西南地区pH影响紫色土硝化作用机制研究
孟瑶,王智慧,罗红燕,蒋先军(西南大学资源环境学院,重庆 400715)
西南地区pH影响紫色土硝化作用机制研究
孟瑶,王智慧,罗红燕*,蒋先军*
(西南大学资源环境学院,重庆 400715)
硝化作用是一个对pH高度敏感的典型过程,但pH影响土壤硝化作用的机制尚不完全明了。本研究以西南地区3种发育于同一母质的不同pH的紫色土(酸性紫色土 pH=5.7,中性紫色土 pH=7.3,石灰性紫色土 pH=8.0)作为供试材料,研究pH对紫色土硝化强度及氨氧化微生物的长期效应;通过人为添加酸(H2SO4)或碱(NaOH)短期改变土壤pH的方法,研究pH对紫色土硝化作用强度、氨氧化微生物活性及丰度的短期效应。结果表明,pH的短期改变对酸性与石灰性紫色土的硝化作用无显著影响(P>0.05),但对中性紫色土的硝化速率影响显著(P<0.05);氨氧化古菌(AOA)的amoA基因在酸性土壤环境中的表达更高(3.04×108/g干土,AOA/AOB=31.8),而氨氧化细菌(AOB)则更适应于石灰性紫色土环境(2.35×108/g干土,AOA/AOB=0.07)。研究表明,紫色土的硝化作用强度受pH的影响显著,且在不同pH土壤中其作用机制各不相同。硝化微生物群落和活性主导了酸性和石灰性紫色土中的硝化作用,而中性紫色土中的硝化作用则是由底物浓度所主导。研究推测长期稳定的pH是影响硝化微生物群落和活性的关键因素,而pH的短期改变则主要影响硝化反应的底物有效性。
硝化作用;氨氧化古菌;氨氧化细菌;土壤pH;实时荧光定量PCR
土壤中的硝化作用是一个对pH高度敏感的典型过程[1]。实验室模拟试验[2-3]与田间试验[4-6]结果均表明:当pH在4.8~8.5的范围内时,硝化作用随着pH的升高而增强。例如,北美白桦森林土壤pH在5.5~7.5范围内,净硝化速率逐渐增大,且在pH 7.5时,土壤净硝化速率是pH 5.5时的4倍[7]。但令人惊讶的是,土壤pH的短期改变(如施用石灰等人为活动)对硝化作用的影响,却有着不同的研究结果。瑞典pH小于4.8的酸性森林土壤中开展的研究表明:施入石灰显著提高了土壤硝化作用,即添加3 t/hm2石灰后,表层土壤的硝态氮浓度是原始土壤的67.5倍[8]。然而,在日本茶园强酸性土壤中,施用CaCO3并没有提高其硝化活性[7]。甚至,有研究发现中国西湖和太湖地区的酸性森林土壤中施用石灰还会显著降低土壤的净硝化率[9]。
土壤pH影响硝化作用的机制至少包括两个方面。首先,pH影响了硝化反应底物的化学形态与浓度,从而影响其有效性[10]。因为硝化作用的底物是NH3分子而非NH4+离子[11-12],而高pH可以使得NH3分子与NH4+转换的化学平衡式趋向于生成NH3分子,从而提高NH3分子的有效性。其次,土壤pH对参与硝化反应的土壤微生物的分布会有所影响。例如:在酸性土壤(pH 4.5~6.9)中,存在大量的AOA(氨氧化古菌 ammonia-oxidizing archaea),但却检测不到AOB(氨氧化细菌 ammonia-oxidizing bacteria)[13],Di等[14]发现在新西兰高氮草地土壤中施氮会增加AOB的数量和土壤硝化活性。
本实验假设长期pH的影响主要针对硝化微生物的群落组成,而pH的短期改变主要针对反应底物有效性;硝化活性则是硝化微生物与底物相互作用的表现,所以不同性质的土壤中,硝化作用对pH的响应有所不同。因此,本实验采集相似母质发育的酸性、中性和石灰性紫色土,研究pH对紫色土硝化强度、氨氧化微生物活性及丰度的长期影响;通过添加酸(H2SO4)或碱(NaOH)的方式,调节3种土壤的pH,研究短期pH变化对紫色土硝化强度、氨氧化微生物活性及丰度的影响,从而探索土壤pH影响紫色土硝化过程的作用机制,为土壤氮素管理策略提供理论支撑。
1 材料与方法
1.1 供试土壤
