马铃薯品种‘青薯168’和‘陇薯3号’块茎愈伤能力的比较
姜红,毕阳,李昌健,王毅,李生娥,刘耀娜,王斌
(甘肃农业大学食品科学与工程学院,兰州 730070)
马铃薯品种‘青薯168’和‘陇薯3号’块茎愈伤能力的比较
姜红,毕阳,李昌健,王毅,李生娥,刘耀娜,王斌
(甘肃农业大学食品科学与工程学院,兰州 730070)
【目的】研究抗/感干腐病的马铃薯品种‘青薯168’和‘陇薯3号’块茎间愈伤能力的差异,从木质素与软木脂积累以及苯丙烷代谢角度探讨导致差异的原因。【方法】以抗病品种‘青薯168’和感病品种‘陇薯3号’块茎为试材,人工模拟损伤后置于常温下进行愈伤,通过测定损伤块茎愈伤期间失重率的变化和块茎在不同愈伤时期接种硫色镰孢(Fusarium sulphureum)后病情指数的变化评价愈伤效果,利用间苯三酚-HCl和甲苯胺蓝-中性红染色法分别观察块茎损伤部位木质素与软木脂的形成,并量化木质化细胞层厚度和软木脂荧光强度以说明木质素和软木脂的沉积状况,通过测定苯丙烷代谢途径的关键酶和产物即苯丙氨酸解氨酶活性和木质素、总酚与类黄酮含量的变化,比较两个品种块茎愈伤能力的不同。【结果】愈伤期间,‘青薯168’的失重率显著低于‘陇薯3号’。第14天时,‘青薯168’和‘陇薯3号’的失重率分别为0.93%和1.96%,‘青薯168’的失重率比‘陇薯3号’低52.3%。同样,块茎在不同愈伤时期接种硫色镰孢后,‘青薯168’的病情指数也显著低于‘陇薯3号’。愈伤第3天接种,‘青薯168’和‘陇薯3号’的病情指数为20.36和71.59,前者的病情指数比后者低71.5%。愈伤期间,‘青薯168’的木质素与软木脂积累速率显著高于‘陇薯3号’。第14天时,‘青薯168’的木质化细胞层厚度和软木脂荧光总强度分别高出‘陇薯3号’47.4%和60.6%。此外,‘青薯168’愈伤期间表现出较高的苯丙烷代谢活性,其苯丙氨酸解氨酶活性和木质素含量在第14天时分别高出‘陇薯3号’77%和65%。两个品种的总酚含量在愈伤后期也存在差异,第14天时,‘青薯168’的总酚含量高出‘陇薯3号’76%。但两个品种间类黄酮含量在愈伤期间的变化不明显。【结论】抗病品种‘青薯168’的愈伤能力显著高于感病品种‘陇薯3号’,抗病品种所具备的良好愈伤能力与其高活力的苯丙烷代谢密切相关。
马铃薯;品种;块茎;愈伤;苯丙烷代谢
0 引言
【研究意义】马铃薯(Solanum tuberosum)在中国粮菜供给中具有重要地位[1]。但其块茎贮藏期间的腐烂损失颇为严重,由各类镰刀菌引起的干腐病是导致马铃薯腐烂的重要病害,块茎腐烂不仅造成巨大的经济损失,而且会积累真菌毒素,带来食用的安全隐患[2]。采收时在马铃薯块茎表面形成的大量伤口是致腐病原物侵入的主要通道[3],但块茎可通过形成愈伤组织来阻止病原物的侵入[4-5]。调查发现,不同品种的马铃薯如‘陇薯3号’、‘陇薯5号’和‘陇薯6号’等在贮藏期间的感病程度不同,耐贮性也就不同[6]。因此,研究不同抗性品种马铃薯块茎的愈伤能力,对于阐明不同品种块茎在贮藏期间的病害程度差异具有重要意义,也可为块茎贮藏提供理论依据。【前人研究进展】不同品种块根和块茎的愈伤能力存在较大差异。例如,‘Kemb 10’和‘SPK004’两个甘薯品种在愈伤期间不能连续形成木质层,木质化评分较低,抗病性不强。而‘Yanshu 1’和‘BP1-SP-2’两个品种则可连续形成木质层,木质化评分较高,抗病性较强[7]。AMAND等[8]分析了18个受损甘薯品种块根愈伤期间的乙烯释放量,发现不同甘薯品种间乙烯释放量、细胞木质化和伤口周皮的形成均存在显著差异,且乙烯释放量与细胞的木质化和周皮的形成存在一定的相关性。DASTMALCHI等[9-10]研究发现,不同品种马铃薯块茎愈伤组织中的极性和非极性提取物存在差异,品种‘Yukon Gold’的提取物中因富含酚类和长链脂肪酸而有利于软木脂的合成,而‘Norkotah Russet’和‘Atlantic’等品种的提取物中因缺乏这些物质而阻碍了软木脂的形成。【本研究切入点】针对单一或不同品种马铃薯块茎愈伤组织形成的生理生化及分子生物学研究已有报道,但对抗/感马铃薯品种间块茎愈伤能力的综合比较却未见报道。【拟解决的关键问题】以西北产区两个对干腐病抗性差异较大的马铃薯品种‘青薯168’(抗病)和品种‘陇薯3号’(感病)为试材,通过人工模拟创伤,在常温下进行愈伤,从组织化学和生理生化水平比较两个品种块茎愈伤期间的愈伤效果、木质素和软木脂积累以及苯丙烷代谢关键酶活性及其产物含量的差异,以期为马铃薯的块茎愈伤提供理论依据。
1 材料与方法
试验于2014—2015年在甘肃农业大学食品学院采后生物学与技术实验室完成。
1.1 材料与仪器
供试马铃薯品种‘青薯168’与‘陇薯3号’于2014年10月采自甘肃省渭源县会川镇,块茎装入网袋后当天运抵甘肃农业大学食品学院采后生物学与技术实验室,于常温(20—25℃,RH 70%—80%)下贮藏备用。供试硫色镰孢(Fusarium sulphureum)由甘肃省农业科学院植物保护研究所提供,PDA培养基上保存待用。
超净工作台(苏净集团苏州安泰空气技术有限公司);立式压力蒸汽灭菌锅(上海申安医疗器械厂);恒温培养箱(上海一恒科技有限公司);正置万能显微镜(OLYMPUS公司);紫外-可见光分光光度计(日本岛津);台式高速冷冻离心机(长沙湘仪离心机有限公司)。
1.2 方法
1.2.1 块茎创伤 参考包改红等[11]的方法并作修改。选取外观整齐,大小一致,无病虫害,无机械损伤的马铃薯块茎,用1%的次氯酸钠消毒清洗后,在每个块茎中部分别切出3块长×宽×深=20 mm×20 mm×2 mm的伤口,每处理用块茎200个,重复3次。
1.2.2 块茎愈伤 参考包改红等[11]的方法。将人工损伤的块茎装入打孔的聚乙烯保鲜袋(25 cm×40 cm,厚度0.02 mm)中,置于常温(20—25℃,RH 70%—80%)条件下,并用黑色塑料薄膜覆盖避光,进行愈伤。
1.2.3 愈伤效果评价 失重率采用重量法测定,每个处理用块茎15个,重复3次。病情指数的测定采用孢子悬浮液的配置参照包改红等[11]的方法,于培养了7 d的硫色镰孢培养皿中加入适量的无菌水(含0.01% Tween-80),用涂布器刮下培养基表面的病原菌孢子,用4层纱布过滤至三角瓶中,振荡20 s,采用血球计数板计数,制备成浓度为1×106CFU/mL的孢子悬浮液。在创伤块茎愈伤的第0、3、5、7、14天时,将已配制好的孢子悬浮液均匀喷洒于块茎表面,晾干后装入打孔的聚乙烯保鲜袋中,于黑暗条件下贮藏,7 d后观察并统计病情指数。每个品种处理选用块茎30个,重复3次。

式中,发病级别评定标准:0级,创伤面不发生干腐病;1级,发生干腐病的面积占创伤面积的1/4;2级,发生干腐病的面积占创伤面积的1/2;3级,发生干腐病的面积占创伤面积的3/4;4级,创伤面完全发生干腐病。
1.3 木质素及软木脂沉积观察
木质素的沉积观察参照ALBA等[12]的方法并修改。用不锈钢刀片垂直于愈伤块茎创伤表面切出0.2—0.3 mm厚的薄片,蒸馏水冲洗3次后,置于载玻片上,滴加1%(w/v)间苯三酚溶液及浓盐酸染色1 min,置于光学显微镜下观察,拍照观察。
软木脂(suberin poly aliphatic,SPA)的沉积观察参照LULAI等[13-14]的方法并修改。用不锈钢刀片垂直于块茎愈伤表面切出0.2—0.3 mm厚的薄片,蒸馏水冲洗3次后,进行如下染色观察。初染:配制0.05%(w/v)的甲苯胺蓝染液,染色45 min,染色过程中轻微振荡使其染色均匀;脱染料:分别用75%酒精、蒸馏水冲洗3次;复染:吸取中性红染液,染色1—1.5 min;除去染料:分别用75%酒精、蒸馏水冲洗3次。将染色的切片置于载玻片上,滴上清水加盖盖玻片,在荧光显微镜下观察,拍照。
块茎损伤表面的木质化细胞层厚度和软木脂总荧光强度分别根据文献[7,15]的方法通过IS Capture与Imagine J图像软件进行测量计算。
1.4 苯丙烷代谢关键酶活性及产物含量的测定
