石蜡断面超疏水机理的探究及其在油水分离方面的应用
刘 滨,徐 都,邱文莲,沈 烈
(高分子合成与功能构造教育部重点实验室,浙江大学高分子科学与工程学系,浙江 杭州 310027)
石蜡断面超疏水机理的探究及其在油水分离方面的应用
刘 滨,徐 都,邱文莲,沈 烈
(高分子合成与功能构造教育部重点实验室,浙江大学高分子科学与工程学系,浙江 杭州 310027)
实验发现石蜡断面具有良好的超疏水性能,石蜡断面的水滴接触角达到152.4±3°,石蜡外表面水滴接触角为108±3°。利用铜网在石蜡表面复制其断面形貌,可快速制备大面积超疏水石蜡表面,接触角高达162.4±3°,滚动角小于3°。利用此超疏水石蜡表面设计出一种自动油水分离装置,可实现油水连续分离,收集正己烷速率可达0.67mL/s。扫描电子显微镜(SEM)观察发现石蜡断面粗糙度很高且存在较多连续和非连续的晶区,而石蜡外表面较光滑。扫描差示量热仪(DSC)和X射线衍射(XRD)证明石蜡具有一定的熔限和较高的结晶度。偏光显微镜(POM)观察石蜡熔体的冷却过程,发现冷却过程中有大量不同尺寸的结晶产生。实验结果表明:石蜡断面和石蜡外表面的超疏水性差别较大的原因在于断面具有较高的粗糙度,而外表面粗糙度较低,断面的高粗糙度来自于石蜡内部存在大量的连续晶相和非晶相。当石蜡断裂时,晶相一方面充当了应力集中点,导致断面出现不规则的裂纹;另一方面,晶相充当了微纳尺度的“填料”;晶相和非晶相在断裂面的凸起也会导致断面粗糙度高。
超疏水; 石蜡; 断面; 结晶相; 油水分离
1 Introduction
Nowadays, petroleum leakage has become an international problem because of its severe damage to the environment. Material scientists make huge efforts to solve this problem, and the key to handle the leakage is to find a sort of material to adsorb the petroleum fast and efficiently, since now, all kinds of materials have been tried to adsorb the petroleum in the past few years, the most promising material described by scientists as the “ultimate sponge” is the aerogel such as the silica aeroge and the graphene aerogel, with millions of tiny pores on its surface making it ideal for absorbing pollutants in water and other areas, it is of high value but not widely used due to the high cost and complicated or lengthy fabrication processes, in addition, this process may not be continuous, which also limit the application of the aerogel because of the efficiency. Convenient low-cost method with a continuous process is in much need, recently, scientists have developed the superhydrophobic materials for the oil-water separation technology.
Superhydrophobic materials with WCAs above 150° play a key role for the fabrication of self-cleaning surfaces, wind-shields, navigation systems, solar panels[1-2], micro-fluidic systems[3], textiles[4], and anti-icing products[5-6], oil-water separation system and so on. The growing demand for superhydrophobic surfaces has prompted extensive and ever-increasing research and development over the last few years.
Most of the superhydrophobic plant surfaces found in nature are composed of hierarchical structures consisting of 3D wax crystallites in which micro- and nanoroughness-scales are combined[7-8]. From the inspiration in nature and a lot of previous work related to the superhydrophobic surfaces, we have learnt that both roughness and hydrophobic chemical composition are important for superhy-drophobic surfaces, usually fabricated by making micro/nano structures on the surfaces of the materials with a low surface energy. Up to now, molding, electro-spinning, region separation, crystal growth, templating, etching, physical or chemical vapor deposition, electro-chemical reaction and deposition, sol-gel processing, self-assembly, hot-pressing and thermal replica molding-peeling have been used for the fabrication of the superhydrophobic surfaces[9-22].S. Pechook uses two kinds of paraffin wax with different molecular weight (Mw) to achieve hierarchical roughness on paraffin wax surfaces[23]. Zhang fabricates superhydrophobic coating with the mixture of beeswax and carnauba wax by a process of emulsion and annealing[24]. These methods are all interesting but a little complex because they all contains a step of increasing the roughness of the surfaces, which is the key step but the most difficult step. With a number of complicated methods invented to increase the roughness, the facile fracture method seemed to be ignored by surface scientists, as we all know, fracture surfaces of some materials are naturally rough, such as the wood, the paper. If the materials are hydrophobic enough, the fracture surfaces may be superhydrophobic with a high possibility.