本实验开展于2014年4月,3种供试紫色土壤分别是釆集于重庆市永川的酸性紫色土(pH 5.7)、中性紫色土(pH 7.3)及采集于四川盐亭的石灰性紫色土(pH 8.0),其基本理化性质见表1。随机选择3个采样点,釆集表层土壤(≤20 cm),采集时尽量保持原状土壤结构。将采回的湿土在室内沿其自然结构将土块小心掰成小土块,除去植物残体石块以及蚯蚓等小动物,自然风干。土样风干后磨细过2 mm筛,供实验分析测定。

表1 供试土壤的基本性质Table 1 Basic properties of the selected soils
1.2 培养实验
称取适量酸性、中性、石灰性紫色土风干土,分别分为3等份。一份只加纯水,其余两份用0.1 mol/L的NaOH溶液或0.5 mol/L的H2SO4溶液调节土壤pH,且保持田间最大持水量60%,置于28 ℃恒温培养箱预培养7 d等待调节的pH达到平衡。经淋洗后过2 mm筛,获得不同pH系列土壤样品:酸性紫色土(pH 5.7、6.5、7.1)、中性紫色土(pH 6.5、7.3、8.1)、石灰性紫色土(pH 6.5、7.4、8.0)。在250 mL三角瓶中分别加入10 g上述9种样品,并加入8 mmol/kg氮源,每个样品均有3个重复,保持田间最大持水量60%,置于28 ℃恒温培养箱进行9 d的正式培养。正式培养的第0天(未加氮源时)、第9天,采集土壤样品进行其硝态氮浓度的测定并计算净硝化速率,且在第0、5、9天采集土壤样品进行氨氧化微生物amoA基因拷贝数的测定。
1.3 测定方法
土壤样品用浓度为2 mol/L的KCl溶液进行浸提,用紫外分光光度法测定浸提液中硝态氮的浓度,并用培养前后硝氮浓度变化计算净硝化速率[15]。
本实验所有样品均采用E.Z.N.A TM Soil DNA kit试剂盒(Omega Bio-tek,美国)提取土壤总DNA,提取步骤按试剂盒说明书操作,提取出的DNA样品保存于-20 ℃冰箱中备用。
随后进行AOB和AOA中amoA基因的实时荧光定量PCR:采用引物amoA-1F (5′-GGGGTTTCTACTGGTGGT-3′) 和amoA-2R (5′-CCCCTCKGSAAAGCCTTCTTC-3′ )[K=G or T;S=G or C]直接扩增总DNA中氨氧化细菌(AOB)amoA的基因片段。采用引物Arch-amoA F (5′-STAATGGTCTGGCTTAGACG-3′) [K=G or T;S=G or C]和Arch-amoA R (5′-GCGGCCATCCATCTGTATGT-3′)直接扩增总DNA中氨氧化古菌(AOA)amoA基因片段。PCR反应体系为20 μL,包括1 μL DNA模板、10 μL SYBR Premix Ex TaqTM Perfect Real Time,前、后引物各0.5 μL (10 μmol/L)及8 μL的灭菌双蒸水。实验空白用灭菌双蒸水代替DNA作为反应模板。AOA、AOB的荧光定量 PCR 扩增程序皆为95 ℃预变性30 s;95 ℃变性30 s,54 ℃退火30 s,72 ℃延伸45 s,40个循环。
1.4 数据处理
测定所得数据采用Excel与Origin 8.6软件进行数据处理和图表绘制,采用SPSS 19.0中的单因素方差分析(ANOVA)进行数据的显著性检验,其中多重比较采用最小显著差数法(LSD)进行检验(P<0.05)。
2 结果与分析
2.1 pH对紫色土中硝化作用的影响
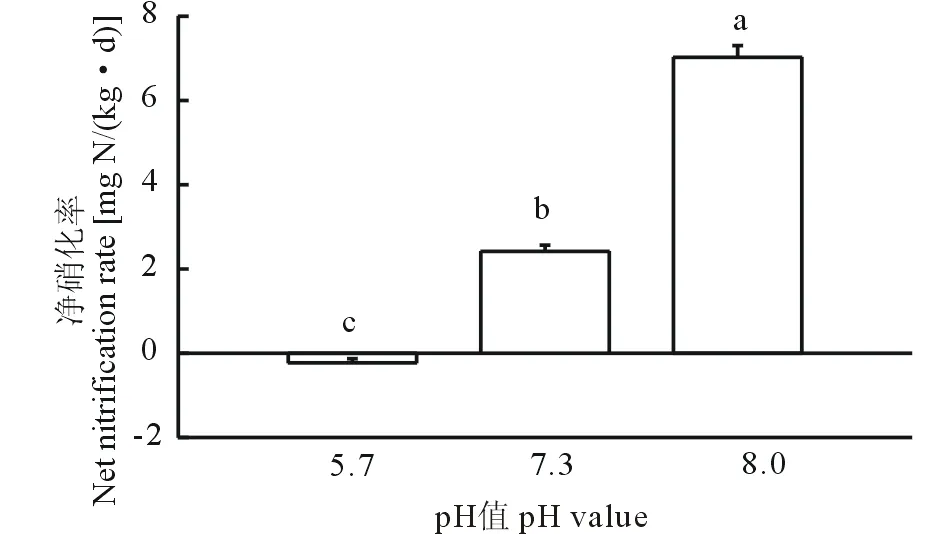
图1 3种不同pH紫色土的硝化作用Fig.1 Nitrification in three purple soil with different pH 不同字母代表P<0.05水平上差异显著。 Different letters mean the significant differences at P<0.05. 误差线表示标准差Error bars represent standard deviations, n=3.下同 The same below.
