Antioxidant and Antiproliferative Activities of Portulaca oleracea L. Seed Oil
GUO Gai, YUE Li, FAN Shaoli, JING Siqun,⋆, YAN Liangjun
(1. College of Life Sciences and Technology, Xinjiang University, Ürümqi 830046, China; 2. Department of Pharmaceutical Sciences, UNT System College of Pharmacy, University of North Texas Health Science Center at Fort Worth, Fort Worth 76107, USA)
Antioxidant and Antiproliferative Activities of Portulaca oleracea L. Seed Oil
GUO Gai1, YUE Li1, FAN Shaoli1, JING Siqun1,⋆, YAN Liangjun2
(1. College of Life Sciences and Technology, Xinjiang University, Ürümqi 830046, China; 2. Department of Pharmaceutical Sciences, UNT System College of Pharmacy, University of North Texas Health Science Center at Fort Worth, Fort Worth 76107, USA)
The purpose of this study was to assess the antioxidant and antitumor activities of Portulaca oleracea L. seed oil (PSO) in vitro and its application to delay rancidity of horse oil. We determined the reducing power of PSO and its scavenging effects on hydroxyl radicals (・OH) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals and assessed its stability during horse oil storage with its addition. The results showed that PSO had remarkable, dose-dependent antioxidant activity, and could effectively prevent lipid oxidation in horse oil. We treated cervical cancer HeLa cells, human esophageal carcinoma Eca-109 cells and human breast adenocarcinoma MCF-7 cells with PSO using non-neoplastic monkey kidney Vero cells as control. The results indicated that PSO significantly inhibited tumor cell proliferation in a time-and dose-dependent manner. Our findings suggest that PSO can be a substitute for synthetic antioxidants in food preservation and may be potentially useful as a food or cosmetic ingredient.
Portulaca oleracea L. seed oil (PSO); antioxidant; anti-proliferation; preservation
Purslane (Portulaca oleracea L.) is widely grown in various places. It is used both as an edible plant and a Chinese traditional medicine[1]. Both the leaves and seeds of purslane can be consumed orally and applied typically to relieve skin irritations[2]. Manystudies have revealed certain pharmacological actions of this plant, such as antibacterial[3-4], hypoglycemic[5], antihypoxia[6], antioxidant effects[7], antitumor activity[8], and neuroprotective effects[9]. As a medicinal and edible wild plant, purslane is generally known as a “longevity food”due to its reputation as a “natural antibiotic”. Purslane has many active ingredients, such as flavonoids[10], alkaloids[11], ω-3 fatty acids, noradrenaline, coumarins, polysaccharides, and other active ingredients[6]. In particular, purslane seeds are more effective in antioxidation thanother herbs[12]. In previous studies, we have extracted purslane seed oil (PSO) with a 17.68% yield using an ultrasound-assisted enzyme hydrolysis in combination with a Soxhlet extraction method. Moreover, we have analyzed distribution of fatty acids and the oil content utilizing a gas chromatography-mass spectrometer (GC-MS)[13], and the results showed that the main fatty acid of PSO were alpha-linolenic acid, linoleic acid and oleic acid with relative content of 40.26%, 29.43% and 15.61%, respectively. Saturated fatty acids represent 13.95% of PSO, and monounsaturated fatty acids account for 16.29% and polyunsaturated fatty acids (PUFAs) occupy 69.69%, respectively. Moreover, the linolenic acid content (40.26%) is much higher in PSO than in camellia seed (0.27%)[14], grape seed (7.3%)[15], and olive (6.09%) oils[14]but is slightly lower than in flaxseed oil (41.22%)[12]. The content of linoleic acid (29.43%) is higher than many other vegetable oils, for instance flaxseed oil (15.44%), camellia seed oil (7.26%), grape seed oil (11.40%), and olive oils (0.56%). It is well known that linolenic acid and linoleic acid belong to ω-3 fatty acid and ω-6 fatty acid, respectively, and they are the essential fatty acids that play important roles in human growth and in preventing disease[16]. Additionally, oils rich in ω-3 fatty acids are good for health[17]. PSO is expected to show superior antioxidant activity and antitumor effects because it has higher ω-3 fatty acid. Thus, it is a good candidate as both a health food and a cosmetic ingredient.
Currently, studies of seed oil are focused on optimization of the extraction process and its in vitro effects of antioxidation[18-19]. Moreover, to the best of our knowledge, there have been no published studies on the antioxidant activity or antiproliferative effect of PSO on cancer cell lines. Therefore, in this study, we tested the antioxidant activity of PSO on free radical scavenging and its inhibitory effects on tumor cell proliferation. Additionally, we also tested PSO as a preserving agent in horse oil storage to explore whether it can be used as a food-preserving agent.