Herein, a facile method to prepare superhy-drophobic functional surfaces with paraffin is presented and just one fracture step is needed. Paraffin is extracted from the oil and consists of short chain alkane with a low surface energy, and usually, the water contact angle of the paraffin is around 110°, while we discovered that the WCAs of the paraffin’s fracture surfaces reach a high level of 154° showing good superhydrophobicity.
2 Experimental
2.1 Materials
Paraffin with a melting point 62-64℃ was provided by Aladdin Industrial Corporation, China, and used for the preparation of the superhydrophobic surfaces. Copper meshes were provided by the Qingfeng company, China. N-hexane was bought from the Shanghai Lingfeng Chemical reagent CO., LTD. Sudan Ⅲ was provided by the Tianjing Zhiyuan Chemical reagent CO., LTD.
2.2 Preparation of the superhydrophobic fracture surfaces
First, we fused paraffin in a mold made of aluminum foil at the temperature of 100℃, and cooled the melt down at the room temperature until it froze with a shape of cuboid. Then the molded paraffin was fractured at the direction perpendicular to the long axis, and the two fracture surfaces formed at the cracked point.
2.3 Fabrication of superhydrophobic surfaces with a sheet of copper mesh
As depicted in Fig.1, a sheet of copper mesh spread out at the bottom of the iron mold with the solid paraffin put on it and the iron mold was heated at 100℃ until all the paraffin fused to the liquid state, after that, the mold was cooled down at room temperature til the paraffin melt froze to the solid state. At last, the solid paraffin was taken out and the copper mesh was peeled off, the newly-fabricated surfaces showed excellent superhydrophobicity.
2.4 Automatic oil-water separation system
Fig.2 displays the fabrication process for the core device of the oil-water separation system. Three pieces of copper mesh were folded in the plasticvessel, the paraffin was fused first (Fig.2a), then the paraffin melt was poured into the vessel (Fig.2b), when the melt froze (Fig.2c), the copper mesh were peeled off and the separation core device was made (Fig.2d). In Fig.3 the oil was driven into the channels of the solid paraffin, and with a straw drawing oil from the center of the core separation device, the oil-water separation process could be continuous and efficient.

Fig.1 Illustration of the process to fabricate superhydrophobic surfaces with copper mesh
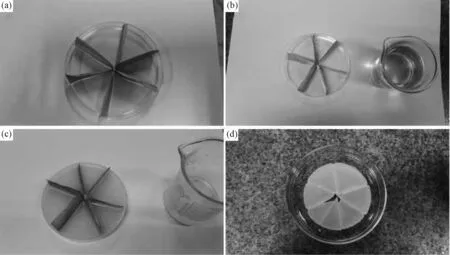
Fig.2 Fabrication process for the core device of the oil-water separation system (a) Folded copper mesh in the plastic vessel; (b) Fuse the paraffin and pour the paraffin melt into the vessel; (c) The copper mesh were peeled off when the melt froze; (d) The prepared separation core device.

Fig.3 Illustration for the automatic oil-water separation system
2.5 Characterization
The WCA and SA were tested with a contact angle analyzer (DATA Physics OCA20, Germany) at room temperature. Differential Scanning Calorimetry (Q200, TA, USA) was conducted at a temperature rising speed of 10℃/min from 0℃ to 100℃. Wax powder were characterized structurally and micro-structurally by X-ray diffraction (Shimadzu, LabX XRD-6000) with a Cu anode sealed tube. Surface imaging was performed using a field-emission scanning electron microscope (Hitachi S-4800, Japan). The process of crystallization was observ-ed with a polarizing microscope (POM) (Olympus Bx 51).