在经过9 d的培养后,3种紫色土的净硝化率差异显著(P<0.05)(图1)。其中,酸性紫色土的净硝化率为负值[-0.23 mg N/(kg·d)],表明该土壤中没有发生明显的硝化作用。而在pH较高的中性紫色土与石灰性紫色土中,其净硝化率分别为2.42、7.02 mg N/(kg·d),发生了较为强烈的硝化作用。结果表明土壤的pH越高,发生的硝化作用越强烈,土壤中的硝化作用随土壤pH的升高而增强。
2.2 pH短期改变对紫色土硝化作用的影响
在经过9 d的培养后,酸性紫色土的净硝化率为:-0.23, -0.22, -0.23 mg N/(kg·d),石灰性紫色土的净硝化率为:7.05, 7.11, 7.02 mg N/(kg·d),显著性分析结果表明酸性土壤中pH的短期上升与石灰性土壤中pH的短期下降对这两种土壤中硝化作用影响并不显著(P>0.05)。而中性紫色土中的净硝化率随着pH的增大,依次为1.23、2.42、3.27 mg N/(kg·d),具有显著性差异(P<0.05)(图2)。
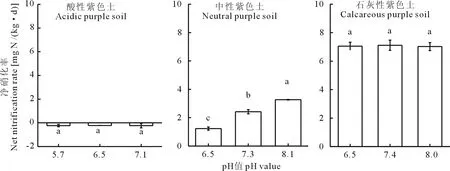
图2 pH短期改变对3种不同pH紫色土硝化作用的影响Fig.2 Effect of short-term pH change on nitrification in three purple soil with different pH
2.3 不同pH紫色土中氨氧化细菌和古菌的丰度
如图3所示,培养过程中,中性紫色土中的AOA丰度先升高再降低,酸性紫色土中AOA丰度培养期间的变化趋势与中性紫色土相似,而石灰性紫色土的AOA丰度在培养期间几乎没有变化,且中性紫色土中的AOA丰度大于酸性和石灰性紫色土。中性紫色土中AOB的丰度先升高再降低,石灰性紫色土中AOB丰度培养期间的变化趋势与中性土相似,而酸性紫色土中的AOB丰度在培养期间几乎没有变化,且石灰性紫色土中的AOB丰度大于中性和酸性紫色土。而且AOA与AOB丰度的比值在酸性、中性、石灰性3种不同pH的紫色土中分别为31.8,1.55和0.07,AOA/AOB大小为:酸性>中性>石灰性。

图3 3种不同pH紫色土中氨氧化微生物的丰度Fig.3 Abundance of ammonia-oxidizing bacteria (AOB) and ammonia-oxidizing archaea (AOA) amoA gene in three purple soil with different pH
2.4 pH的短期改变对紫色土中硝化微生物丰度的影响
如图4所示,短期改变酸性及石灰性紫色土中的pH,其AOA丰度并无显著变化。但在中性紫色土中,pH的短期改变使得AOA呈下降趋势,且在pH上升为8.1的中性紫色土中培养第5天时,AOA的丰度极显著下降,由原样土的9.79×108/g干土下降至2.53×108/g干土。AOB丰度在酸性紫色土中pH改变前后无显著变化,石灰性紫色土pH的短期降低使AOB有增长趋势,而在中性土中,pH的上升使得其AOB丰度增大,pH下降则对AOB丰度无明显影响(图5)。
3 讨论
pH的短期变化对酸性与石灰性土壤中的硝化活性无显著影响,而对中性土壤有显著影响。该结果表明不同pH的紫色土可能有不同的硝化反应机制。

图4 3种不同pH紫色土pH短期改变后对AOA丰度的影响Fig.4 Effect of short-term pH change on abundance of ammonia-oxidizing archaea (AOA) amoA gene in three purple soil with different pH

