基质金属蛋白酶9及其组织型抑制因子1在高血压大鼠血液及组织中的变化
李焕敏,李春光
(1. 南方医科大学第三附属医院神经内科,广州 510630; 2.南方医科大学珠江医院神经内科,广州 510280)
研究报告
基质金属蛋白酶9及其组织型抑制因子1在高血压大鼠血液及组织中的变化
李焕敏1,李春光2*
(1. 南方医科大学第三附属医院神经内科,广州 510630; 2.南方医科大学珠江医院神经内科,广州 510280)
目的 探讨肾血管性高血压大鼠(RHR) 血清、脑及血管组织中基质金属蛋白酶9(MMP-9) 和组织型基质金属蛋白酶抑制因子1(TIMP-1)的表达以及血压与两者的关系。方法 雄性SD大鼠80只随机分成RHR组(40只) 和假手术组(40只)。RHR组采用双肾-双夹法制作高血压大鼠模型,鼠尾测压仪测定血压。根据Longa 5评分法和病理学结果确定脑卒中;Western blot和免疫组化测定脑组织和血管中MMP-9和TIMP-1的表达,双抗体夹心ELISA 检测血清MMP-9和TIMP-1水平。结果 与假手术组比较,RHR组大鼠术后2、4、6、8、10、12 周血压均明显升高[(157±9.0)比(128±7.0), (176±10.0)比(122±6.0), (194±8.0)比(117±6.5), (202±12.0)比(124±8.0), (218±15.0)比(126.±8.5), (224±20.0)比(129.±9.0) mmHg,均P< 0.05];术后12周,RHR大鼠血清MMP-9高于假手术组[(783.4±109.79)比(573.4±109.59) ng/mL,P<0.05];而RHR大鼠血清TIMP-1低于假手术组[(313.02±83.9)比(976.19±191.1) pg/mL,P<0.05]。同时,RHR大鼠脑组织和血管MMP-9的表达均明显高于假手术组 (均P<0.05),而TIMP-1的表达则均明显低于假手术组(均P<0.05)。Pearson相关分析显示收缩压与血清、脑及血管组织中的MMP- 9水平均呈正相关(r=0.557,r=0.774和r=0.661,均P<0.05),而与TIMP-1水平均呈负相关(r=-0.481,r=-0.535和r=-0.685,均P<0.01)。结论 肾血管性高血压大鼠血清、脑和血管组织中MMP-9表达升高,而TIMP-1表达降低。两者在血液和组织中的变化趋势保持一致,血压的升高与血液和组织中MMP-9的升高和TIMP-1的降低相关。
肾血管性高血压大鼠;基质金属蛋白酶9;组织型基质金属蛋白酶抑制因子1
高血压是心脑血管病的主要危险因素,基质金属蛋白酶-9(matrix metalloproteinase-9,MMP-9)在高血压相关的心脑血管疾病的病理生理过程中发挥重要作用[1-3]。循环中MMP-9水平可预测冠心病和脑卒中患者的心血管疾病死亡率[4, 5]。在无心血管疾病的人群中,升高的MMP-9与急性心血管事件和/或高血压相关[6]。因此,循环中MMP-9的升高可能使健康人群更易罹患心脑血管疾病。