3 Results and Discussion
3.1 Mechanism of the superhydrophobic fracture surface
The as-prepared fracture surfaces exhibit remarkable water repellency with water droplets keeping typical spherical shape on the surface as shown in the optical image in Fig 4a, the WCA is 152.4±1° (Fig.4b). The contact angle of outer surfaces is 108±2° as shown by the Fig.4f, the outer surfaces are not superhydrophobic (Fig.4e). As the chemical compositions of the two different kinds of surfaces are the same, the roughness of the two different kinds of paraffin’s surfaces must be different. Fig.4c presents the SEM images of fracture surface, the fracture surface of paraffin is quite rougher than outer surfaces formed naturally shown by the Fig.4g. A number of bulges appear at the fracture surface with an average size of 40μm and with higher magnification we could see small edges with width ranging from 10μm to 30μm (Fig.4d). This leads to a high roughness, however, from Fig.4g we find the outer surface is smooth with few bulge on it, the higher magnification Fig.4d shows us a surface with the edges of the lamellas emerging from inside the paraffin. Paraffin has a low surface energy, it is easy to get superhydrophobic surfaces when the surfaces of paraffin become rougher, this could be explained by the Wenzel equation.
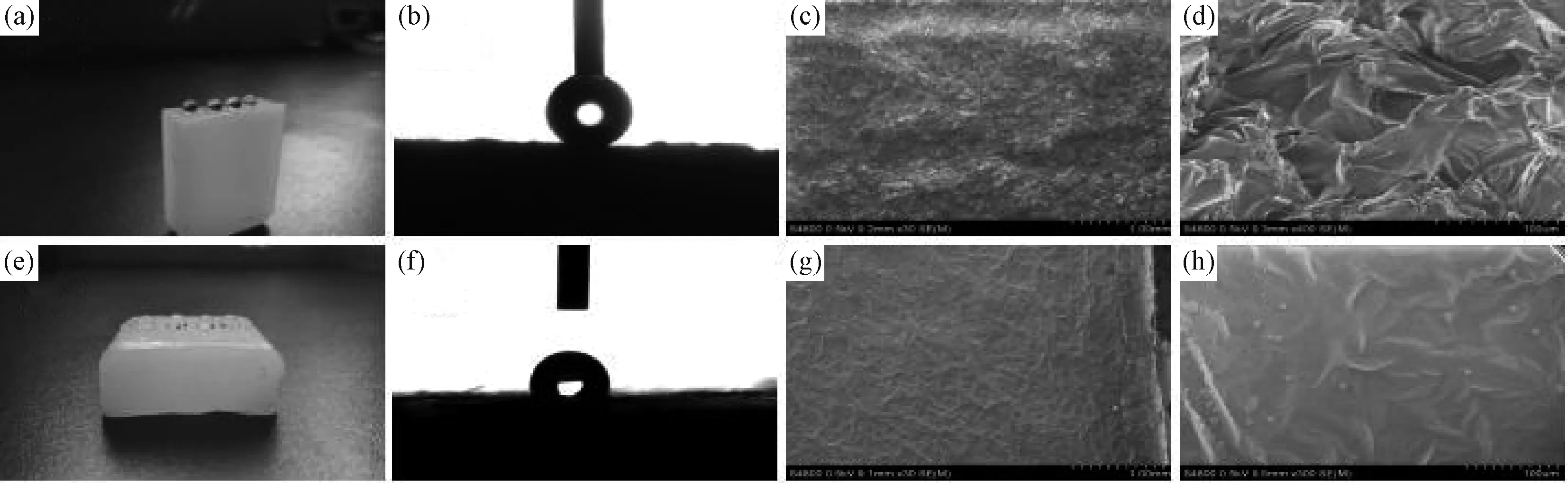
Fig.4 (a) Water droplets stay spherical on the fracture surface of paraffin. (b) WCA of fracture surface(WCA=152.4±1°). (c,d) Scanning electron microscopy (SEM) images of the morphology of the fracture surface. (f) Water droplets spread out on the other surfaces of paraffin.(e) WCA of the other surfaces of paraffin(WCA=108.5±2°). (g,h) SEM images of the morphology of the outer surfaces
θ—contact angle;r—roughness factor;γSL—solid-liquid surface tension;γLV—liquid surface tension;γSV—solid surface tensionγSL,γLVandγSVare determined by the chemical composition, to the fracture surfaces and the outer surfaces, chemical compositions are the same, while the roughness are different, the fracture surfaces are rougher than the outer surfaces, so the roughness factorris bigger and the contact angle becomes bigger as well.