取样参照吴觉天等[16]的方法。在创伤后的0、3、5、7和14 d时,取皮下2—3 mm厚的愈伤组织2 g,用锡箔纸包好,液氮冷冻,粗酶液提取之前保存在-80℃超低温冰箱中待用。
1.4.1 苯丙氨酸解氨酶(phenylalnine ammonialyase, PAL)活性 参照YIN等[17]的方法。称取愈伤组织2 g,用3 mL 0.05 mol·L-1的硼酸缓冲液(含1% PVPP,5 mmol·L-1β-琉基乙醇,pH 8.8)冰浴研磨成匀浆,4℃、15 000×g离心30 min,取上清液用于酶活测定。酶促反应体系:将500 μL粗酶液加入0.05 mol·L-1的硼酸缓冲液中(pH 8.8)孵育,再加入20 mmol·L-1的L-苯丙氨酸,37℃保温30 min,充分混合后290 nm下测定吸光值,作为反应初始值(OD0)。再在37℃下反应1 h,测定其OD/290nm,作为终止值(OD1)。对照组以硼酸缓冲液代替酶液。每分钟内OD/290 nm变化0.01为1个酶活性单位(U),以U·g-1FW表示。重复3次。
1.4.2 木质素、总酚及类黄酮含量 木质素含量的测定参照YIN等[17]方法。取2 g愈伤组织,在预冷的5 mL 95%乙醇中研磨成匀浆状,然后4℃,14 000 ×g离心30 min,弃去上清液,将沉淀物用95%乙醇冲洗3次,再用乙醇∶正己烷=1∶2(V/V)冲洗3次,再次收集沉淀在60℃烘箱中干燥24 h,将干燥物转移至离心管中,溶于1 mL 25%溴化乙酰冰醋酸溶液,70℃恒温水浴30 min,最后加入1 mL 2 mol·L-1NaOH中止反应。再加2 mL冰醋酸和0.1 mL 7.5 mol·L-1羟胺盐酸,离心,取上清液0.5 mL,用冰醋酸定容至5 mL,在280 nm下测定吸光值,样品重复3次。木质素含量以FW表示。
总酚及类黄酮含量的测定参照参照YIN等[17]方法。取2 g愈伤组织,于预冷的5mL 1% HCl-甲醇溶液中,冰浴研磨成匀浆进行提取,然后于4℃下12 000×g离心30 min,收集上清液分别于280、325 nm下测定吸光值。总酚与类黄酮含量分别以OD280·g-1FW和OD325·g-1FW表示。重复3次。
1.5 数据统计与分析
全部数据采用Excel 2007计算平均值和标准误(±SE),采用SPSS 17.0进行Duncan’s多重比较检验分析。
2 结果
2.1 马铃薯块茎抗/感品种愈伤期间失重率和病情指数
愈伤期间,两个品种块茎的失重率均逐渐升高,‘陇薯3号’的失重率显著高于‘青薯168’(图1-A)。第14天时‘陇薯3号’的失重率是‘青薯168’的2.1倍。两个品种块茎在不同愈伤时期接种硫色镰孢后的病情指数均逐渐降低。愈伤前期‘陇薯3号’的病情指数显著高于‘青薯168’(图1-B),第3和5天时,该品种的病情指数分别是‘青薯168’的3.5和3.6倍。第7天时,两个品种病情指数接近,无显著差异,之后逐渐趋于一致。
2.2 马铃薯块茎抗/感品种愈伤期间木质素和软木脂的积累
两个品种块茎愈伤组织的木质素沉积随愈伤时间的延长而增加(图2-A)。但‘青薯168’的木质素沉积速度明显快于‘陇薯3号’。前者的木质素染色较深,细胞形成层数较多。第14天时,‘青薯168’形成愈伤组织的细胞层数约为4—5层,细胞较小且排列紧密,而‘陇薯3号’的仅为2—3层,细胞较大且排列疏松。同样,两个品种块茎愈伤组织中的软木脂沉积也在不断增加(图2-B)。‘青薯168’的木栓化程度明显快于‘陇薯3号’。前者的荧光强度较高,细胞形成层数较多。第14天时,‘青薯168’的愈伤组织有5—6层细胞发出强烈荧光,而‘陇薯3号’只有2—3层细胞发出微弱荧光。
愈伤期间,两个品种块茎愈伤组织的木质化细胞层厚度均不断增加(图3-A)。‘青薯168’的木质层厚度显著大于‘陇薯3号’。第14天时,‘青薯168’的木质层厚度高出‘陇薯3号’47.4%。同样,两个品种愈伤组织中的软木脂总荧光强度也持续增加(图3-B),第14天时,‘青薯168’的荧光强度高出‘陇薯3号’60.6%。
2.3 马铃薯块茎抗/感品种愈伤期间苯丙氨酸解氨酶(PAL)活性以及木质素、总酚和类黄酮含量
愈伤期间,两个品种块茎愈伤组织中的PAL活性明显存在差异(图4-A)。‘青薯168’的PAL活性呈缓慢上升趋势,而‘陇薯3号’呈现先下降,然后基本保持稳定的趋势。但‘青薯168’的PAL活性显著高于‘陇薯3号’。第14天时,高出‘陇薯3号’77%。随着愈伤时间的延长,两个品种愈伤组织中的木质素含量均逐渐上升,但‘青薯168’的木质素含量显著高于‘陇薯3号’(图4-B)。第7天时,‘青薯168’的木质素含量高出‘陇薯3号’65%。两个品种愈伤组织中的总酚含量在愈伤期间也存在差异(图4-C)。‘青薯168’的总酚含量不断增加,而‘陇薯3号’的基本稳定。但‘青薯168’的总酚含量在中期和后期均显著高于‘陇薯3号’,第14天时含量高出‘陇薯3号’76%。两个品种的类黄酮含量在愈伤期间的变化趋势基本相同,均随愈伤时间的延长而不断增加(图4-D)。但在愈伤前期差异显著,第0和3天时,‘青薯168’的类黄酮含量分别高出‘陇薯3号’43.5%和16.3%。而第5天以后,两个品种间的类黄酮含量基本保持一致。
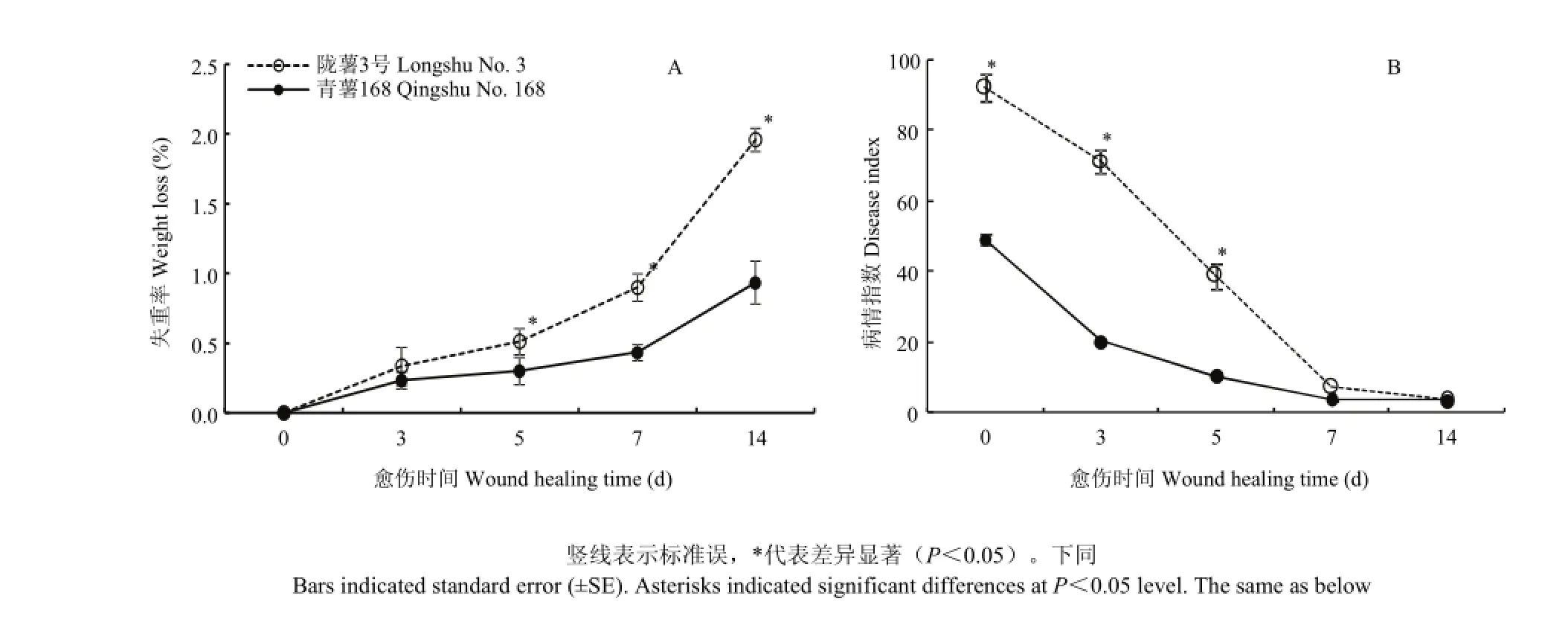
图1 马铃薯品种‘青薯168’和‘陇薯3号’块茎愈伤期间失重率(A)和病情指数(B)Fig. 1 Weight loss (A) and disease index (B) of potato tuber cultivars ‘Qingshu No. 168’ and ‘Longshu No. 3’ during wound healing
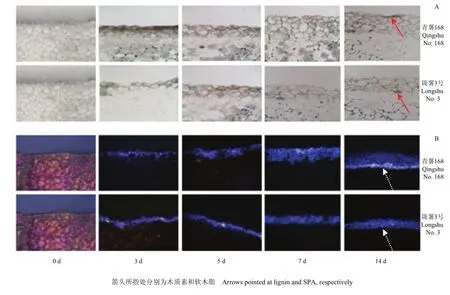
图2 马铃薯品种‘青薯168’和‘陇薯3号’块茎愈伤期间木质素(A)与软木脂(B)的积累Fig. 2 The accumulation of lignin (A) and SPA (B) of potato tuber cultivars ‘Qingshu No. 168’ and ‘Longshu No. 3’ during wound healing
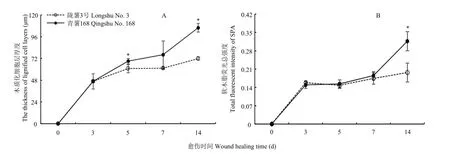
图3 马铃薯品种‘青薯168’和‘陇薯3号’块茎愈伤期间木质化细胞层厚度(A)和软木脂荧光总强度(B)Fig. 3 Thickness of lignified cell layers (A) and total fluorescent intensity of SPA (B) of potato tuber cultivars ‘Qingshu No. 168’ and ‘Longshu No. 3’ during wound healing
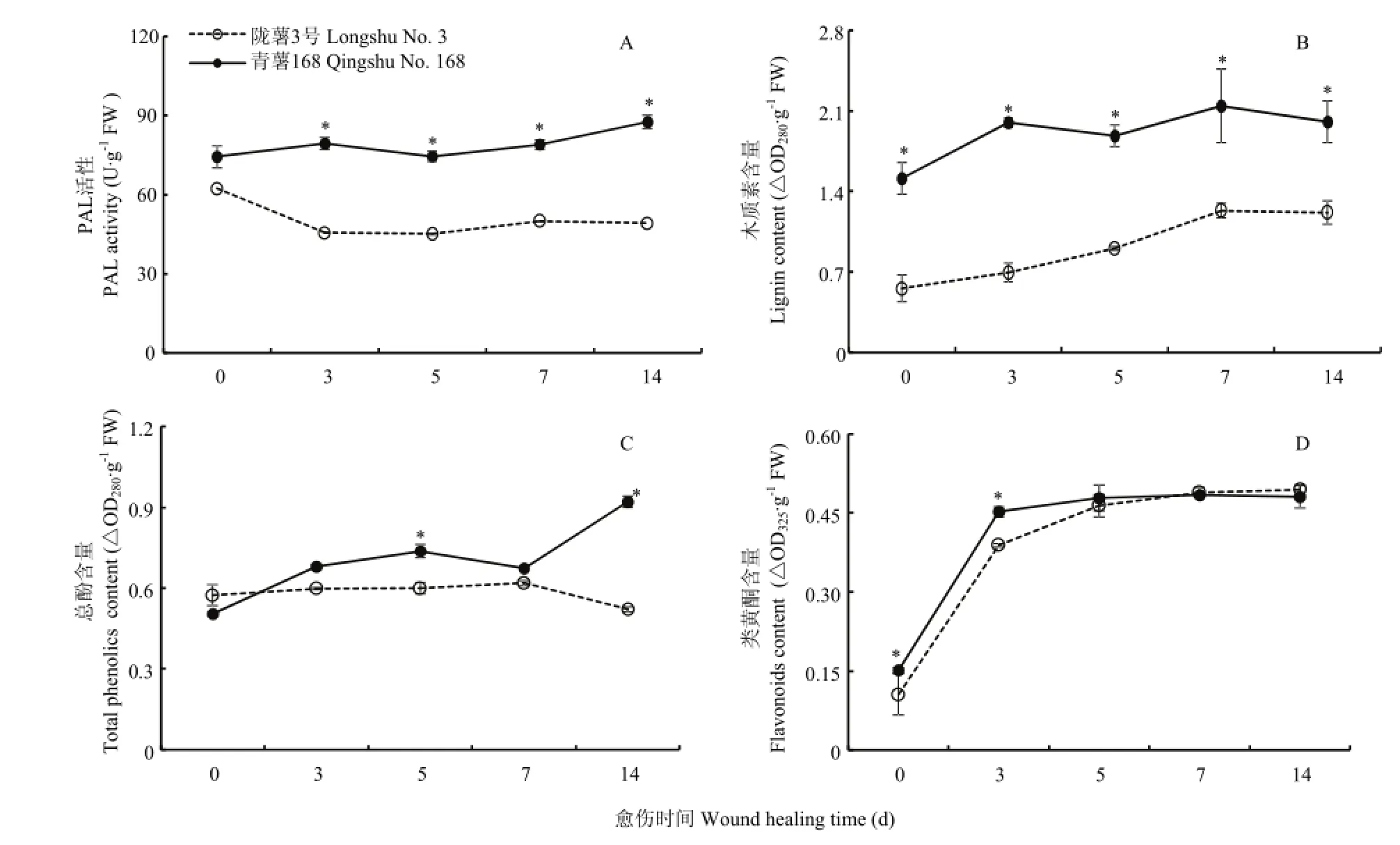
图4 马铃薯品种‘青薯168’和‘陇薯3号’块茎愈伤期间苯丙氨酸解氨酶活性(A)以及木质素(B)、总酚(C)和类黄酮含量(D)Fig. 4 Activity of PAL (A), content of lignin (B), total phenolics (C) and flavonoids (D) of potato tuber cultivars ‘Qingshu No. 168’and ‘Longshu No. 3’ during wound healing
3 讨论
马铃薯块茎受不同程度的损伤后,伤诱导的栓化作用能够使块茎伤口自动愈合,在伤口表面新生成一层完整周皮,从而可以较好地维持块茎品质,抵抗病原物的侵染[18-19]。本研究发现不同品种间块茎的愈合能力存在较大差异。抗病品种‘青薯168’的失重率、病情指数显著低于感病品种‘陇薯3号’,而‘青薯168’的木质素与软木脂沉积速率及苯丙烷代谢活性显著高于‘陇薯3号’,表明抗病品种比感病品种具有更强的愈伤能力。