TIMP-1(tissue inhibitor of metalloproteinase-1,TIMP-1)是MMP-9 主要的内源性抑制剂[7],TIMP-1 通过与MMP-9 的锌离子结合而覆盖在其活性中心部位,通过空间位阻效应使底物不能与活性中心结合从而发挥抑制作用。在生理状态下,两者在体内同步表达,以1∶1 的形式构成复合体。在病理条件下这种平衡被打破会导致相应的病理损害[8]。本研究拟通过制作肾血管性高血压大鼠(renovascular hypertensive rats,RHR) 模型,观察RHR模型血清、脑组织及血管中MMP-9和TIMP-1的变化,探讨高血压对MMP-9和TIMP-1的影响。
1 材料与方法
1.1 实验动物
选用SPF级雄性SD大鼠80 只,体重80~120 g,鼠龄2月龄,将大鼠随机分成RHR组40只和假手术组40只。均购于广东省医学实验动物中心【SCXK(粤)2013-0002】,以普通颗粒性大鼠饲料(蛋白质23%、脂肪4.7%、钠盐0.24%)喂养,饮用自来水,12 h循环灯光,恒定湿度,室温(23±3)℃,实验大鼠饲养及组织取材均于中山大学实验动物中心实验设备内进行【SYXK(粤)2012-0081】。
1.2 方法
1.2.1 高血压模型大鼠制作
RHR组大鼠按双肾-双夹法制作。具体方法如下:手术前12 h禁食,用10%水合氯醛(250 mg/kg)腹腔注射麻醉,仰卧固定,常规消毒铺巾,经腹部正中纵行切口,依次分离双侧肾动脉,用自制“Ω”环形银夹(内径为0.2 mm)分别钳夹双侧肾动脉起始部,使肾动脉置于银夹的环形结构中,夹子能够沿动脉滑动,并确认双肾无明显的瘀血、坏死或苍白,整个手术过程不损伤肾、肝、乳糜池及肾静脉。对腹膜和肌肉用连续缝合,皮肤用间断缝合。术后腹腔注射庆大霉素素(8000 U/kg)预防感染。假手术组大鼠只开腹分离肾动脉,不放置银夹,其余步骤同前。术后4~6 h恢复喂食,术后注意保暖,每天早晚观察大鼠肢体活动及呼吸、进食、伤口愈合情况。在饲养过程中出现脑卒中症状及体征的大鼠均被剔除并进行更换。术后4 周收缩压>150 mmHg 为造模成功。假手术组仅打开腹腔,不上银夹,其他步骤同RHR组。两组术后均用8000 U/ kg 庆大霉素腹腔内注射预防感染。术后4~6 h恢复喂食,术后注意保暖,每天早晚观察大鼠肢体活动及呼吸、进食、伤口愈合情况。
1.2.2 血压测定
将大鼠于37℃温箱中预热约15 min,预热后采用BP-98A型Softron大鼠心率血压计经尾动脉测量血压。每只大鼠测3次,取平均值作为该大鼠的血压值。术前3 d连续测血压作为基础血压,术后2、4、6、8、10、12 周固定时间点各测1次。
1.2.3 标本制作
大鼠腹腔麻醉后经升主动脉快速灌注肝素化生理盐水(50 U/mL) 120 mL (滴速为10 mL/min) 以清除血管内血液,断头取脑,分离主动脉,脑组织在冰箱-20℃冷冻10 min后由嘴侧至尾侧每2 mm切成一冠状薄片,先肉眼观察出血情况,再TTC染色观察梗死情况。每亚组随机取8只大鼠进行Western blot检测:切取主动脉及部分额叶,快速置入液氮中,成固体状后放-80℃冰箱保存。将其余大鼠脑及主动脉组织于4%多聚甲醛溶液中固定,常规脱水,石蜡包埋,连续切片进行免疫组化和HE染色,镜检观察。