Fig.5 DSC spectra of the paraffin (10℃/ min from 0℃ to 100℃). The two exothermal peaks show that there mainly exist two different kinds of paraffin crystals
Known that the roughness leads to the huge difference between the two kinds of paraffin surfaces and the fracture surfaces are much rougher than the outer surfaces. Concerning the crystallization of paraffin, it is induced that the crystallization in the paraffin determines the roughness when the solid paraffin is fractured. DSC curve of Fig.5 proves that paraffin has a huge exothermal peak and a smaller exothermal peak, which indicates a high crysta-llinity, the two exothermal peaks show that there mainly exist two different sizes of paraffin crystals. Paraffin is a mixture of molecules with different lengths, when the paraffin melt cools down, molecules with short chain crystallize at a high temperature, while molecules with long chain crystallize fast at a lower temperature, the crystals of the long chain molecules are less perfect than the crystals of the short chain molecules because of the low mobility, that is the reason peak at 62℃ is much more intense than the peak around 50℃.
In the XRD spectra of paraffin in Fig.6, intense spikes show that there exist crystals inside the paraffin, the crystals has a strongly preferred orientation of growth at a 2 theta of 21.6°(110). At the 2 theta around 25°, there is a less strong peak, combined with the exothermal peak around 50℃ in the DSC curve of Fig.5, it can be concluded that paraffin is poly-crystalline with crystals of different sizes inside.

Fig.6 XRD spectra of the paraffin shows a strongly preferred orientation at a 2 theta of 21.6°(110). Diffraction was carried out with Cu Kα. It can be concluded that paraffin is poly-crystalline with crystals of different sizes

Fig.7 Cooling process of paraffin observed by the POM. Crystals appear and the dimensions of the crystals increase with the lowering of temperature. The scale bar is 50μm
It could be seen from the POM images of Fig. 7 that when the paraffin melt cools down, large quantities of crystals appear and the dimensions of the crystals increase with the lowering of temperature, in this process crystals with all sizes form at last.
The POM images present that quite a lot of crystals form in the solid paraffin when cooling down. SEM was employed to study the fracture surface. As the solid paraffin is easy to melt in the rising energy of the electron beam, a low voltage of 0.5KV was chosen, avoiding the damage to the morphology of the fracture surface caused by the electron-beam focusing. Fig.8 displays the outcome, the white points in Fig.8a represent the small crystallization regions on the fracture surface as we induced, and the continuous white region in the fracture surface of Fig.8b and Fig.8c shows the crystallization region. From the Fig.8b to Fig.8c, the white region fused because of the electron-beam focusing, this is an evidence that the white region is actually the crystallization region. The continuous crystallization region and the scattered small crystallization regions are revealed in the Fig.8d, also, the crystallization regions gathered together in Fig.8e. Fig.8f is the high magnification SEM picture of one scattered small crystallization region, an average amorphous region width of 2μm is presented within the crystallization regions. Considering the rough morphology of the fracture surface of Fig.4c and Fig.4d, we draw a conclusion that when the solid paraffin is fractured, the crystals play two kinds of role, one is the role of stress concentration points (crystals are harder than the amorphous area, the stress concentration points lead to different directions of crazes), leading to the irregularity of the fracture surfaces; the other role is that the crystals function as micro-size “fillers” in the paraffin, “fillers” emerge on the fracture surfaces and the fracture surfaces is rougher than the outer surface because of the different sizes of the crystals.