受损马铃薯块茎的伤口应答和愈伤涉及许多生物过程,其中最为重要的就是伤诱导的栓化。该过程包括初级栓化和次级栓化两个时期,前者是伤口处形成‘封闭层’,而后者是‘封闭层’下形成‘伤口周皮’[3]。栓化完成后,伤口处会积累大量的脂质聚合物、软木脂和木质素等成分,这些物质在阻止水分蒸腾、营养损失及抵抗病原物方面发挥着重要作用[20]。愈伤期间,感病品种‘陇薯3号’的失重率显著高于抗病品种‘青薯168’(图1-A),这可能与感病品种周皮的形成速度较慢,软木脂的积累速度较低有关。已有报道表明,软木脂是由沉积在细胞壁上的聚酚类物质(SPP)和沉积在细胞壁与细胞膜之间的聚脂类物质(SPA)通过甘油交联、蜡质填充而形成的杂聚物,这两类物质对病原物的抵抗作用存在差异,SPP可抵抗细菌性病害,而SPA只对真菌性病害表现抗性[21-23]。当SPA在伤后5—7 d沉积至第一层细胞时,块茎就具备了抵抗真菌侵染的能力[14]。本研究所观察到的块茎病情指数随着愈伤时间延长而降低的结果与之相符。此外,块茎愈伤组织积累的脂质和木质素以及周皮细胞层的厚度均会显著影响块茎对病原物侵染的抗性[24]。因此,抗病品种‘青薯168’所表现出的良好愈伤效果与其愈伤组织中木质素和SPA的快速积累(图2-A、2-B)以及较大的木质化细胞层厚度与软木脂荧光强度(图3-A、3-B)密切相关。JARVINEN等[23]发现,红皮抗病品种‘Asterix’和白皮感病品种‘Nikola’块茎的软木脂组织中SPA的成分与含量的变化在贮藏期间存在差异。由此表明,不同品种马铃薯块茎的愈伤能力与其愈伤组织中木质素与软木脂的积累速度密切相关。
蛋白组学的研究结果指出,马铃薯块茎的愈伤涉及细胞增大和分裂、细胞结构、信号转导、能量代谢和次生代谢等多种生化过程[25]。其中以苯丙烷途径为代表的多种次生代谢在块茎周皮的形成中起着重要作用[26-28]。苯丙氨酸解氨酶(PAL)作为植物体内合成总酚、类黄酮及木质素等物质的关键酶[29-30],在愈伤组织的形成中发挥着重要作用。该酶为软木脂的合成提供了酚前体[31],如阿魏酸和羟基肉桂酸衍生物等[32-34]。PAL在参与木质素形成的同时也参与了防御机制的诱导[35-36]。本研究显示,抗病品种‘青薯168’较高的PAL活性是导致该品种愈伤组织中木质素与软木脂快速积累的关键(图4-A),该结果与SHAO等[37]在‘红富士’和‘嘎啦’苹果果实愈伤期间的观察结果类似。愈伤组织中积累的酚类物质和木质素还可能会直接毒杀病原物并参与物理屏障的形成[38-39]。有报道表明,过氧化物酶和H2O2参与了愈伤结构的形成[40],在过氧化物酶和H2O2的介导下酚酸类物质的聚合才能实现[41]。同样,MAHGOUBA等[42]也发现马铃薯抗病品种可产生更多的次生代谢产物,其总酚含量和过氧化物酶活性较高,木栓层较厚,能更好地氧化羟基肉桂醇生成木质素和交联细胞壁,从而具备较强的抗病性。由此表明,活性氧和过氧化物酶在马铃薯块茎愈伤组织形成中发挥了重要作用,但具体的作用机理尚有待今后进一步研究。
4 结论
抗/感马铃薯品种块茎的愈伤能力存在较大差异,抗病品种的愈伤能力要显著高于感病品种。抗病品种所具备的良好愈伤能力与其高活力的苯丙烷代谢密切相关,在苯丙氨酸解氨酶的作用下,块茎愈伤组织中快速积累酚前体,在过氧化物酶和H2O2参与下聚合为木质素与软木脂,促进了愈伤结构的形成。
[1] DASTMALCHI K, WANG I, STARK R E. Potato wound-healing tissues: A rich source of natural antioxidant molecules with potential for food preservation.Food Chemistry, 2016, 210: 473-480.
[2] XUE H L, BI Y, WEI J M, TANG Y M, ZHAO Y, WANG Y. Effect of cultivars,Fusariumstrains and storage temperature on trichothecenes production in inoculated potato tubers.Food Chemistry, 2014, 151: 236-242.
[3] LULAI E C. Skin-set, wound healing and related defects//Potato Biology and Biotechnology:Advances and Perspectives, 2007: 471-500.
[4] LULAI E C, SUTTLE J C, PEDERSON S M. Regulatory involvement of abscisic acid in potato tuber wound-healing.Journal of Experimental Botany, 2008, 59(6): 1175-1186.
[5] LULAI E C, NEUBAUER J D. Wound-induced suberization genes are differentially expressed, spatially and temporally, during closing layer and wound periderm formation.Postharvest Biology and Technology, 2014, 90: 24-33.
[6] 孙小娟, 李永才, 毕阳, 刘瑾, 尹艳. 西北地区马铃薯贮藏期病害调查分析. 中国马铃薯, 2009, 23(6): 364-365.
SUN X J, LI Y C, BI Y, LIU J, YIN Y. Investigation and analysis of disease on potato tubers during storage in Northwest China.Chinese Potato Journal, 2009, 23(6): 364-365. (in Chinese)
[7] VAN OIRSCHOT Q E A, REES D, AKED J, KIHURANI A. Sweetpotato cultivars differ in efficiency of wound healing.Postharvest Biology and Technology, 2006, 42(1): 65-74.
[8] St AMAND P C, RANDLE W M. Ethylene production as a possible indicator of wound healing in roots of several sweet potato cultivars.Euphytica, 1991, 53(2): 97-102.
[9] DASTMALCHI K, KALLASH L, WANG I, PHAN V C, HUANG W, SERRA O, STARK R E. Defensive armor of potato tubers: nonpolar metabolite profiling, antioxidant assessment, and solid-state NMR compositional analysis of suberin-enriched wound-healing tissues.Journal of Agricultural and Food Chemistry, 2015, 63(30): 6810-6822.
[10] DASTMALCHI K, CAI Q, ZHOU K, HUANG W L, SERRA O, STARK R E. Solving the jigsaw puzzle of wound-healing potato cultivars: metabolite profiling and antioxidant activity of polar extracts.Journal of Agricultural and Food Chemistry, 2014, 62(31): 7963-7975.