1.2.4 Western blot
样品制备,BCA法测量蛋白浓度,配胶(5%浓缩胶,12%分离胶),上样(20 μg)。①电泳:100V恒压15 min,150 V恒压电泳至溴酚蓝刚出胶底部止。②转膜:制作电转“三明治”恒压100 V, MMP-9(90 min),TIMP-1(30 min)。内参:GAPDH(40 min)。封闭:转膜结束后,将膜取下,用TBST漂洗3次,每次5 min,根据目的蛋白和GAPDH蛋白大小将膜切开,将膜置于封闭液中;TBST洗膜;结合一抗:分别将MMP-9(购自Cell Signaling Technology公司)和TIMP-1抗体(购自Santa Cruz公司)用5%脱脂奶粉封闭液按1∶750稀释后、GAPDH抗体按1∶10 000稀释后,将PVDF膜放置其中,4℃过夜孵育;TBST洗膜5 min,三次。结合二抗:将膜转移到含有辣根过氧化酶(HRP)标记的羊抗兔多克隆抗体的新鲜封闭液中,室温下放置在脱色摇床上,室温平缓摇动40 min;TBST洗膜5 min,三次。蒸馏水漂洗膜2 min,弃去液体。共洗三次。将杂交膜置于一透明塑料板上,注意不要让膜干燥。用一干净移液器将化学荧光发光底物均匀地加到膜的表面,并使反应持续5 min。用试剂盒提供的滤纸吸去膜表面多余的底物溶液,放至暗盒,显影。
1.2.5 免疫组化
每组随机选取8只大鼠,将大鼠脑和主动脉经过石蜡包埋后在切片机上连续切片作MMP-9和TIMP-1免疫组化染色。具体步骤如下:用二甲苯脱蜡,梯度酒精水化,3% H2O2封闭内源性过氧化物酶,微波加热抗原修复,10% 正常羊血清孵育,滴加兔抗鼠MMP-9(购自Cell Signaling Technology公司)或TIMP-1一抗(1∶100)(购自Santa Cruz公司),37℃孵育50 min,0.01 mol/L PBS漂洗,滴加生物素化羊抗兔二抗(购自Dako公司),37℃孵育40 min,0.01 mol/L PBS漂洗,DAB 显色5~10 min,蒸馏水漂洗,苏木精复染,梯度酒精脱水,二甲苯透明,中性树脂封片观察。免疫组化阳性细胞胞质或胞核内可见棕黄色颗粒或团块状物质,在400 倍光镜下随机选取脑皮层和主动脉的20 个互不重叠的视野拍照,所选区域占整个动脉环和脑皮层的20%~30%,并且使批间变异小于4%。利用Image-Pro plus 6.0软件计算平均光密度。
1.2.6 血清MMP-9和TIMP-1水平测定
术后12周,将大鼠用10%水合氯醛(300 mg/kg) 腹腔注射麻醉后开腹经下腔静脉采血,EDTA-K2抗凝,离心20 min(3000 r/min),收集上清,采用ELISA法(购自上海西唐生物科技有限公司) 测定血清MMP-9和TIMP-1水平。
1.3 统计分析

2 结果
2.1 RHR模型构建及血压变化