The results demonstrate that the high roughness leads to the good superhydrophobicity of the fracture surface, from the DSC spectra, XRD spectra and the

Fig.8 (a) High magnification SEM pictures of the fracture surfaces. There are quantities of white points on the fracture surface. (b) The continuous crystallization region in the fracture surface. (c) The crystals melt in the fracture surface when the electron-beam focused on continuous crystallization region. (d) The continuous crystallization region and the scattered small crystallization regions. (e) Crystallization regions gathered together. (f) The high magnification SEM picture of one scattered small crystallization region
SEM images it is inferred that the crystals inside the paraffin cause the high roughness of the fracture surfaces when the paraffin is fractured. In the Fig.9, there mainly exist two regions in the solid paraffin: the amorphous region and the crystallization region, hollows and bulges appear on the fracture surfaces when fractured (Fig.9a), leading to a high roughness (Fig.9b). As the paraffin’s fracture surface is rough, combined with the low surface energy of the paraffin, water cannot penetrate into the gaps of the rough surface, and air exist in the gaps and hollows of the rough surfaces. The trapped air reduces the contact area between the water droplet and the solid surface, which improves the superhydrophobicity of the surface greatly, the fracture surfaces gain the superhydrophobicity, in contrast with the outer surface (Fig.9c).

Fig.9 Mechanism for the superhydrophobicity of paraffin’s fracture surface. The solid paraffin consists of two regions: (a) The amorphous region and the crystallization region, hollows and bulges appear on the fracture surfaces when fractured. (b) Water droplet stays spherical on the fracture surface. (c) Water droplet stays half spherical on the outer surface of the solid paraffin
3.2 Superhydrophobic surfaces fabricated with a sheet of copper mesh
A sheet of copper mesh was utilized to increase the roughness of the paraffin’s surface. Fig.10a and Fig.10e exhibit the water droplets staying spherical on the surface of the paraffin with copper mesh peeled off, observed from the Fig.10b and Fig.10f, regular lattices on the surface bring about a high roughness. Here two surfaces made with different sizes of copper mesh were displayed. With micro-size structures, surfaces in Fig.10c and Fig.10g are as rough as the morphology of the fracture surfaces in Fig.4d. They show high WCA(WCA=160.2±2°) (Fig.10d) and low sliding angle (SA<2°) (Fig.10h), indicating good superhydrophobicity. Through this way large-area superhydrophobic surface can be made with less time and simple process at a low cost. Morphology of paraffin fracture surface was successfully replicated by this means. When copper mesh is peeled off, each grid of the mesh produces a small fracture surface with rough morphology, enabling the whole surface to be water-repellent.
3.3 Automatic oil-water separation process
In Fig.11a, the red oil floating on the water is n-hexane dyed with Sudan III, which was driven into the channels in the solid paraffin by the lipophilicity of the paraffin, while water was stopped outside the solid paraffin because of the superhydrophobicity of the narrow channels with a width of 0.2cm in the solid paraffin (Fig.11b), as a result, the n-hexane finally assembled at the center of the core separation device made of the solid paraffin, and with a straw drawing oil from the center of the core separation device, the oil-water separation process could be continuous. 2mL red oil was driven into the core device in 3 seconds without a straw, this system could separate the oil and water in the condition of layered oil/water mixture.
