.81(2010)320-332.
[2]J.M.Sun,P.Chen,Resourcing utilization of high alumina fly ash,Adv.Mater.Res.652-654(2013)2570-2575.
[3]H.Q.Li,J.B.Hui,C.Y.Wang,W.J.Bao,Z.H.Sun,Extraction of alumina from coal fly ash by mixed-alkaline hydrothermalmethod,Hydrometallurgy147-148(2014)183-187.
[4]G.H.Bai,T.Teng,A.G.Wang,J.G.Qin,P.Xu,P.C.Li,Alkali desilicated coal fly ash as substitute of bauxite in lime-soda sintering process for aluminum production,Trans.Nonferrous Met.Soc.20(2010)169-175.
[5]X.B.Xu,Y.B.Zhu,S.Zhang,Z.K.Liang,X.X.Chen,Y.B.Gong,Research on optimizing process of pre-desilication of high-aluminum fly ash,Light Met.7(2013)18-21.
[6]Z.H.Sun,W.J.Bao,H.Q.Li,J.B.Hui,C.Y.Wang,Q.Tang,Mineral phase change of highalumina fly ash during desilication and extraction of Al2O3by alkali dissolution process,Chin.J.Process.Eng.13(2013)403-407.
[7]C.Feng,Y.Yao,Y.Li,X.M.Liu,H.H.Sun,Thermal activation on calcium silicate slag from high-alumina fly ash:A technical report,Clean Technol.Environ.Policy16(2014)667-672.
[8]J.J.Chen,J.J.Thomas,H.F.W.Taylor,H.M.Jennings,Solubility and structure of calcium silicate hydrates,Cem.Concr.Res.34(2004)1499-1519.
[9]D.R.Moorehead,E.R.Mccartney,Hydrothermal formation of calcium silicate hydrates,J.Am.Ceram.Soc.48(1965)565-569.
[10]S.Garrault-Gauffinet,A.Nonat,Experimental investigation of calcium silicate hydrate(C-S-H)nucleation,J.Cryst.Growth200(1999)565-574.
[11]N.Meller,C.Hall,J.S.Phipps,A new phase diagram for the CaO-Al2O3-SiO2-H2O hydro ceramic system at 200 degrees C,Mater.Res.Bull.40(2005)715-723.
[12]E.B.Nelson,G.L.Kalousek,Effect of Na2O on calcium silicate hydrates at elevated temperature,Cem.Concr.Res.7(1977)687-694.
[13]Y.Z.Xi,L.S.D.Glasser,Hydrothermal study in the system Na2O-CaO-SiO2-H2O at 300°C,Cem.Concr.Res.14(1984)741-748.
[14]K.Baltakys,R.Siauciunas,The influence of γ-Al2O3and Na2O on the formation of gyrolite in the stirring suspension,J.Mater.Sci.41(2006)4799-4805.
[15]E.A.Blakeman,J.A.Gard,C.G.Ramsay,H.F.W.Taylor,Studies on the system sodium oxide-calcium oxide-silica-water,J.Chem.Technol.Biotechnol.24(1974)239-245.
[16]W.Nocuń-Wczelik,Effect of Na and Al on the phase composition and morphology of autoclaved calcium silicate hydrates,Cem.Concr.Res.29(1999)1759-1767.
[17]I.G.Richardson,The calcium silicate hydrates,Cem.Concr.Res.38(2008)137-158.
[18]X.L.Hu,K.Yanagisawa,A.Onda,K.Kajiyoshi,Stability and phase relations of dicalcium silicate hydrates under hydrothermal conditions,J.Ceram.Soc.Jpn.224(2006)174-179.
[19]L.Black,K.Garbev,P.Stemmermann,K.R.Hallam,G.C.Allen,Characterisation of crystalline C-S-H phases by X-ray photoelectron spectroscopy,Cem.Concr.Res.33(2003)899-911.
[20]J.A.Gard,H.F.W.Taylor,The crystal structure of foshagite,Acta Crystallogr.13(1960)785-793.
[21]R.A.Rashid,R.Shamsudin,M.A.A.Habid,A.Jalar,In-vitro bioactivity of wollastonite materials derived from limestone and silica sand,Ceram.Int.40(2014)6847-6853.
[22]W.Li,Z.L.Jin,Z.H.Zhang,Application and synthesis of inorganic whisker materials,Prog.Chem.15(2003)264-274.
[23]M.Q.Li,Y.F.Chen,S.Q.Xia,J.H.Li,H.X.Liang,Microstructure and processing of ultralight calcium silicate insulation material,J.Chin.Ceram.Soc.28(2000)401-406.
[24]G.O.Assarsson,Hydrothermal reactions between calcium hydroxide and amorphous silica:The reactions between 180°and 220°,J.Phys.Chem.61(1957)473-479.
[25]S.Y.Hong,F.P.Glasser,Phase relations in the CaO-SiO2-H2O system to 200°C at saturated steam pressure,Cem.Concr.Res.34(2004)1529-1534.
[26]F.Lu,B.Zhao,R.Li,W.D.Ruan,Crystal growth of barium nitrate on thiol-terminated self-assembled monolayers and a Raman spectroscopic investigation of the crystal facets,J.Cryst.Growth426(2015)33-37.
[27]R.Li,J.S.Gandhi,R.Pillai,R.Forrest,D.Starikov,A.Bensaoula,Epitaxial growth of(111)-oriented ZrxTi1-xN thin films onc-plane Al2O3substrates,J.Cryst.Growth404(2014)1-8.
[28]H.F.Xu,P.R.Buseck,TEM investigation of the domain structure and superstructure in hillebrandite,Ca2SiO3(OH)2,Am.Mineral.81(1996)1371-1374.
 Chinese Journal of Chemical Engineering2017年10期
Chinese Journal of Chemical Engineering2017年10期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of carboxymethyl cellulose on dissolution kinetics of carboxymethyl cellulose-sodium carbonate two-component tablet
- Aluminum impregnated silica catalyst for Friedel-Crafts reaction:Influence of ordering mesostructure☆
- Microencapsulation of stearic acid with polymethylmethacrylate using iron(III)chloride as photo-initiator for thermal energy storage☆
- Novel efficient procedure for biodiesel synthesis from waste oils with high acid value using 1-sulfobutyl-3-methylimidazolium hydrosulfate ionic liquid as the catalyst☆
- Toluene degradation by a water/silicone oil mixture for the design of two phase partitioning bioreactors
- Effects of anaerobic composting on tetracycline degradation in swine manure☆