大鼠在造模过程中无死亡,造模后饲养过程中死亡5只,其中2例死于术后大出血,3例死于肾衰竭,予以补充复制相同数量的模型。两组的基础血压无显著差异,动态观察显示手术后第2周RHR组大鼠血压即开始升高,直至12周趋于稳定,在饲养过程中假手术组大鼠的血压无明显变化。如图1所示,RHR组术后2、4、6、8、10、12 周血压均明显高于假手术组(均P<0.05)。
2.2 血清MMP-9和TIMP-1水平
术后12周,RHR大鼠血清MMP-9高于假手术组[(783.4±109.79)比(573.4±109.59) ng/mL,P<0.05]。
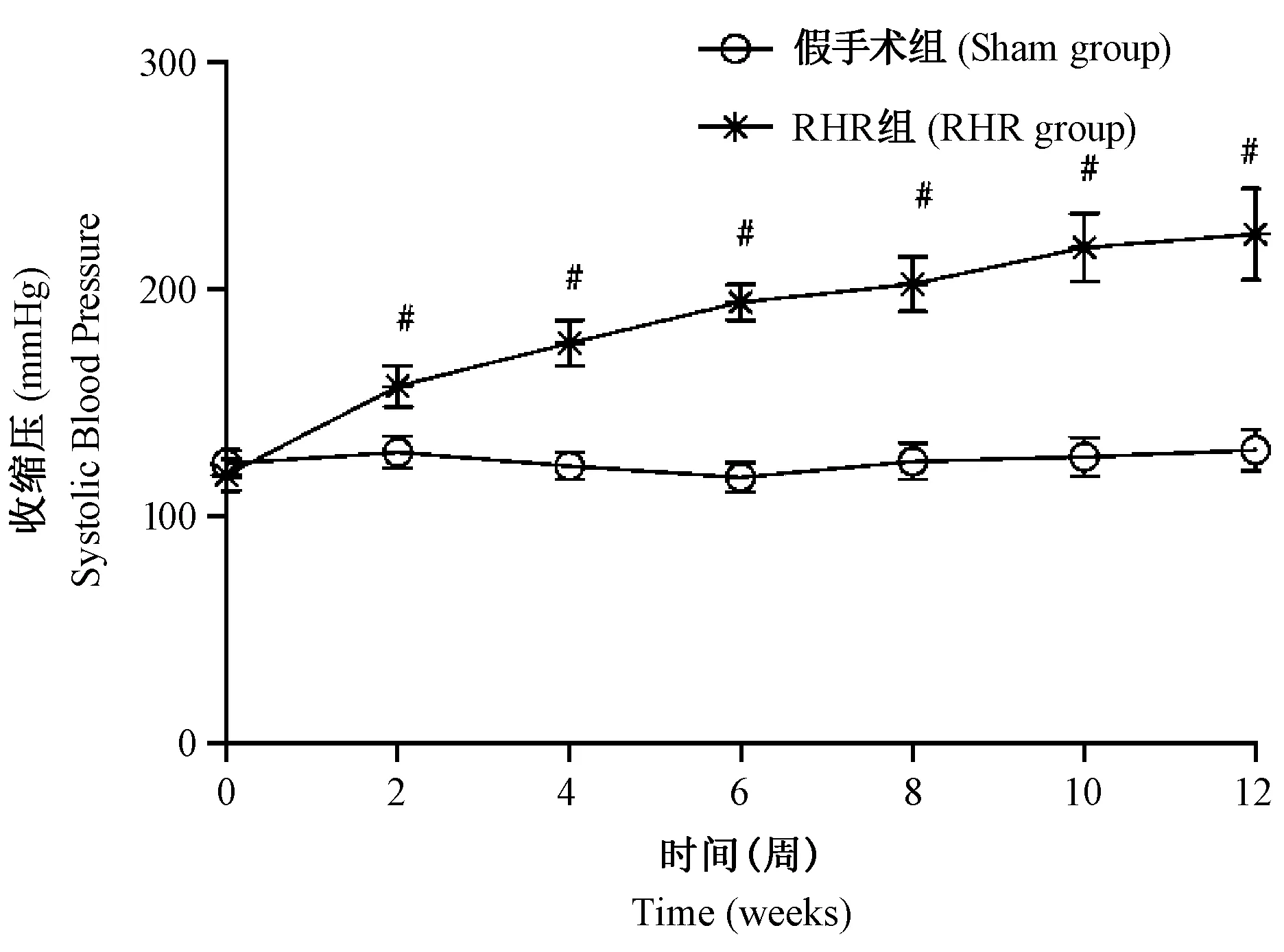
注:与假手术组比较,#P<0.05。图1 两组大鼠12周内不同时间点血压变化Note.Compared with the sham group,#P<0.05.Fig.1 Changes of SBP in the rats at different time-points in 12weeks
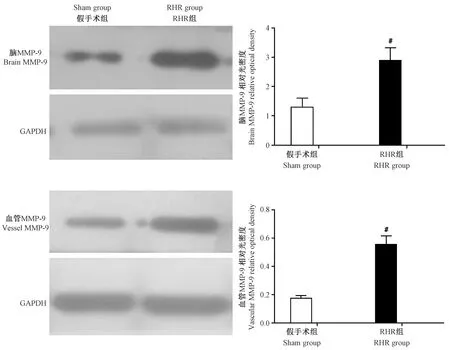
注:与假手术组比较,#P<0.05。图2 各组大鼠脑和主动脉MMP-9表达变化情况Note.Compared with the sham group,#P<0.05.Fig.2 Changes of the MMP-9 protein expression in the brain and vessels of rats in each group
2.3 脑和主动脉MMP-9和TIMP-1的表达
而RHR大鼠血清TIMP-1低于假手术组[(313.02±83.9)比(976.19±191.1) pg/mL,P<0.05]。术后12 周,Western blot 和免疫组化的结果证实RHR组脑和主动脉组织中MMP-9 的表达均高于假手术组(P<0.05),见图2~3;RHR组脑和主动脉组织中TIMP-1的表达则均低于假手术组(P<0.05),见图4~5。
2.4 血压与MMP-9和TIMP-1的相关分析
剔除观察过程中发生脑卒中大鼠,分别对收缩压与血清、脑组织和血管中MMP-9和TIMP-1的关系进行相关分析,如图6~8所示,收缩压与血清及组织中MMP-9表达呈正相关(r=0.557,r=0.774和r=0.661,均P<0.05),与血清及组织中TIMP-1表达呈负相关(r=-0.481,r=-0.535和r=-0.685,均P<0.01)。

注:与假手术组比较,#P<0.05。脑标尺:20 μm,血管标尺:200 μm。图3 各组大鼠脑和主动脉MMP-9免疫组化(×400)Note.Compared with the sham group,#P<0.05. Scale bar=20 μm for brain and 200 μm for blood vessels.Fig.3 Changes of MMP-9 expression and distribution in the brain and vessels of rats in each group