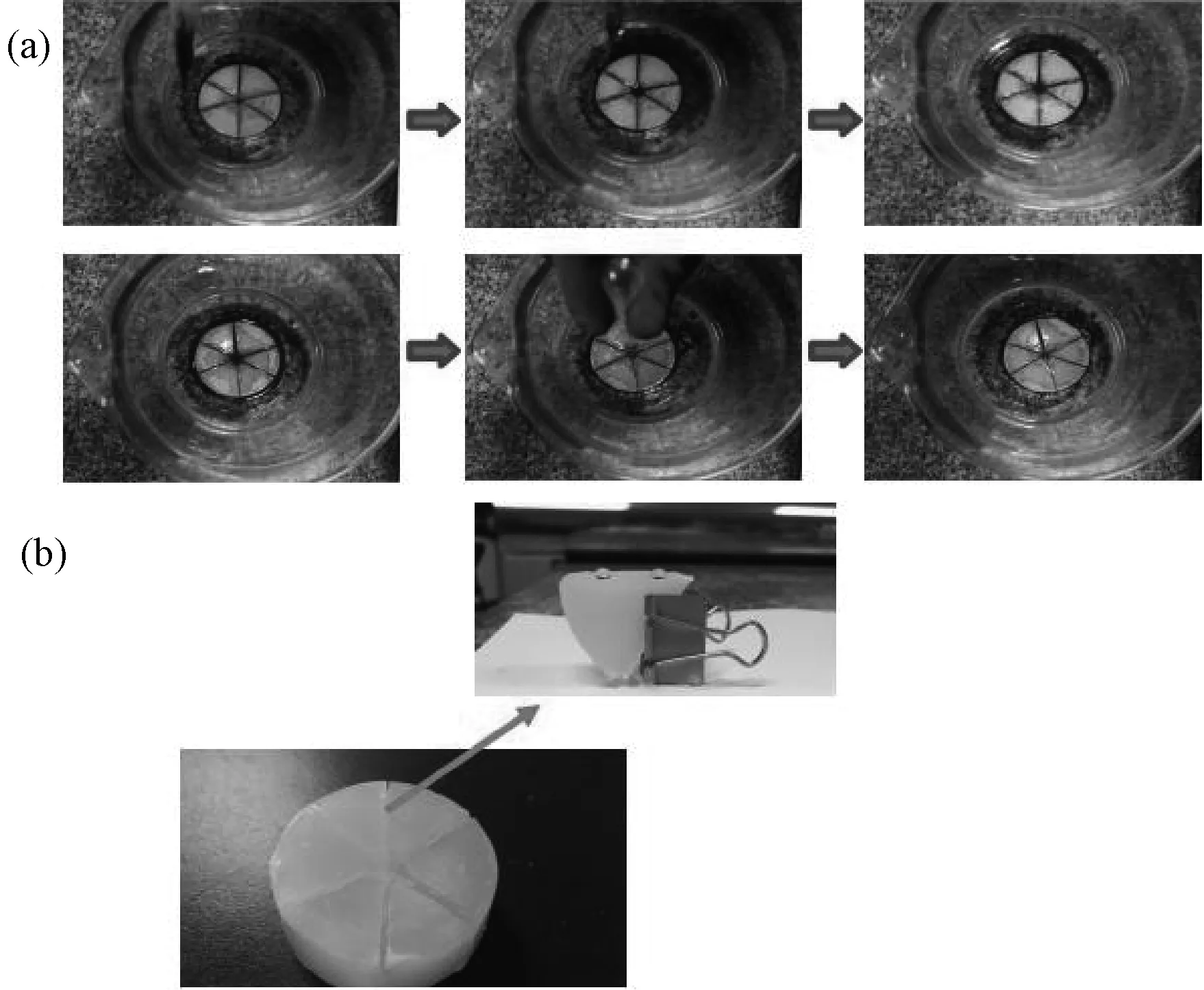
Fig.11 (a) Automatic oil-water separation process. (b) Water droplets stay spherical on the surfaces of the channels. The width of the channel is less than 0.2cm
4 Conclusion
The unique superhydrophobicity of the fracture paraffin surfaces was studied. The experimental results lead to the following conclusions:
1. Superhydrophobicity of the paraffin fracture surface was discovered, the WCA reaches 152.4±3°, higher than the 108±3° of the outer surface. This discovery provides a new perspective for the fabrication methods of superhydrophobic surface, the solid paraffin is just a model for us to utilize the high roughness of the fracture surface.