图5 3种不同pH紫色土pH短期改变对AOB丰度的影响Fig.5 Effect of short-term pH change on abundance of ammonia-oxidizing bacterial(AOB) amoA gene in three purple soil with different pH
已有研究均发现pH在4.8~8.5的范围内时,土壤的硝化速率随着pH的增加而提高[16-18]。本实验中的石灰性紫色土的硝化作用最强,而酸性紫色土中几乎不发生硝化作用,与以往的研究结果[4-5,19]一致。但是,也有研究结果表明低pH的土壤中也会发生硝化作用[20-21]。例如,Nugroho等[22](2005)在荷兰和芬兰的9种酸性森林土(pH 2.9~3.4)中发现了强烈的硝化作用[5 mg N/(kg·d)];且He等[23]在pH 3.7的土壤中获得了细菌和古菌的amoA基因,说明强酸性土壤中有发生硝化作用的可能。而在本实验pH为5.7的土壤中却没有发生显著的硝化作用,经计算得到pH为5.7的土壤中NH3分子的浓度为500 nmol N/kg 干土(NH3+H+↔NH4+; pKa=9.25),是Nugroho等[22]试验中土壤(pH 3.4)NH3分子浓度的200倍。但在加拿大酸性森林土(pH 2.9~3.4)中却发生了强烈的硝化作用,所以酸性紫色土没有发生显著的硝化反应并非缺少反应底物;而更可能是与相关硝化微生物的活性有关。同时,酸性紫色土采用NaOH调高pH后,反应底物NH3分子浓度呈数量级增加,但净硝化率并没有增加;这一实验结果也为该猜测提供了充分证据。
pH的短期降低对碱性土壤中的净硝化作用也无显著影响。硝化反应速率主要受氨单加氧酶(ammonia monooxygenase, AMO)催化的氨氧化速率控制[20,24-25]。因此,硝化反应符合酶促反应动力学方程,如果底物浓度高于酶促反应常数[26],则限制反应速率的是酶活性,而不是反应底物浓度。因此,碱性紫色土采用H2SO4调低pH后,反应底物NH3分子浓度呈数量级降低,但净硝化速率并没有降低;这一实验结果说明制约碱性紫色土硝化作用的因子不是氮源,而是相关硝化微生物的活性。
pH值的短期改变对酸性和碱性土壤的硝化作用没有影响,但对中性土壤的硝化作用影响显著。pH短期上升,硝化作用显著增强,pH短期下降,硝化作用随之显著减小。这表明底物浓度(NH3)控制着中性紫色土的硝化速率。
氨氧化细菌(AOB)以及氨氧化古菌(AOA)都拥有氨单加氧酶amoA基因编码,这意味着AOB和AOA都在硝化作用起着关键作用[27-31]。越来越多的证据表明,土壤pH值在7.0以上时,硝化作用是由氨氧化细菌主导[14,32-33],而氨氧化古菌则更可能在酸性土壤中主导了硝化作用[33-35]。本实验研究结果显示了3种不同pH值紫色土中氨氧化微生物的生态位差异显著。AOA主要分布于酸性紫色土,而AOB主要分布于碱性紫色土;而在中性紫色土(pH=7.4)中,AOA和AOB的丰度都很高,这可能意味着AOB和AOA在中性紫色土中共存,且都可能是硝化作用的推动者。
4 结论
综上所述,本研究可得出以下结论:1)pH短期改变对酸性与石灰性土壤中的硝化作用无显著影响,但对中性土壤影响显著,说明不同pH的紫色土具有不同的硝化作用机制;2)紫色土中氨氧化微生物的生态位分布差异显著,且受到pH的影响。AOA主要分布于酸性紫色土,而AOB主要分布于碱性紫色土;而在中性紫色土中,AOA和AOB共存。
References:
[1] Strayer R F, Lin C J, Alexander M. Effect of simulated acid rain on nitrification and nitrogen mineralization in forest soils. Journal of Environmental Quality (USA), 1981, 10: 547-551.