注:与RHR组比较,#P<0.05。图4 各组大鼠脑和主动脉TIMP-1表达情况Note.Compared with the RHR group,#P<0.05.Fig.4 Changes of TIMP-1 protein expression in the brain and vessels of rats in each group
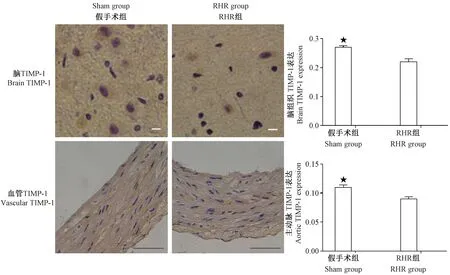
注:与RHR组比较,★P<0.05,脑标尺:20 μm,血管标尺:200 μm。图5 各组大鼠脑和主动脉TIMP-1免疫组化Note.Compared with the RHR group,★P<0.05.Scale bar=20 μm for brain and 200 μm for vessels.Fig.5 Changes of the TIMP-1 expression and distribution in the brain and vessels of rats in each group(Immunohistochemical staining)

图6 RHR组大鼠术后12 周收缩压与血清MMP-9和TIMP-1水平的相关分析Fig.6 Correlation analysis between the systolic blood pressure and serum MMP-9 and TIMP-1 levels in the rats of RHR group
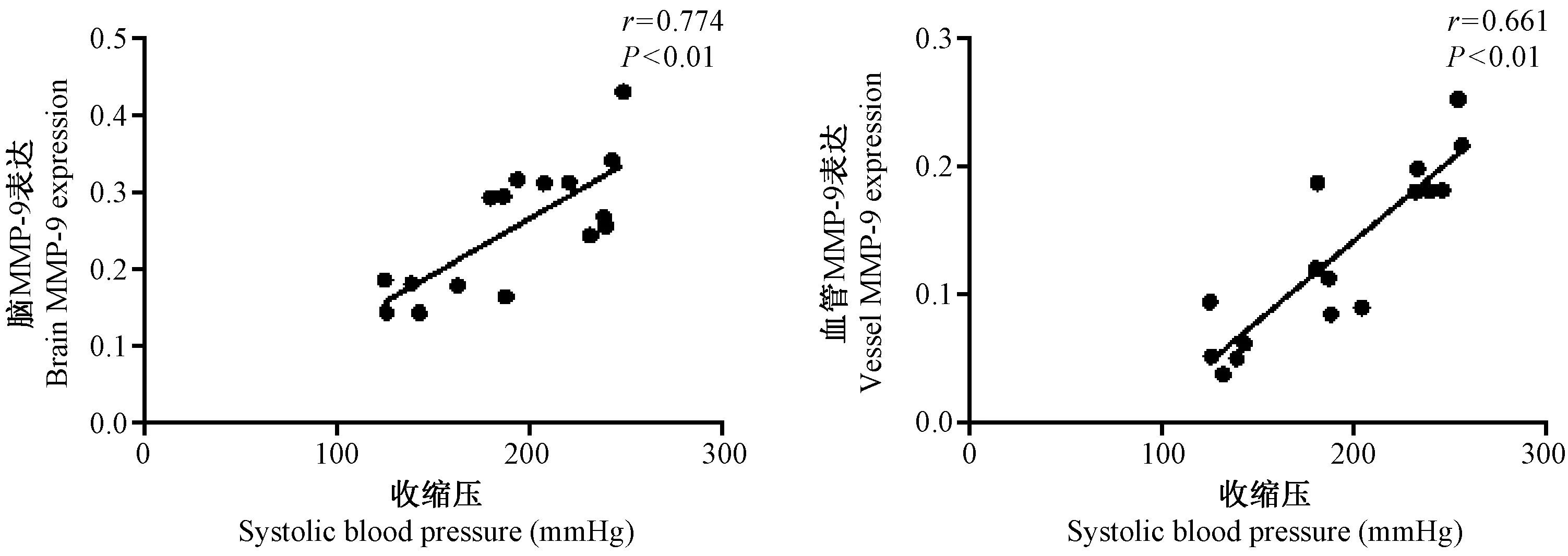
图7 RHR组大鼠术后12 周收缩压血压与脑及主动脉MMP-9蛋白表达的相关分析Fig.7 Correlation analysis between the systolic blood pressure and brain and vascular MMP-9 protein expression in the rats of RHR group
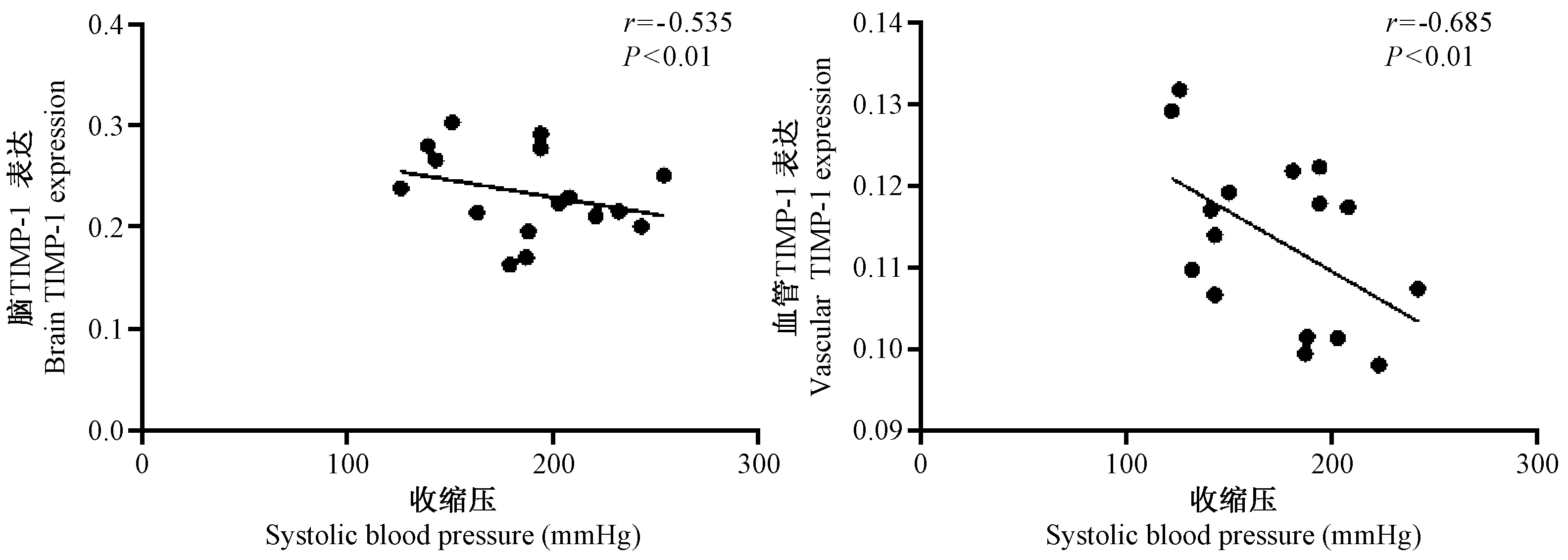
图8 RHR组大鼠术后12 周收缩压血压与脑及主动脉TIMP-1蛋白表达的相关分析Fig.8 Correlation analysis between the systolic blood pressure and brain and vascular TIMP-1 protein expression in the rats of RHR group
3 讨论
高血压是心脑血管疾病最重要的危险因素。MMP-9 是基质金属蛋白酶系家族中降解细胞外基质(ECM)的主要蛋白溶解酶,具有降解I、IV、V型胶原及明胶、软骨蛋白多糖、层粘蛋白、纤维连接蛋白等能力。MMP-9参与了高血压性血管重塑过程[9]。此外,MMP-9 在动脉粥样硬化的形成、发展和破裂过程中发挥关键作用[10, 11]。MMP-9 还与动脉粥样硬化斑块内出血有关[12]。TIMP-1是MMP-9 主要的内源性抑制剂[7],在小鼠模型中通过升高TIMP-1可减轻动脉粥样硬化的进展[13]。研究证实,MMP-9在脑动脉瘤的发展过程中发挥关键作用,通过抑制MMP-9 的基因表达可抑制弹性蛋白酶诱导的脑动脉瘤发生[14]。灭活TIMP-1 的基因可促进动脉瘤形成[15],而促进局部TIMP-1 表达可阻止动脉瘤进展和破裂[16]。最近研究证实MMP-9通过破坏脑血管的基底膜导致血管破裂参与高血压脑出血的发病过程[17, 18]。