2. The main cause for the superhydrophobicity of the fracture surface is the surface roughness. High roughness of paraffin fracture surface is caused by the continuous crystalline region and the scattered crystalline regions. When being fractured, the crystalline regions function as the concentration points and micro-size “fillers”, leading to a rough morphology.
3. Superhydrophobic surfaces with a WCA of 162.4±3° and a sliding angle (SA) lower than 3° were fabricated by replicating the morphology of the fracture surface with a sheet of copper mesh. It’s convenient for the fabrication of large-area superhydrophobic surface.
4. Automatic oil-water separation system using the superhydrophobic surfaces obtained with the copper mesh was designed. Water was repelled outside while oil was driven into the core device automatically. With a working speed of 0.67mL/s, this system separated the oil and water efficiently.
Reference
[1] H. M. Shang, Y. Wang, S. J. Limmer, T. P. Chou, K. Takahashi, G. Z. Cao. Optically transparent superhydrophobic silica-based films [J]. Thin Solid Films, 2005, 472(1): 37~43.
[2] A. Nakajima, K. Hashimoto, T. Watanabe, et al. Transparent superhydrophobic thin films with self-cleaning properties[J]. Langmuir, 2000, 16(17): 7044~7047.
[3] B. Bhushan, Y. C. Jung, J. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces[J]. Journal of Physics: Condensed Matter, 2008, 20(22): 225010.
[4] H. F. Hoefnagels, D. Wu, G. de With, W. Ming. Biomimetic superhydrophobic and highly oleophobic cotton textiles[J]. Langmuir, 2007, 23(26): 13158~13163.
[5] L. Mishchenko, B. Hatton, V. Bahadur, et al. Design of ice-free nanostructured surfaces based on repulsion of impacting water droplets[J]. ACS nano, 2010, 4(12): 7699~7707.
[6] P. Kim, T. S. Wong, J. Alvarenga, et al. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance[J]. ACS nano, 2012, 6(8): 6569~6577.
[7] K. Koch, H. F. Bohn, W. Barthlott. Hierarchically Sculptured Plant Surfaces and Superhydrophobicity[J]. Langmuir, 2009, 25(24): 14116~14120.
[8] C. Neinhuis and W. Barthlott. Characterization and distribution of water-repellent, self-cleaning plant surfaces[J]. Annals of Botany, 1997, 79(6): 667~677.
[9] P. Roach, N. J. Shirtcliffe, M. I. Newton. Progess in superhydrophobic surface development[J]. Soft matter, 2008, 4(2): 224~240.
[10] X. Song, J. Zhai, Y. Wang, L. Jiang. Fabrication of superhydrophobic surfaces by self-assembly and their water-adhesion properties[J]. The Journal of Physical Chemistry B, 2005, 109(9): 4048~4052.
[11] B. Qian, Z. Shen. Fabrication of superhydrophobic surfaces by dislocation selective chemical etching on aluminum, copper, and zinc substrates[J]. Langmuir, 2005, 21(20): 9007~9009.
[12] L. Huang, S. P. Lau, H. Y. Yang, et al. Stable superhydrophobic surface via carbon nanotubes coated with a ZnO thin film[J]. The Journal of Physical Chemistry B, 2005, 109(16): 7746~7748.
[13] K. K. S. Lau, J. Bico, K. B. K. Teo, et al. Superhydrophobic carbon nanotube forests[J]. Nano letters, 2003, 3(12): 1701~1705.
[14] N. J. Shirtcliffe, G. McHale, M. I. Newton, et al. Dual-Scale Roughness Produces Unusually Water-Repellent Surfaces[J]. Advanced Materials, 2004, 16(21): 1929~1932.