[2] Robertson G P. Factors regulating nitrification in primary and secondary succession. Ecology, 1982, 63: 1561-1573.
[3] Tietema A, De Boer W, Riemer L,etal. Nitrate production in nitrogen-saturated acid forest soils: vertical distribution and characteristics. Soil Biology & Biochemistry, 1992, 24: 235-240.
[4] Dancer W S, Peterson L A, Chesters G. Ammonification and nitrification of N as influenced by soil pH and previous N treatment. Soil Science Society of America Proceedings, 1973, 37: 67-69.
[5] Persson T, Wireén A. Nitrogen mineralization and potential nitrification at different depths in acid forest soils. Plant and Soil, 1995, 168-169: 55-65.
[6] Ste-Marie C, Pare D. Soil, pH and N availability effects on net nitrification in the forest floors of a range of boreal forest stands. Soil Biology & Biochemistry, 1999, 31: 1579-1589.
[7] Hayatsu M, Kosuge N. Autotrophic nitrification in acid tea soils. Soil Science and Plant Nutrition, 1993, 39: 209-217.
[8] Bäckman J S, Hermansson A, Tebbe C C,etal. Liming induces growth of a diverse flora of ammonia-oxidizing bacteria in acid spruce forest soil as determined by SSCP and DGGE. Soil Biology & Biochemistry, 2003, 35: 1337-1347.
[9] Yao H, Gao Y, Nicol G W,etal. Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils. Applied and Environmental Microbiology, 2011, 77: 4618-4625.
[10] Kemmitt S J, Wright D, Goulding K W T,etal. pH regulation of carbon and nitrogen dynamics in two agricultural soils. Soil Biology & Biochemistry, 2006, 38: 898-911.
[11] Burton S A, Prosser J I. Autotrophic ammonia oxidation at low pH through urea hydrolysis. Applied and Environmental Microbiology, 2001, 67: 2952-2957.
[12] Stark J M, Firestone M K. Kinetic characteristics of ammonium-oxidizer communities in a california oak woodland-annual grassland. Soil Biology & Biochemistry, 1996, 28: 1307-1317.
[13] Hansel C M, Fendorf S, Jardine P M,etal. Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Applied and Environmental Microbiology, 2008, 74(5): 1620-1633.
[14] Di H J, Cameron K C, Shen J P,etal. Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nature Geoscience, 2009, 2: 621-624.
[15] Davidson E A, Hart S C, Firestone M K. Internal cycling of nitrate in soils of a mature coniferous forest. Ecology, 1992, 73(4): 1148-1156.
[16] Wood P M. Mechanisms for biological ammonia oxidation[M]//Cole J A, Ferguson S J. The Nitrogen and Sulphur Cycles. SGM Special Publication, Cambridge University Press, 1988: 219-243.
[17] Killham K. Nitrification in coniferous forest soils. Plant and Soil, 1990, 128: 31-44.
[18] Paul E A, Clack F E. Soil Microbiology and Biochemistry[M]. San Diego: Academic Press, 1989.
[19] Curtin D, Campbell C A, Jalil A. Effects of acidity on mineralization: pH-dependence of organic matter mineralization in weakly acidic soils. Soil Biology & Biochemistry, 1998, 30: 57-64.
[20] De Bore W, Kowalchuk G A. Nitrification in acid soils: micro-organisms and mechanisms. Soil Biology & Biochemistry, 2001, 33: 853-866.
[21] Nugroho R A, Röling W F M, Laverman A M,etal. Low nitrification rates in acid Scots pine forest soils are due to pH-related factors. Microbial Ecology, 2007, 53: 89-97.
[22] Nugroho R A, Röling W F M, Laverman A M,etal. Presence of Nitrosospira cluster 2 bacteria corresponds to N transformation rates in nine acid Scots pine forest soils. FEMS Microbiology Ecology, 2005, 53: 473-481.
[23] He J, Shen J, Zhang L. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environmental Microbiology, 2007, 9: 2364-2374.
[24] Ardakani M S, Schulz R K, McLaren A D. A kinetic study of ammonium and nitrite oxidation in a soil field plot. Soil Science Society of America Proceedings, 1974, 38: 273-277.
[25] Jiang Q Q, Bekken L R. Comparison of nitrosospira strains isolated from terrestrial environments. FEMS Microbiology Ecology, 1999, 30: 171-186.