[11] 包改红, 毕阳, 李永才, 吴觉天, 寇宗红, 葛永红, 王毅, 王蒂. 不同愈伤时间对低温贮藏期间马铃薯块茎采后病害及品质的影响.食品工业科技, 2013, 34(11): 330-334.
BAO G H, BI Y, LI Y C, WU J T, KOU Z H, GE Y H, WANG Y, WANG D. Effect of curing duration on incidence and quality of potato tuber during storage at low temperature.Science and Technology ofFood Industry, 2013, 34(11): 330-334. (in Chinese)
[12] ALBA C M, DE FORCHETTI S M, TIGIER H A. Phenoloxidase of peach (Prunus persica) endocarp: Its relationship with peroxidases and lignification.Physiologia Plantarum, 2000, 109(4): 382-387.
[13] LULAI E C, MORGAN W C. Histochemical probing of potato periderm with neutral red: a sensitive cytofluorochrome for the hydrophobic domain of suberin.Biotechnic and Histochemistry, 1992, 67(4): 185-195.
[14] LULAI E C, CORSINI D L. Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosumL.) wound-healing.Physiological and Molecular Plant Pathology, 1998, 53(4): 209-222.
[15] 韩晶, 骞爱荣, 胡丽芳, 王哲, 于丹, 张蓉, 商澎. 基于免疫荧光图像的微管蛋白半定量分析. 第四军医大学学报, 2009, 30(24): 2901-2904.
HAN J, QIAN A R, HU L F, WANG Z, YU D, ZHANG R, SHANG P. Semi-quantitative analysis methods of tubulin based on the fluorescent images.Journal of the Fourth Military Medical University, 2009, 30(24): 2901-2904. (in Chinese)
[16] 吴觉天, 毕阳, 李永才, 包改红, 葛永红, 王蒂. 不同愈伤时间对常温贮藏期间马铃薯干腐病和品质的影响. 食品工业科技, 2013, 34(14): 332-334, 357.
WU J T, BI Y, LI Y C, BAO G H, GE Y H, WANG D. Wound curing in different time on dry rot and quality of potatoes during storage at room temperature.Science and Technology of Food Industry, 2013, 34(14): 332-334, 357. (in Chinese)
[17] YIN Y, LI Y C, BI Y, CHENG S J, LI Y, C, YUAN L, WANG Y, WANG D. Postharvest treatment withβ-aminobutyric acid induces resistance against dry rot caused byFusarium sulphureumin potato tuber.Agricultural Sciences in China, 2010, 9(9): 1372-1380.
[18] BERNARDS M A. Demystifying suberin.Canadian Journal of Botany, 2002, 80(3): 227-240.
[19] VISHWANATH S J, DELUDE C, DOMERGUE F, ROWLAND O. Suberin: biosynthesis, regulation, and polymer assembly of a protective extracellular barrier.Plant Cell Reports, 2015, 34(4): 573-586.
[20] SOLIDAY C L, KOLATTUKUDY P E, DAVIS R W. Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosumL.).Planta, 1979, 146(5): 607-614.
[21] BERNARDS M A, LEWIS N G. The macromolecular aromatic domain in suberized tissue: a changing paradigm.Phytochemistry, 1998, 47(6): 915-933.
[22] SCHREIBER L, FRANKE R, HARTMANN K. Wax and suberin development of native and wound periderm of potato (Solanum tuberosumL.) and its relation to peridermal transpiration.Planta, 2005, 220(4): 520-530.
[23] JARVINEN R, RAUHALA H, HOLOPAINEN U, KALLIO H. Differences in suberin content and composition between two varieties of potatoes (Solanum tuberosumL.) and effect of post-harvest storage to the composition.LWT-Food Science and Technology, 2011, 44(6): 1355-1361.
[24] MORRIS S C, FORBES-SMITH M R, SCRIVENB F M. Determination of optimum conditions for suberization, wound periderm formation, cellular desiccation and pathogen resistance in woundedSolanum tuberosumtubers.Physiological and Molecular Plant Pathology, 1989, 35(2): 177-190.
[25] CHAVES I, PINHEIRO C, PAIVA1 J A, PLANCHON S, SERGEANT K, RENAUT J, GRAÇA J A, COSTA G, COELHO A V, RICARDO C P. Proteomic evaluation of wound-healing processes in potato (Solanum tuberosumL.) tuber tissue.Proteomics, 2009, 17(9): 4154-4175.
[26] FRANKE1 R, HÖFER R, BRIESEN I, EMSERMANN M, EFREMOVA N, YEPHREMOV A, SCHREIBER L. TheDAISYgene fromArabidopsisencodes a fatty acid elongase condensing enzyme involved in the biosynthesis of aliphatic suberin in roots and the chalaza-micropyle region of seeds.The Plant Journal, 2009, 57(1): 80-95.
[27] YANG W L, BERNARDS M A. Metabolite profiling of potato (Solanum tuberosumL.) tubers during wound-induced suberization.Metabolomics, 2007, 3(2): 147-159.
[28] YANG W L, BERNARDS M A. Wound-induced metabolism in potato (Solanum tuberosum) tubers.Plant Signaling and Behavior, 2006, 1(2): 59-66.
[29] STADNIK M J, BUCHENAUER H. Inhibition of phenylalanine ammonia-lyase suppresses the resistance induced by benzothiadiazole in wheat toBlumeria graminisf. sp.tritici.Physiological and Molecular Plant Pathology, 2000, 57(1): 25-34.
[30] BORG-OLIVIER O, MONTIES B. Lignin, suberin, phenolic acids and tyramine in the suberized, wound-induced potato periderm.Phytochemistry, 1993, 32(3): 601-606.
[31] KOLATTUKUDY P E. Biopolyester membranes of plants: cutin and suberin.Science, 1980, 208(4447): 990-1000.
[32] HAMMERSCHMIDT R. Rapid deposition of lignin in potato tuber tissue as a response to fungi non-pathogenic on potato.PhysiologicalPlant Pathology, 1984, 24(1): 33-42.
[33] BERNARDS M A, SUSAG L M, BEDGAR D L, ANTEROLA A M, LEWIS N G. Induced phenylpropanoid metabolism during suberization and lignification: a comparative analysis.Journal of Plant Physiology, 2000, 157(6): 601-607.
[34] BERNARDS M A, LOPEZ M L, ZAJICEK J, LEWIS N G. Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin.The Journal of Biological Chemistry, 1995, 270(13): 7382-7386.
[35] VALENTINES M C, VILAPLANA R, TORRES R, USALL J, LARRIGAUDIERE C. Specific roles of enzymatic browning and lignification in apple disease resistance.Postharvest Biology and Technology, 2005, 36(3): 227-234.
[36] THOMAS R, FANG X, RANATHUNGE K, ANDERSON T R, PETERSON C A, BERNARDS M A. Soybean root suberin: anatomical distribution, chemical composition, and relationship to partial resistance toPhytophthora sojae.Plant Physiology, 2007, 144(1): 299-311.
[37] SHAO X F, KANG T, TU S C, SU J, ZHAO Y. Effects of heat treatment on wound healing in Gala and Red Fuji apple fruits.Journalof Agricultural and Food Chemistry, 2010, 58(7): 4303-4309.
[38] GHANEKAR A S, PADWAL-DESAI S R, NADKARNI G B. The involvement of phenolics and phytoalexins in resistance of potato to soft rot.Potato Research, 1984, 27(2): 189-199.
[39] BACK K. Hydroxycinnamic acid amides and their possible utilization for enhancing agronomic traits.The Plant Pathology Journal, 2001, 17(3): 123-127.
[40] ARRIETA-BAEZ D, STARK R E. Modeling suberization with peroxidase-catalyzed polymerization of hydroxycinnamic acids: cross-coupling and dimerization reactions.Phytochemistry, 2006, 67(7): 743-753.
[41] RAZEM F A, BERNARDS M A. Hydrogen peroxide is required for poly (phenolic) domain formation during wound-induced suberization.Journal of Agricultural and Food Chemistry, 2002, 50(5): 1009-1015.
[42] MAHGOUBA H A M, EISAB G S A, YOUSSEFC M A H. Molecular, biochemical and anatomical analysis of some potato (Solanum tuberosumL.) cultivars growing in Egypt.Journal of Genetic Engineering and Biotechnology, 2015, 13(1): 39-49.