本研究发现在高血压大鼠模型的血清、脑和主动脉中MMP-9水平明显升高。这与既往研究发现高血压大鼠脑及血清中MMP-9升高的结果相一致[19]。在AngII 诱导的高血压模型中,在传导血管MMP-9的活性增高[20]。具体机制可能是通过管腔内高压力、氧化应激等机制实现的[21]。同时,研究发现RHR模型血清、脑和主动脉组织中TIMP-1水平均降低。既往研究发现高血压患者血液TIMP-1降低[22,23]。相关性分析发现收缩压与血清、脑及主动脉组织中MMP-9表达均呈正相关,说明持续的高血压可能诱导MMP-9表达,升高的MMP-9可能参与高血压大鼠脑和血管的病理损害过程。同时,相关性分析发现收缩压与血清、脑及主动脉组织中TIMP-1表达均呈负相关,表明持续的高血压可诱导TIMP-1表达降低。上述结果表明高血压可诱导MMP-9/TIMP-1在血液和组织的表达失衡,从而导致MMP-9的净效益升高,两者的失衡可能与高血压性心脑血管病的病理生理过程有关。两种标志物在血液和组织中的变化趋势一致,关于两个标志物在血液与组织之间是否存在交互关系需要进一步深入研究。
在临床上,心脑血管疾病的患者会服用很多药物,那么是否有药物会影响MMP-9和TIMP-1的水平呢?研究发现在众多的抗高血压药物中,氨氯地平[24]、依那普利[25]和氯沙坦[26]可降低MMP-9。而非洛地平和地尔硫卓则对MMP-9无影响[27];乐卡地平[28]、依那普利[23]、赖诺普利[22]和氯沙坦[23]对TIMP-1无影响,而坎地沙坦不仅可降低MMP-9而且可升高TIMP-1[22]。他汀类药物是心脑血管疾病防治的常用药物,研究发现他汀可下调MMP-9的表达[29]。这为我们的临床用药选择提供了一定的参考。
本研究发现高血压大鼠血液和组织中MMP-9/TIMP-1表达失衡,MMP-9/TIMP-1失衡可能与高血压及相关心脑血管疾病有关。MMP-9和TIMP-1可能成为相关的药物干预靶点,下调MMP-9和升高TIMP-1的药物可能会发挥保护作用。MMP-9和TIMP-1在血液和组织中的变化趋势保持一致,因此,血液中MMP-9和TIMP-1的变化有可能被用作评估高血压及相关心脑血管疾病发病风险的生物标志物,这使两者具备临床应用前景。本文的不足之处在于未能动态检测术后血压与血清MMP-9/TIMP-1变化的相关性,从而使论据更具说服力,这需要在今后的实验中进一步验证。
[1] Kelly D, Cockerill G, Ng LL, et al. Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study[J]. Eur Heart J 2007;28:711-718.