[15] H. Yan, K. Kurogi, H. Mayama, K. Tsuji. Environmentally Stable Super Water-Repellent Poly (alkylpyrrole) Films[J]. Angewandte Chemie International Edition, 2005, 44(22): 3453~3456.
[16] J. T. Han, D. H. Lee, C.Y. Ryu, K. Cho. Fabrication of superhydrophobic surface from a supramolecular organosilane with quadruple hydrogen bonding[J]. Journal of The American Chemical Society, 2004, 126(15): 4796~4797.
[17] M. Hikita, K. Tanaka, T. Nakamura, T. Kajiyama, A. Takahara. Super-liquid-repellent surfaces prepared by colloidal silica nanoparticles covered with fluoroalkyl groups[J]. Langmuir, 2005, 21(16): 7299~7302.
[18] J. Feng, B. Y. Huang, M. Q. Zhong. Fabrication of superhydrophobic and heat-insulating antimony doped tin oxide/polyurethane films by cast replica micromolding[J]. Journal of Colloid and Interface Science, 2009, 336(1): 268~272.
[19] 叶霞, 周明, 蔡兰, 等. 超疏水固体表面的制备及其量化表征[J]. 材料科学与工程学报, 2007, 25(04): 644~648.
[20] 曹丰, 管自生, 李东旭. 类荷叶表面疏水结构的材料表面制备[J]. 材料科学与工程学报, 2007, 25(04): 602~605+615.
[21] 齐连怀, 杨清香, 汤凯, 等. 超疏水硅橡胶涂层的制备[J]. 材料科学与工程学报, 2014, 32(02): 272~275.
[22] 冯杰,林飞云,钟明强. 钢辊热压微模塑-可控剥离法构建聚乙烯超疏水表面[J]. 材料科学与工程学报, 2010, 28(06): 835~838+906.
[23] S. Pechook, B. Pokroy. Bioinspired hierarchical superhydrophobic structures formed by n-paraffin waxes of varying chain lengths [J]. Soft Matter, 2013, 9(24): 5710~5715.
[24] W. W. Zhang, P. Lu, L. Y. Qian, H. N. Xiao. Fabrication of superhydrophobic paper surface via wax mixture coating[J]. Chemical Engineering Journal, 2014, 250: 431~436.
Superhydrophobicity of Paraffin Fracture Surfaces and the Application in Fabricating Automatic Oil-Water Separation System
LIU Bin, XU Du, QIU Wenlian, SHEN Lie
(MOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China)
Superhydrophobicity of the paraffin fracture surface was discovered, the water contact angle (WCA) reaches 152.4±3°, higher than the 108±3° of the outer surface. Superhydrophobic paraffin surfaces with a WCA of 162.4±3° and a sliding angle (SA) lower than 3° were fabricated by replicating the morphology of the fracture surface with a sheet of copper mesh. A device with a separating speed of 0.67mL/s for continuous oil-water separation was designed. The morphology of the obtained surface was investigated by scanning electron microscope (SEM). Differential scanning calorimetry (DSC), X-ray diffraction (XRD) and polarizing microscopy (POM) tested the narrow melting temperature range and high crystallinity of paraffin. Results show that the main cause for the superhydrophobicity of the fracture surface is the surface roughness. High roughness of paraffin fracture surface is caused by the continuous crystalline region and the scattered crystalline regions. When being fractured, the crystalline regions function as the concentration points and micro-size “fillers”, leading to a rough morphology.
superhydrophobicity; paraffin; fracture surface; crystallization region; oil-water separation
TH145 Document code:A
1673-2812(2017)02-0195-08
Foundation item:This work was financial supported by the Zhejiang Provincial Natural Science Foundation of China (LY13E030001)
10.14136/j.cnki.issn 1673-2812.2017.02.006
Received date:2015-12-07;Modified date:2016-03-01
Biography:Liu Bin (1990-), material master, Email: 21329042@zju.edu.cn.Corresponding author:Shen Lie, E-mail: shenlie@zju.edu.cn.