(责任编辑 岳梅)
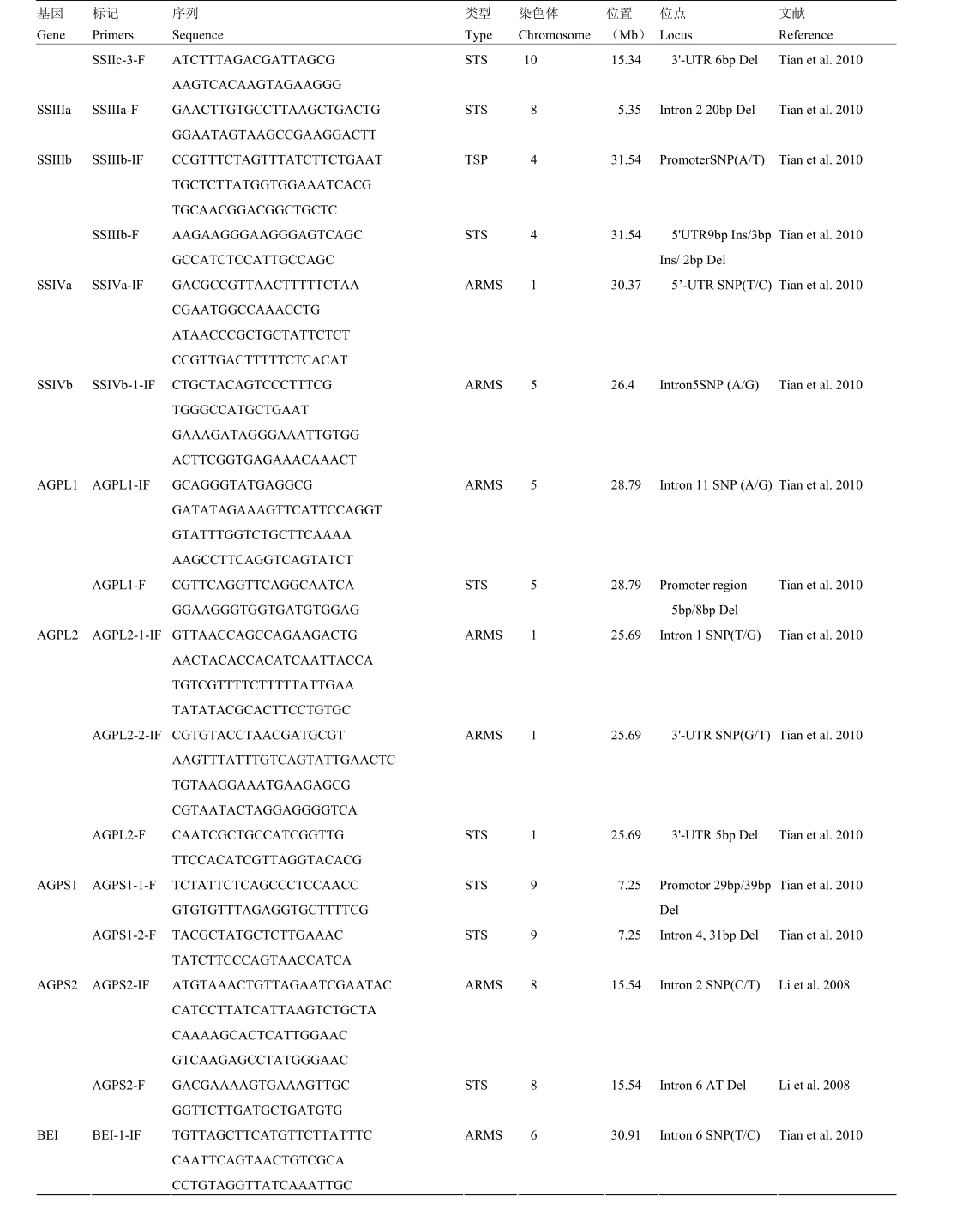
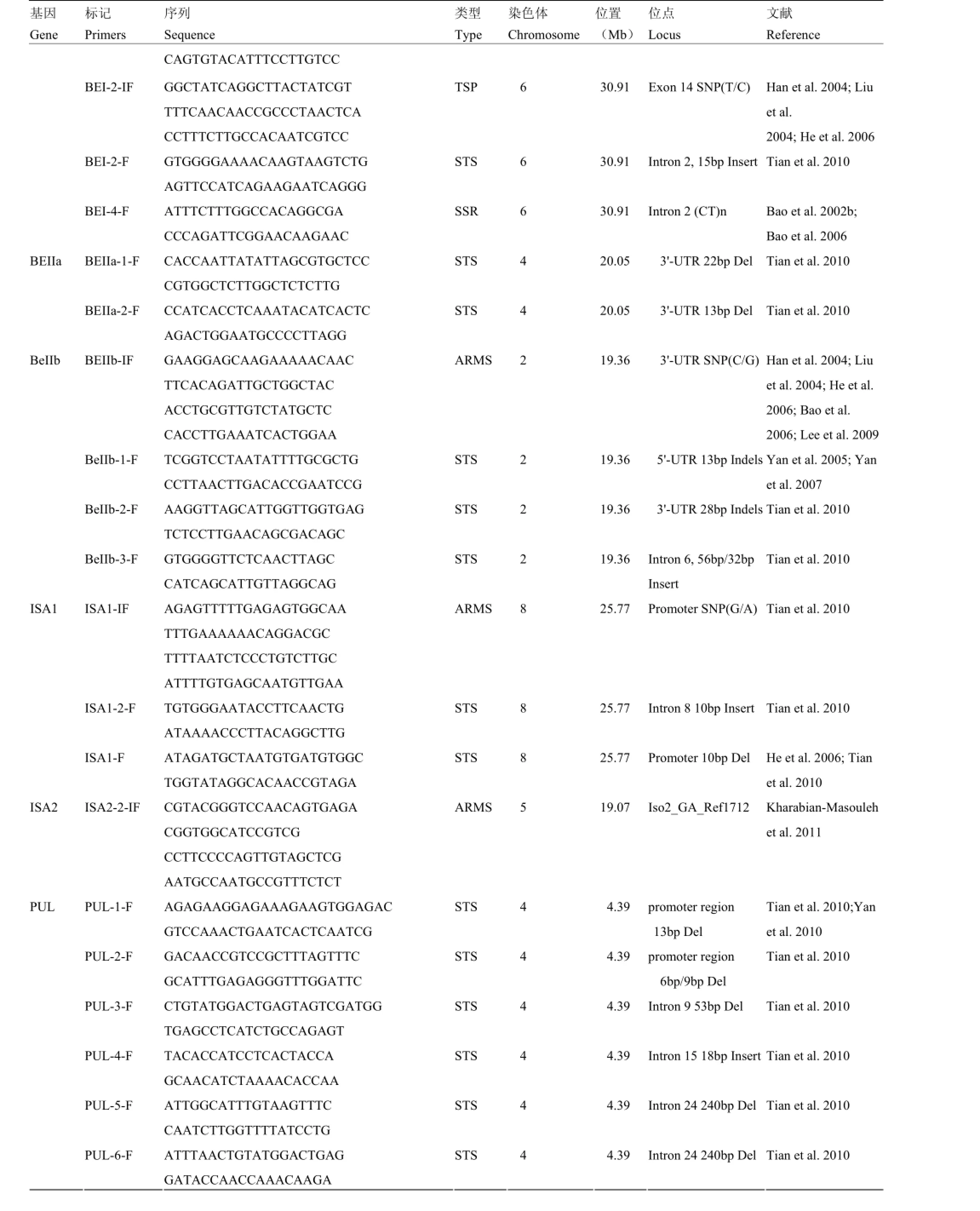
附表1 本研究筛选用于性状-标记关联分析的分子标记
Supplementary table 1 The molecular markers used for trait-marker association analysis in this study
Comparison of Healing Ability on Potato Tuber Cultivars‘Qingshu No. 168’ and ‘Longshu No. 3’
JIANG Hong, BI Yang, LI ChangJian, WANG Yi, LI ShengE, LIU YaoNa, WANG Bin
(College of Food Science and Engineering, Gansu Agricultural University, Lanzhou 730070)
【Objective】The objective of this study is to find out the differences of tuber healing ability of resistant and susceptible potato cultivars against dry rot (‘Qingshu No. 168’ and ‘Longshu No. 3’), and explore the reasons causing differences in the levels of lignin and suberin accumulation and phenylpropanoid metabolism.【Method】The resistant cultivar ‘Qingshu No. 168’ and susceptible cultivar ‘Longshu No. 3’ were used as materials. The damage of tubers was artificially simulated and heal was conducted at ambient temperature. The healing effect was assessed by determining the weight loss and disease index of wounded tubers that were inoculated with Fusarium sulphureum at every healing time point. Phloroglucinol-HCl staining and toluidin blue O-neutral red staining were used to observe lignin and suberin deposition, and the lignified cell layers and suberin fluorescent intensity were quantified. Moreover, the key enzyme and metabolites of phenylpropanoid metabolism pathway such asphenylalanine ammonia lyase activity, and the contents of lignin, total phenol and flavonoid were determined to compare the differences of healing ability between the two cultivars.【Result】The weight loss of ‘Qingshu No. 168’ was significantly lower than that of ‘Longshu No. 3’ during healing. The weight loss of ‘Qingshu No. 168’ and ‘Longshu No. 3’ was 0.93% and 1.96% after 14 days of healing, and the weight loss of ‘Qingshu No. 168’ was 52.3% lower than that of ‘Longshu No. 3’. The disease index of ‘Qingshu No. 168’ was also significantly lower than ‘Longshu No. 3’ after inoculated withF. sulphureumduring healing. After 3 days of healing for inoculated tubers, the disease index of ‘Qingshu No. 168’ and ‘Longshu No. 3’ was 20.36 and 71.59, respectively, the former was 71.5% lower than the later. During healing, ‘Qingshu No. 168’ had higher accumulation rate of lignin and suberin than ‘Longshu No. 3’. After healing for 14 days, the thickness of lignified cell layers and total fluorescent intensity of suberin of ‘Qingshu No. 168’ were 47.4% and 60.6% higher than ‘Longshu No. 3’. In addition, ‘Qingshu No. 168’ showed more phenylpropanoid metabolism activity than ‘Longshu No. 3’. The activity of phenylalanine ammonialyase and the lignin content of‘Qingshu No. 168’ were 77% and 65% higher than ‘Longshu No. 3’after healing for 14 days, respectively. The total phenolics contents of the two cultivars showed a significant difference at late stage of healing, ‘Qingshu No. 168’ was 76% higher than‘Longshu No. 3’ after healing for 14 days. However, no significant difference was found in the flavonoids content between the two cultivars during healing.【Conclusion】The resistant cultivar ‘Qingshu No. 168’ showed stronger healing ability than susceptible cultivar ‘Longshu No. 3’. A stronger healing ability of resistant cultivar is related with its higher phenylpropanoid metabolism activity.