[2] Gargiulo S, Sottero B, Gamba P, et al. Plaque oxysterols induce unbalanced up-regulation of matrix metalloproteinase-9 in macrophages through redox-sensitive signaling pathways: Implications regarding the vulnerability of atherosclerotic lesions[J]. Free Radic Biol Med,2011, 51(4):844-855.
[3] Jin D, Sheng J, Yang X, et al.Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm[J]. Surg Neurol, 2007, 68(Suppl 2):S11-16.
[4] Blankenberg S, Rupprecht HJ, Poirier O, et al. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease[J]. Circulation 2003, 107:1579-1585.
[5] Montaner J, Molina CA, Monasterio J, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke[J]. Circulation 2003, 107:598-603.
[6] Timms PM, Mannan N, Hitman GA, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? [J]. QJM 2002, 95(12):787-796.
[7] Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade[J]. Crit Rev Biochem Mol Biol. 2013.48(3):222-272.
[8] Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease[J]. Biochem Pharmacol. 2008.75(2):346-359.
[9] Derosa G, D’Angelo A, Ciccarelli L, et al. Matrix metalloproteinase-2, -9, and tissue inhibitor of metalloproteinase-1 in patients with hypertension[J]. Endothelium 2006,13(3): 227-231.
[10] Rodriguez-Feo JA, Hellings WE, Moll FL, et al. Caveolin-1 influences vascular protease activity and is a potential stabilizing factor in human atherosclerotic disease[J]. PLoS One.2008.3(7):e2612.
[11] Loftus IM, Naylor AR, Goodall S, et al. Increased matrix metalloproteinase-9 activity in unstable carotid plaques: a potential role in acute plaque disruption[J]. Stroke. 2000,31(1):40-47.
[12] Jiang XB, Wang JS, Liu DH, et al. Overexpression of matrix metalloproteinase-9 is correlated with carotid intraplaque hemorrhage in a swine model[J]. J Neurointerv Surg. 2013.5(5):473-477.
[13] Rouis M, Adamy C, Duverger N, et al. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E-deficient mice[J]. Circulation. 1999.100(5):533-540.
[14] Aoki T, Kataoka H, Morimoto M, et al. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats[J]. Stroke. 2007,38(1):162-169.
[15] Silence J, Collen D, Lijnen HR. Reduced atherosclerotic plaque but enhanced aneurysm formation in mice with inactivation of the tissue inhibitor of metalloproteinase-1 (TIMP-1) gene[J]. Circ Res. 2002,90(8):897-903.
[16] Allaire E, Forough R, Clowes M, et al. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model[J]. J Clin Invest.1998,102(7):1413-1420.
[17] Wakisaka Y, Chu Y, Miller JD, et al. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice[J]. J Cerebr Blood Flow MeTab. 2010,30(1): 56-69.
[18] Wakisaka Y, Chu Y, Miller JD, et al. Critical role for copper/zinc-superoxide dismutase in preventing spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice[J].Stroke.2010.41(4):790-797.
[19] 刘淑云,张平,聂亚雄,等. 易卒中型肾血管性高血压大鼠脑组织及血清中基质金属蛋白酶9的表达[J]. 中华高血压杂志,2011, 19(5): 464-468.
[20] Flamant M, Placier S, Dubroca C, et al. Role of matrix metalloproteinases in early hypertensive vascular remodeling[J]. Hypertension.2007,50(1):212-218.
[21] Lehoux S, Lemarie CA, Esposito B, et al.Pressure-induced matrix metalloproteinase-9 contributes to early hypertensive remodeling[J]. Circulation.2004,109:1041-1047.
[22] Onal IK, Altun B, Onal ED, et al. Serum levels of MMP-9 and TIMP-1 in primary hypertension and effect of antihypertensive treatment[J].Eur J Intern Med,2009,20:369-372.
[23] Li-Saw-Hee FL, Edmunds E, Blann AD, et al. Matrix metalloproteinase-9 and tissue inhibitor metalloproteinase-1 levels in essential hypertension. Relationship to left ventricular mass and anti-hypertensive therapy[J].Int J Cardiol, 2000, 75:43-47.
[24] Zervoudaki A, Economou E, Stefanadis C, et al. Plasma levels of active extracellular matrix metalloproteinases 2 and 9 in patients with essential hypertension before and after antihypertensive treatment[J]. J Hum Hypertens, 2003, 17:119-124.