[26] Levine I. Physical Chemistry[M]. New York: Kingsport, 1983: 542.
[27] Francis C A, Roberts K J, Beman J M,etal. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proceedings of the National Academy of Science, 2005, 102: 14683-14688.
[28] Lam P, Jensen M M, Lavik G,etal. Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proceedings of the National Academy of Science, 2007, 104: 7104-7109.
[29] Leininger S, Urich T, Schloter M,etal. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature, 2006, 442: 806-809.
[30] Nugroho R A, Röling W F M, van Straalen N M,etal. Changes in nitrification and bacterial community structure upon cross-inoculation of Scots pine forest soils with different initial nitrification rates. Soil Biology and Biochemistry, 2009, 41: 243-250.
[31] Wuchter C, Abbas B, Coolen M J L,etal. Archaeal nitrification in the ocean. Proceedings of the National Academy of Science, 2006, 103: 1231-12322.
[32] Jia Z J, Conrad R. Bacteria rather than archaea dominate microbial ammonia oxidation in an agricultural soil. Environment Microbiology, 2009, 11: 1658-1671.
[33] Jiang X J, Hou X Y, Zhou X,etal. pH regulates key players of nitrification in paddy soils. Soil Biology & Biochemistry, 2015, 81: 9-16.
[34] Erguder T H, Boon N, Wittebolle L,etal. Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiology Reviews, 2009, 33: 855-869.
[35] Wessén E, Nyberg K, Jansson J K,etal. Responses of bacterial and archaeal ammonia oxidizers to soil organic and fertilizer amendments under long-term management. Applied Soil Ecology, 2010, 45: 193-200.
Mechanisms research on how pH affects nitrification in purple soils of Southwest China
MENG Yao, WANG Zhi-Hui, LUO Hong-Yan*, JIANG Xian-Jun*
CollegeofResourcesandEnvironment,SouthwestUniversity,Chongqing400715,China
Nitrification processes are highly sensitive to soil pH but the mechanisms through which pH affects soil nitrification are not fully understood. In the present study, three types of purple soils with different pH values were identified from the same parent material in southwest China (acid purple soil pH=5.7, neutral purple soil pH=7.3, calcareous purple soil pH=8.0). These soil types were used to investigate the long-term effects of soil pH on nitrification and ammonia oxidizers and the short-term effects of soil pH on nitrification and the activities and abundance of ammonia oxidizers. Tests were undertaken by artificially adding acid (H2SO4) or alkali (NaOH) to change soil pH. The results showed that short-term pH changes had no significant effect (P>0.05) on nitrification in acidic and calcareous purple soils whereas they had a significant effect (P<0.05) on nitrification in the neutral purple soil. The geneamoAof ammonia-oxidizing archaea (AOA) was expressed much more fully in the acid soil (3.04×108/g dry soil, AOA/AOB=31.8), whereas ammonia-oxidizing bacteria (AOB) were more adapted to the calcareous purple soil environment (2.35×108/g dry soil, AOA/AOB=0.07). These results indicate that soil pH can significantly affect nitrification in purple soils and that different pH levels have different mechanisms. The activities and community of nitrifying microorganisms were the dominant factors for nitrification in acidic and calcareous purple soils, while substrate concentration was the dominant factor in neutral purple soil. The key factor affecting the activity and community of nitrifying microorganisms is the long-term stable soil pH, while short-term pH changes mainly influence the substrate availability of nitrification.
nitrification; ammonia-oxidizing archaea(AOA); ammonia-oxidizing bacteria(AOB); soil pH; real-time quantitative PCR
10.11686/cyxb2016164
http://cyxb.lzu.edu.cn
2016-04-19;改回日期:2016-06-28
国家自然科学基金项目(41271267, 41301315)和重庆市自然科学基金 (Cstc2012JJA80024)资助。
孟瑶(1992-),女,广西桂林人,在读硕士。E-mail: 937196302@qq.com*通信作者Corresponding author. E-mail: hongyan5282@163.com, jiangxj@swu.edu.cn
孟瑶, 王智慧, 罗红燕, 蒋先军. 西南地区pH影响紫色土硝化作用机制研究. 草业学报, 2017, 26(4): 73-79.
MENG Yao, WANG Zhi-Hui, LUO Hong-Yan, JIANG Xian-Jun. Mechanisms research on how pH affects nitrification in purple soils of Southwest China. Acta Prataculturae Sinica, 2017, 26(4): 73-79.