potato; cultivars; tuber; wound healing; phenylpropanoid metabolism
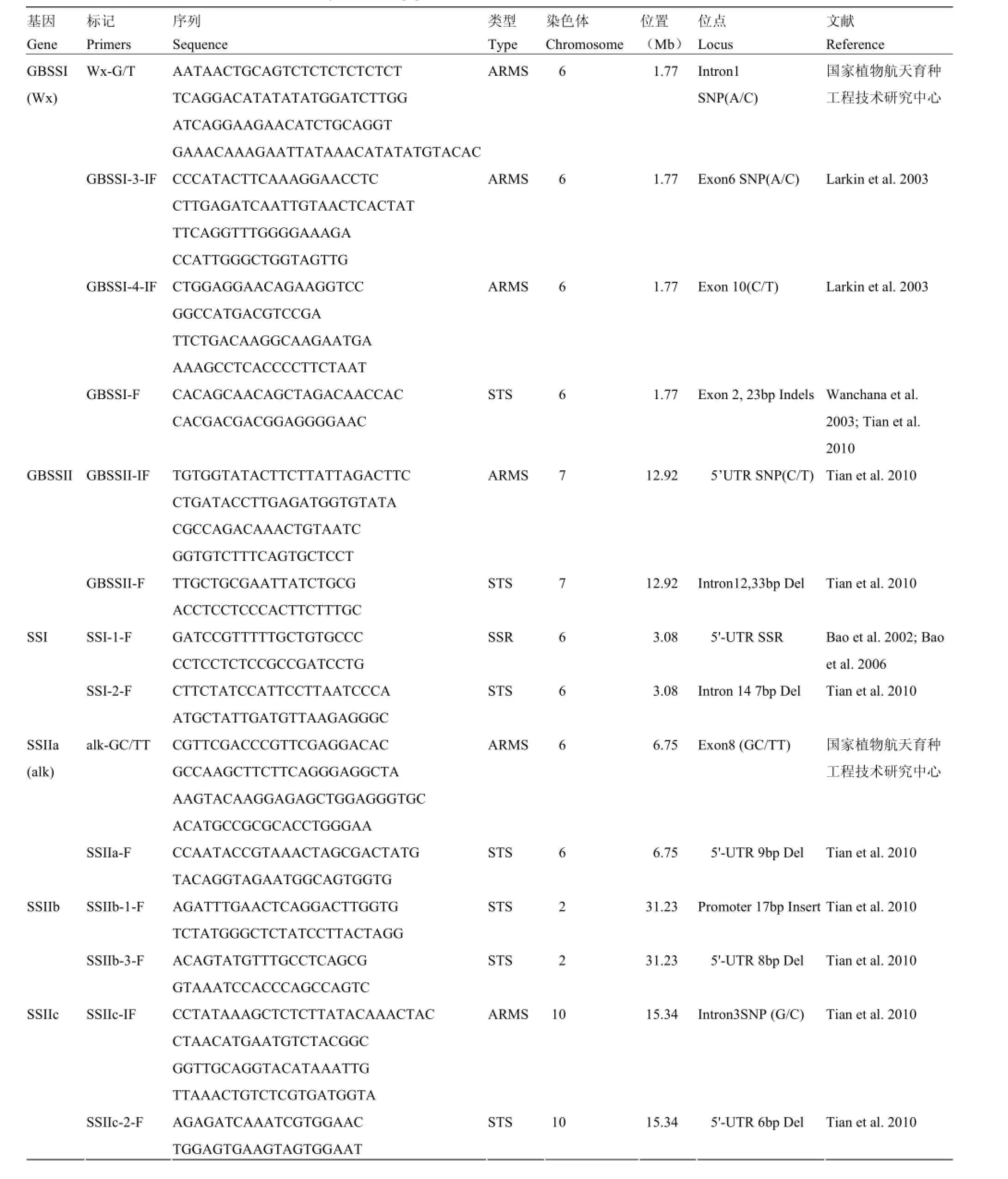
第一部分:淀粉合成相关基因等位标记Part I: Allelic markers related to starch biosynthesizing genes
基因Gene标记Primers序列Sequence类型Type染色体Chromosome位置(Mb)位点Locus文献Reference SSIIc-3-F ATCTTTAGACGATTAGCG AAGTCACAAGTAGAAGGG STS 10 15.34 3'-UTR 6bp Del Tian et al. 2010 SSIIIa SSIIIa-F GAACTTGTGCCTTAAGCTGACTG GGAATAGTAAGCCGAAGGACTT STS 8 5.35 Intron 2 20bp Del Tian et al. 2010 SSIIIb-IF CCGTTTCTAGTTTATCTTCTGAAT TGCTCTTATGGTGGAAATCACG TGCAACGGACGGCTGCTC TSP 4 31.54 PromoterSNP(A/T) Tian et al. 2010 SSIIIb SSIIIb-F AAGAAGGGAAGGGAGTCAGC GCCATCTCCATTGCCAGC STS 4 31.54 5'UTR9bp Ins/3bp Ins/ 2bp Del Tian et al. 2010 SSIVa SSIVa-IF GACGCCGTTAACTTTTTCTAA CGAATGGCCAAACCTG ATAACCCGCTGCTATTCTCT CCGTTGACTTTTTCTCACAT ARMS 1 30.37 5’-UTR SNP(T/C) Tian et al. 2010 SSIVb SSIVb-1-IF CTGCTACAGTCCCTTTCG TGGGCCATGCTGAAT GAAAGATAGGGAAATTGTGG ACTTCGGTGAGAAACAAACT ARMS 5 26.4 Intron5SNP (A/G) Tian et al. 2010 AGPL1-IF GCAGGGTATGAGGCG GATATAGAAAGTTCATTCCAGGT GTATTTGGTCTGCTTCAAAA AAGCCTTCAGGTCAGTATCT ARMS 5 28.79 Intron 11 SNP (A/G) Tian et al. 2010 AGPL1 AGPL1-F CGTTCAGGTTCAGGCAATCA GGAAGGGTGGTGATGTGGAG STS 5 28.79 Promoter region 5bp/8bp Del Tian et al. 2010 AGPL2-1-IF GTTAACCAGCCAGAAGACTG AACTACACCACATCAATTACCA TGTCGTTTTCTTTTTATTGAA TATATACGCACTTCCTGTGC ARMS 1 25.69 Intron 1 SNP(T/G) Tian et al. 2010 AGPL2-2-IF CGTGTACCTAACGATGCGT AAGTTTATTTGTCAGTATTGAACTC TGTAAGGAAATGAAGAGCG CGTAATACTAGGAGGGGTCA ARMS 1 25.69 3'-UTR SNP(G/T) Tian et al. 2010 AGPL2 AGPL2-F CAATCGCTGCCATCGGTTG TTCCACATCGTTAGGTACACG STS 1 25.69 3'-UTR 5bp Del Tian et al. 2010 AGPS1-1-F TCTATTCTCAGCCCTCCAACC GTGTGTTTAGAGGTGCTTTTCG STS 9 7.25 Promotor 29bp/39bp Del Tian et al. 2010 AGPS1 AGPS1-2-F TACGCTATGCTCTTGAAAC TATCTTCCCAGTAACCATCA STS 9 7.25 Intron 4, 31bp Del Tian et al. 2010 AGPS2-IF ATGTAAACTGTTAGAATCGAATAC CATCCTTATCATTAAGTCTGCTA CAAAAGCACTCATTGGAAC GTCAAGAGCCTATGGGAAC ARMS 8 15.54 Intron 2 SNP(C/T) Li et al. 2008 AGPS2 AGPS2-F GACGAAAAGTGAAAGTTGC GGTTCTTGATGCTGATGTG STS 8 15.54 Intron 6 AT Del Li et al. 2008 BEI BEI-1-IF TGTTAGCTTCATGTTCTTATTTC CAATTCAGTAACTGTCGCA CCTGTAGGTTATCAAATTGC ARMS 6 30.91 Intron 6 SNP(T/C) Tian et al. 2010
基因Gene标记Primers序列Sequence类型Type染色体Chromosome位置(Mb)位点Locus文献Reference CAGTGTACATTTCCTTGTCC BEI-2-IF GGCTATCAGGCTTACTATCGT TTTCAACAACCGCCCTAACTCA CCTTTCTTGCCACAATCGTCC TSP 6 30.91 Exon 14 SNP(T/C) Han et al. 2004; Liu et al. 2004; He et al. 2006 BEI-2-F GTGGGGAAAACAAGTAAGTCTG AGTTCCATCAGAAGAATCAGGG STS 6 30.91 Intron 2, 15bp Insert Tian et al. 2010 BEI-4-F ATTTCTTTGGCCACAGGCGA CCCAGATTCGGAACAAGAAC SSR 6 30.91 Intron 2 (CT)n Bao et al. 2002b; Bao et al. 2006 BEIIa-1-F CACCAATTATATTAGCGTGCTCC CGTGGCTCTTGGCTCTCTTG STS 4 20.05 3'-UTR 22bp Del Tian et al. 2010 BEIIa BEIIa-2-F CCATCACCTCAAATACATCACTC AGACTGGAATGCCCCTTAGG STS 4 20.05 3'-UTR 13bp Del Tian et al. 2010 BEIIb-IF GAAGGAGCAAGAAAAACAAC TTCACAGATTGCTGGCTAC ACCTGCGTTGTCTATGCTC CACCTTGAAATCACTGGAA ARMS 2 19.36 3'-UTR SNP(C/G) Han et al. 2004; Liu et al. 2004; He et al. 2006; Bao et al. 2006; Lee et al. 2009 BeIIb-1-F TCGGTCCTAATATTTTGCGCTG CCTTAACTTGACACCGAATCCG STS 2 19.36 5'-UTR 13bp Indels Yan et al. 2005; Yan et al. 2007 BeIIb-2-F AAGGTTAGCATTGGTTGGTGAG TCTCCTTGAACAGCGACAGC STS 2 19.36 3'-UTR 28bp Indels Tian et al. 2010 