[25] Schieffer B, Bunte C, Witte J, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease[J]. J Am Coll Cardiol,2004, 44:362-368.
[26] Derosa G, Maffioli P, Ferrari I, et al. Different actions of losartan and ramipril on adipose tissue activity and vascular remodeling biomarkers in hypertensive patients[J].Hypertens Res 2011, 34:145-151.
[27] Zervoudaki A, Economou E, Pitsavos C, et al. The effect of Ca2+channel antagonists on plasma concentrations of matrix metalloproteinase-2 and -9 in essential hypertension[J]. Am J Hypertens 2004, 17:273-276.
[28] Martinez ML, Lopes LF, Coelho EB, et al. Lercanidipine reduces matrix metalloproteinase-9 activity in patients with hypertension[J]. J Cardiovasc Pharmacol,2006, 47:117-122.
[29] Souza-Costa DC, Sandrim VC, Lopes LF, et al.Anti-inflammatory effects of atorvastatin: modulation by the T-786C polymorphism in the endothelial nitric oxide synthase gene[J]. Atherosclerosis, 2007, 193:438-444.
Changes of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1in serum and tissues in hypertensive rats
LI Huan-min1, LI Chun-guang2*
(1. Department of Neurology, Third Affiliated Hospital of Southern Medical University, Guangzhou 510630,China; 2.Department of Neurology, Zhujiang Hospital of Southern Medical University,Guangzhou 510280)
Objective To investigate the expressions of metalloproteinase-9 (MMP-9)in serum, brain and aorta matrix and tissue inhibitor of metalloproteinase-1(TIMP-1) in renovascular hypertensive rats (RHR),and to evaluate the association between blood pressure and levels of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of metalloproteinase-1(TIMP-1).Methods Eighty healthy male SD rats were randomly divided into RHR group (n=40) and sham-operated group (n=40). Hypertension was induced by two-kidney, two-clip (2K-2C)clamps.Systolic blood pressure (SBP) was measured every 2 weeks during 12 weeks using a tail pressure meter. Stroke was confirmed by Longa’s five-point scale and pathological examination. The expressions of MMP- 9 and TIMP-1 in the brain and aorta tissues were detected by Western blot and immunohistochemistry. The levels of serum MMP-9 and TIMP-1 were measured by double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA). Results Compared with the sham-operated group, SBP stayed significantly elevated in the RHR group at 2, 4, 6, 8, 10 and 12weeks after the operation [(157±9.0) vs. (128±7.0), (176±10.0) vs. (122±6.0), (194±8.0)vs. (117±6.5), (202±12.0)vs. (124±8.0), (218±15.0) vs. (126.±8.5),and (224±20.0)vs. (129.±9.0) mm Hg, allP< 0.05]. 12 weeks after the surgery, the level of serum MMP-9 in the RHR group was kept significantly higher than that in the sham-operated group [(783.4±109.79)vs. (573.4±109.59) ng/mL,P<0.05],and the serum TIMP-1 level was lower in the RHR group than that in the sham-operated group[(313.02±83.9) vs. (976.19±191.1) pg/mL,P<0.05]. MMP-9 expressions were significantly higher in the brain and aorta in the RHR group than that in the sham-operated group(bothP<0.05), and TIMP-1 expressions were lower than that in the sham-operated group(bothP<0.05).The Pearson correlation analysis showed that MMP-9 levels in serum,brain and aorta were positively correlated with systolic blood pressure (r=0.557,r=0.774 andr=0.661,allP<0.05), and TIMP-1 levels were negatively correlated with systolic blood pressure(r=-0.481,r=-0.535 andr=-0.685,allP<0.01). Conclusions Hypertension induces increased MMP-9 and decreased TIMP-1 in serum, brain and aorta in renovascular hypertensive rats. There are consistent alterations of circulating and tissue MMP-9 and TIMP-1 levels in renovascular hypertensive rats. There is a relationship between increased blood pressure and high MMP-9 and low TIMP-1 in serum and tissues.
Renovascular hypertensive rats; Stroke; Matrix metalloproteinase-9; Tissue inhibitor of metalloproteinase-1
LI Chun-guang,E-mail: lichunguang2005@126.com
国家自然科学基金项目(编号:81500985);南方医科大学科研启动项目(编号:PY2014N080)。
李焕敏(1980-),女,主治医师,硕士研究生,研究方向:神经重症及脑血管病。E-mail: lihuanmin_2004@126.com
李春光(1980-),男,主治医师,博士研究生,研究方向:脑血管病。E-mail: lichunguang2005@ 126.com
Q95-33
A
1005-4847(2017) 02-0138-08
10.3969/j.issn.1005-4847.2017.02.005
2016-09-18