BeIIb BeIIb-3-F GTGGGGTTCTCAACTTAGC CATCAGCATTGTTAGGCAG STS 2 19.36 Intron 6, 56bp/32bp Insert Tian et al. 2010 ISA1-IF AGAGTTTTTGAGAGTGGCAA TTTGAAAAAACAGGACGC TTTTAATCTCCCTGTCTTGC ATTTTGTGAGCAATGTTGAA ARMS 8 25.77 Promoter SNP(G/A) Tian et al. 2010 ISA1-2-F TGTGGGAATACCTTCAACTG ATAAAACCCTTACAGGCTTG STS 8 25.77 Intron 8 10bp Insert Tian et al. 2010 ISA1 ISA1-F ATAGATGCTAATGTGATGTGGC TGGTATAGGCACAACCGTAGA STS 8 25.77 Promoter 10bp Del He et al. 2006; Tian et al. 2010 ISA2 ISA2-2-IF CGTACGGGTCCAACAGTGAGA CGGTGGCATCCGTCG CCTTCCCCAGTTGTAGCTCG AATGCCAATGCCGTTTCTCT ARMS 5 19.07 Iso2_GA_Ref1712 Kharabian-Masouleh et al. 2011 PUL-1-F AGAGAAGGAGAAAGAAGTGGAGAC GTCCAAACTGAATCACTCAATCG STS 4 4.39 promoter region 13bp Del Tian et al. 2010;Yan et al. 2010 PUL-2-F GACAACCGTCCGCTTTAGTTTC GCATTTGAGAGGGTTTGGATTC STS 4 4.39 promoter region 6bp/9bp Del Tian et al. 2010 PUL-3-F CTGTATGGACTGAGTAGTCGATGG TGAGCCTCATCTGCCAGAGT STS 4 4.39 Intron 9 53bp Del Tian et al. 2010 PUL-4-F TACACCATCCTCACTACCA GCAACATCTAAAACACCAA STS 4 4.39 Intron 15 18bp Insert Tian et al. 2010 PUL-5-F ATTGGCATTTGTAAGTTTC CAATCTTGGTTTTATCCTG STS 4 4.39 Intron 24 240bp Del Tian et al. 2010 PUL PUL-6-F ATTTAACTGTATGGACTGAG GATACCAACCAAACAAGA STS 4 4.39 Intron 24 240bp Del Tian et al. 2010
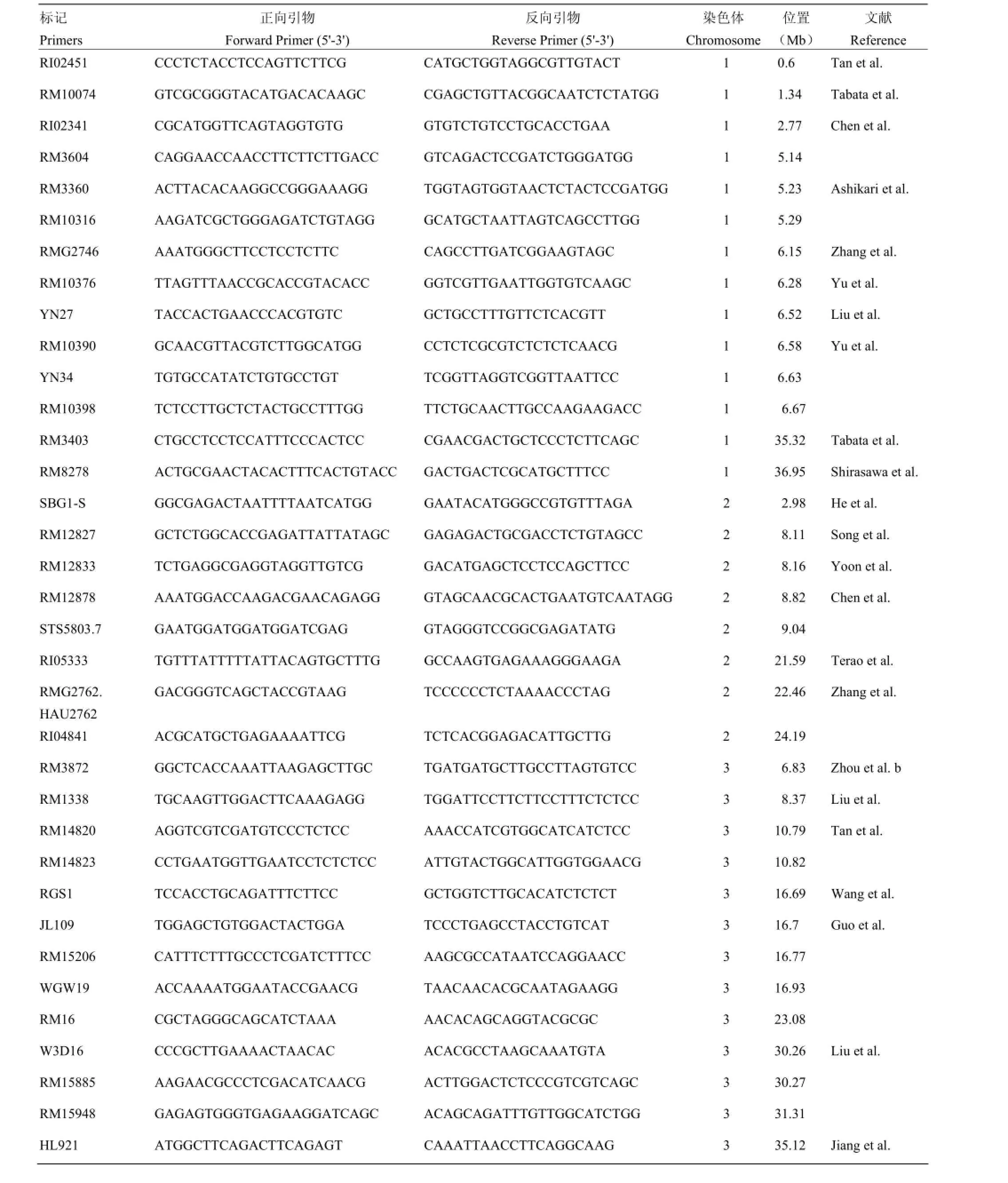
第二部分:稻米籽粒表型相关连锁分子标记Part II: Linkage markers related to phenotype of rice grain
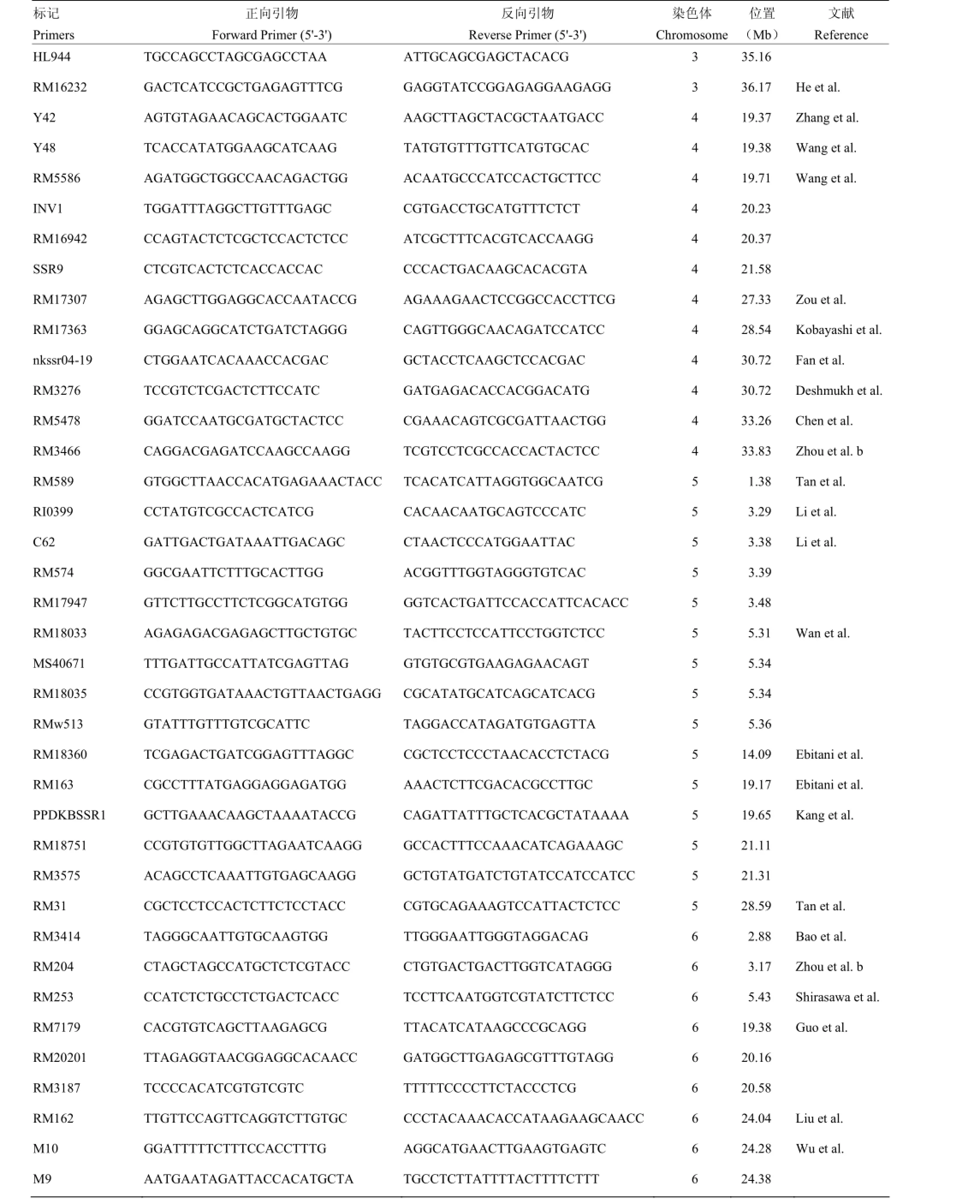
标记Primers正向引物Forward Primer (5'-3')反向引物Reverse Primer (5'-3')染色体Chromosome位置(Mb)文献Reference HL944 TGCCAGCCTAGCGAGCCTAA ATTGCAGCGAGCTACACG 3 35.16 RM16232 GACTCATCCGCTGAGAGTTTCG GAGGTATCCGGAGAGGAAGAGG 3 36.17 He et al. Y42 AGTGTAGAACAGCACTGGAATC AAGCTTAGCTACGCTAATGACC 4 19.37 Zhang et al. Y48 TCACCATATGGAAGCATCAAG TATGTGTTTGTTCATGTGCAC 4 19.38 Wang et al. RM5586 AGATGGCTGGCCAACAGACTGG ACAATGCCCATCCACTGCTTCC 4 19.71 Wang et al. INV1 TGGATTTAGGCTTGTTTGAGC CGTGACCTGCATGTTTCTCT 4 20.23 RM16942 CCAGTACTCTCGCTCCACTCTCC ATCGCTTTCACGTCACCAAGG 4 20.37 SSR9 CTCGTCACTCTCACCACCAC CCCACTGACAAGCACACGTA 4 21.58 RM17307 AGAGCTTGGAGGCACCAATACCG AGAAAGAACTCCGGCCACCTTCG 4 27.33 Zou et al. RM17363 GGAGCAGGCATCTGATCTAGGG CAGTTGGGCAACAGATCCATCC 4 28.54 Kobayashi et al. nkssr04-19 CTGGAATCACAAACCACGAC GCTACCTCAAGCTCCACGAC 4 30.72 Fan et al. RM3276 TCCGTCTCGACTCTTCCATC GATGAGACACCACGGACATG 4 30.72 Deshmukh et al. RM5478 GGATCCAATGCGATGCTACTCC CGAAACAGTCGCGATTAACTGG 4 33.26 Chen et al. RM3466 CAGGACGAGATCCAAGCCAAGG TCGTCCTCGCCACCACTACTCC 4 33.83 Zhou et al. b RM589 GTGGCTTAACCACATGAGAAACTACC TCACATCATTAGGTGGCAATCG 5 1.38 Tan et al. RI0399 CCTATGTCGCCACTCATCG CACAACAATGCAGTCCCATC 5 3.29 Li et al. C62 GATTGACTGATAAATTGACAGC CTAACTCCCATGGAATTAC 5 3.38 Li et al. RM574 GGCGAATTCTTTGCACTTGG ACGGTTTGGTAGGGTGTCAC 5 3.39 RM17947 GTTCTTGCCTTCTCGGCATGTGG GGTCACTGATTCCACCATTCACACC 5 3.48 RM18033 AGAGAGACGAGAGCTTGCTGTGC TACTTCCTCCATTCCTGGTCTCC 5 5.31 Wan et al. MS40671 TTTGATTGCCATTATCGAGTTAG GTGTGCGTGAAGAGAACAGT 5 5.34 RM18035 CCGTGGTGATAAACTGTTAACTGAGG CGCATATGCATCAGCATCACG 5 5.34 RMw513 GTATTTGTTTGTCGCATTC TAGGACCATAGATGTGAGTTA 5 5.36 RM18360 TCGAGACTGATCGGAGTTTAGGC CGCTCCTCCCTAACACCTCTACG 5 14.09 Ebitani et al. RM163 CGCCTTTATGAGGAGGAGATGG AAACTCTTCGACACGCCTTGC 5 19.17 Ebitani et al. PPDKBSSR1 GCTTGAAACAAGCTAAAATACCG CAGATTATTTGCTCACGCTATAAAA 5 19.65 Kang et al. RM18751 CCGTGTGTTGGCTTAGAATCAAGG GCCACTTTCCAAACATCAGAAAGC 5 21.11 RM3575 ACAGCCTCAAATTGTGAGCAAGG GCTGTATGATCTGTATCCATCCATCC 5 21.31 RM31 CGCTCCTCCACTCTTCTCCTACC CGTGCAGAAAGTCCATTACTCTCC 5 28.59 Tan et al. RM3414 TAGGGCAATTGTGCAAGTGG TTGGGAATTGGGTAGGACAG 6 2.88 Bao et al. RM204 CTAGCTAGCCATGCTCTCGTACC CTGTGACTGACTTGGTCATAGGG 6 3.17 Zhou et al. b RM253 CCATCTCTGCCTCTGACTCACC TCCTTCAATGGTCGTATCTTCTCC 6 5.43 Shirasawa et al. RM7179 CACGTGTCAGCTTAAGAGCG TTACATCATAAGCCCGCAGG 6 19.38 Guo et al. RM20201 TTAGAGGTAACGGAGGCACAACC GATGGCTTGAGAGCGTTTGTAGG 6 20.16 RM3187 TCCCCACATCGTGTCGTC TTTTTCCCCTTCTACCCTCG 6 20.58 RM162 TTGTTCCAGTTCAGGTCTTGTGC CCCTACAAACACCATAAGAAGCAACC6 24.04 Liu et al. M10 GGATTTTTCTTTCCACCTTTG AGGCATGAACTTGAAGTGAGTC 6 24.28 Wu et al. M9 AATGAATAGATTACCACATGCTA TGCCTCTTATTTTACTTTTCTTT 6 24.38
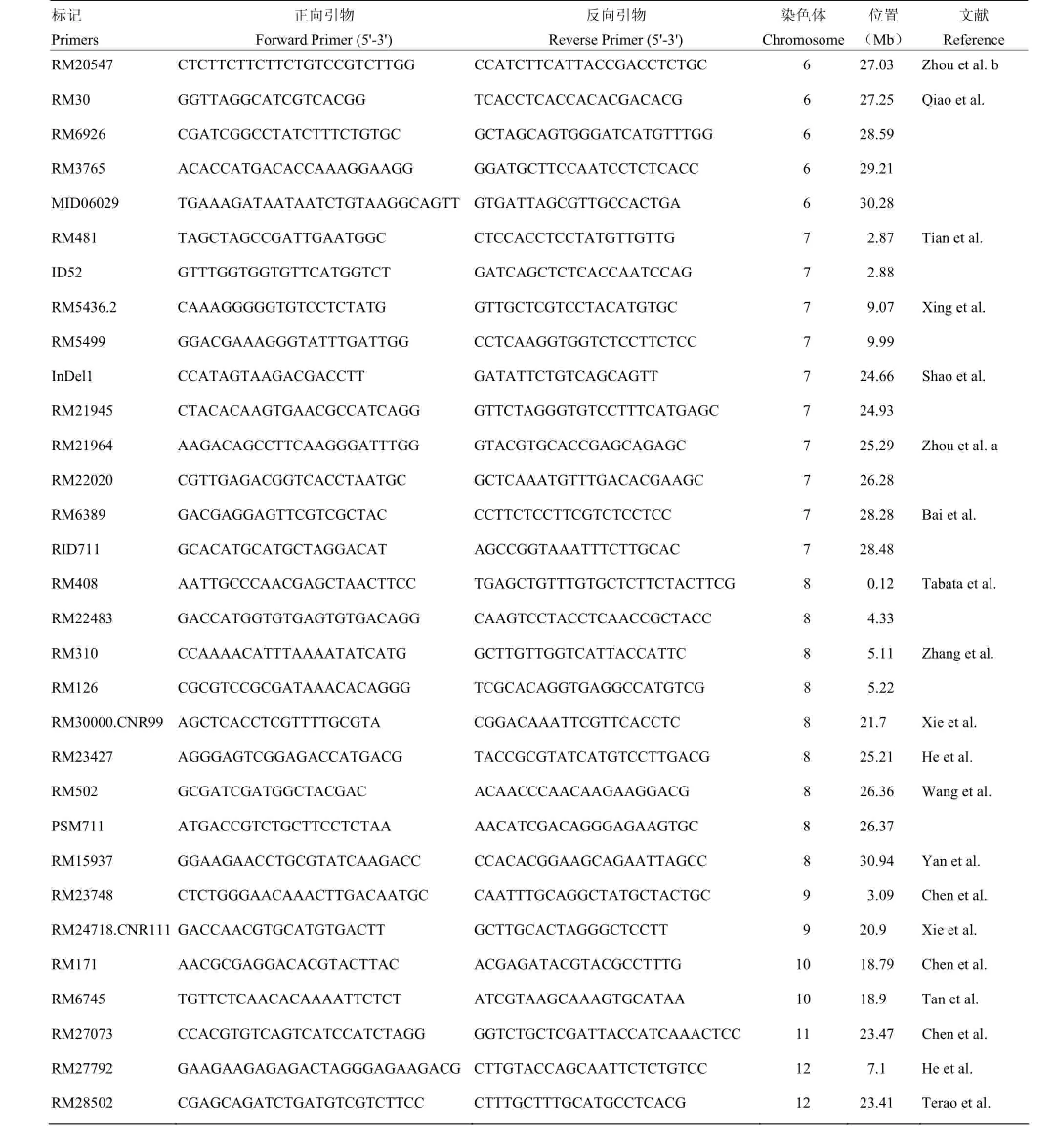
标记Primers正向引物Forward Primer (5'-3')反向引物Reverse Primer (5'-3')染色体Chromosome位置(Mb)文献Reference RM20547 CTCTTCTTCTTCTGTCCGTCTTGG CCATCTTCATTACCGACCTCTGC 6 27.03 Zhou et al. b RM30 GGTTAGGCATCGTCACGG TCACCTCACCACACGACACG 6 27.25 Qiao et al. RM6926 CGATCGGCCTATCTTTCTGTGC GCTAGCAGTGGGATCATGTTTGG 6 28.59 RM3765 ACACCATGACACCAAAGGAAGG GGATGCTTCCAATCCTCTCACC 6 29.21 MID06029 TGAAAGATAATAATCTGTAAGGCAGTTGTGATTAGCGTTGCCACTGA 6 30.28 RM481 TAGCTAGCCGATTGAATGGC CTCCACCTCCTATGTTGTTG 7 2.87 Tian et al. ID52 GTTTGGTGGTGTTCATGGTCT GATCAGCTCTCACCAATCCAG 7 2.88 RM5436.2 CAAAGGGGGTGTCCTCTATG GTTGCTCGTCCTACATGTGC 7 9.07 Xing et al. RM5499 GGACGAAAGGGTATTTGATTGG CCTCAAGGTGGTCTCCTTCTCC 7 9.99 InDel1 CCATAGTAAGACGACCTT GATATTCTGTCAGCAGTT 7 24.66 Shao et al. RM21945 CTACACAAGTGAACGCCATCAGG GTTCTAGGGTGTCCTTTCATGAGC 7 24.93 RM21964 AAGACAGCCTTCAAGGGATTTGG GTACGTGCACCGAGCAGAGC 7 25.29 Zhou et al. a RM22020 CGTTGAGACGGTCACCTAATGC GCTCAAATGTTTGACACGAAGC 7 26.28 RM6389 GACGAGGAGTTCGTCGCTAC CCTTCTCCTTCGTCTCCTCC 7 28.28 Bai et al. RID711 GCACATGCATGCTAGGACAT AGCCGGTAAATTTCTTGCAC 7 28.48 RM408 AATTGCCCAACGAGCTAACTTCC TGAGCTGTTTGTGCTCTTCTACTTCG 8 0.12 Tabata et al. RM22483 GACCATGGTGTGAGTGTGACAGG CAAGTCCTACCTCAACCGCTACC 8 4.33 RM310 CCAAAACATTTAAAATATCATG GCTTGTTGGTCATTACCATTC 8 5.11 Zhang et al. RM126 CGCGTCCGCGATAAACACAGGG TCGCACAGGTGAGGCCATGTCG 8 5.22 RM30000.CNR99 AGCTCACCTCGTTTTGCGTA CGGACAAATTCGTTCACCTC 8 21.7 Xie et al. RM23427 AGGGAGTCGGAGACCATGACG TACCGCGTATCATGTCCTTGACG 8 25.21 He et al. RM502 GCGATCGATGGCTACGAC ACAACCCAACAAGAAGGACG 8 26.36 Wang et al. PSM711 ATGACCGTCTGCTTCCTCTAA AACATCGACAGGGAGAAGTGC 8 26.37 RM15937 GGAAGAACCTGCGTATCAAGACC CCACACGGAAGCAGAATTAGCC 8 30.94 Yan et al. RM23748 CTCTGGGAACAAACTTGACAATGC CAATTTGCAGGCTATGCTACTGC 9 3.09 Chen et al. RM24718.CNR111 GACCAACGTGCATGTGACTT GCTTGCACTAGGGCTCCTT 9 20.9 Xie et al. RM171 AACGCGAGGACACGTACTTAC ACGAGATACGTACGCCTTTG 10 18.79 Chen et al. RM6745 TGTTCTCAACACAAAATTCTCT ATCGTAAGCAAAGTGCATAA 10 18.9 Tan et al. RM27073 CCACGTGTCAGTCATCCATCTAGG GGTCTGCTCGATTACCATCAAACTCC 11 23.47 Chen et al. RM27792 GAAGAAGAGAGACTAGGGAGAAGACGCTTGTACCAGCAATTCTCTGTCC 12 7.1 He et al. RM28502 CGAGCAGATCTGATGTCGTCTTCC CTTTGCTTTGCATGCCTCACG 12 23.41 Terao et al.
10.3864/j.issn.0578-1752.2017.04.017
2016-08-01;接受日期:2016-09-12
国家自然科学基金(地区科学基金项目)(31460412)、甘肃农业大学盛彤笙科技创新基金(GSAU-STS-1432)、国家科技支撑计划(2012BAD06B00)
联系方式:姜红,E-mail:1473240451@qq.com。通信作者毕阳,E-mail:biyang@gsau.edu.cn。通信作者王毅,E-mail:wangyi@gsau.edu.cn