1 Materials and Methods
1.1 Material and reagents
Fresh, mature purslane seeds were provided by Xinjiang Yuansen Agriculture Science and Technology Development Co. Ltd.. PSO containing 40.26% alpha-linolenic acid and 29.43% linoleic acid was obtained by an ultrasound-assisted enzyme hydrolysis in combination with a Soxhlet extraction method. The optimal preparation conditions of PSO were as follows:13 for the hydrolysis process, 2% complex enzyme was used (the ratio of neutral protease to cellulase was 1:1), the liquidsolid ratio was 5:1, pH 5.0, and the hydrolysis time was 2 h; for the sonication process, 40 W ultrasonic power was used, an ultrasonic bath temperature was set to 55 ℃, and the sonication time was 15 min. Petroleum ether was used as a solvent for the Soxhlet extraction.
Fresh, untreated horse fat was obtained from the local market in Yili, Xinjiang, China. Liquid horse oil was obtained from the horse fat using a steam melting method followed by a refining process consistent with our previous studies[20]. Horse fat provides the raw material of which 31.08% of the lipids are unsaturated fatty acids, notably, palmitoleic acid (3.71%) and oleic acid (27.37%).
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT), dimethyl sulfoxide dimethyl sulfoxide (DMSO), 1,1-diphenyl-2-picrylhydrazyl (DPPH), tertiary butylhydroquinone (TBHQ) that used in vitro studies was purchased from Sigma Chemical Co..
HeLa cells, Eca109 cells, MCF-7 cells and Vero cells were came from Xinjiang Biological Resources Gene Engineering Key Laboratory Xinjiang University.
1.2 Instruments and equipment
Benchmark microplate reader Bio-Rad(USA); XD-101 inverted biological microscope Nanjing Jiangnan Yongxin Optical Co. Ltd.; 3423 CO2incubator THERMO Series(USA); WAC-47 sterilizer Beijing Qinye Yongye Technology Co. Ltd.; clean bench Suzhou Antai Air Technology Co. Ltd.; Neofuge13R-high speed refrigerated centrifuge Wuhan Aisipei Scientific Instrument Co. Ltd..
1.3 Methods
1.3.1 In vitro antioxidant potential of PSO
TBHQ is a frequently used synthetic antioxidant. Therefore, it was used as positive substance to evaluate the antioxidant activity of PSO in this study. The antioxidant activity of PSO was tested by various ways, including DPPHfree radical scavenging, hydroxyl radical (・OH) scavenging, and reducing power tests. PSO was dissolved in an absolute ethanol to formulate the desired final concentrations. Same concentrations of TBHQ and PSO were set in each test. All the experiments were repeated three times, and the mean values were calculated.
1.3.1.1 ・OH scavenging activity assay
The phenanthroline-Fe2+oxidation previously reported by Xiao Jie et al.[21]was used as to measure ・OH scavenging activity. More specifically, sodium phosphate buffer (4 mL, pH 7.4) were added to a test tube and blended with 1.5 mL of 5 mmol/L phenanthroline solution. Then, 1 mL of FeSO4solution (7.5 mmol/L) and 1 mL of a series of concentrations (0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, and 7.0 mg/mL) of PSO sample solutions were added to the solution in sequence. At last, double distilled water (1.5 mL) and 1.0 mL H2O2(0.1%) were added in mixture solution. At 536 nm, final solutions was incubated at 37 ℃ for 60 min, then its absorbance was measured with a ultraviolet (UV)-visible spectrophotometer. TBHQ and deionized water were used as positive control and blank control, respectively. Antioxidant effects were expressed using half maximal inhibitory concentration (IC50) value, which was the concentration of PSO that caused 50% inhibition of ・OH formation.
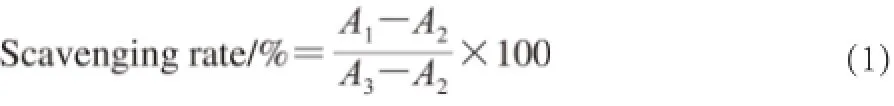
Where A1is the measured absorbance after adding the PSO sample and the H2O2; A2is the measured absorbance after adding H2O2without PSO sample; A3is the measured absorbance without POS sample and without H2O2.
1.3.1.2 DPPH free radical scavenging activity assay
The scavenging activity of DPPH free radical was tested in the light of the method described by Ting et al.[22], with slight modifications. Briefly, 2 mL of 2×10-4mol/mL DPPH was added to 2 mL series of concentrations (3.0, 6.0, 10.0, 15.0, 20.0, 25.0 and 30.0 mg/mL) of PSO in sequence. At room temperature, the mixture solution was placed for 30 min in the shade, and then its absorbance was measured at 517 nm with spectrophotometer. Distilled water was used as a control taking the place of the sample solutions. TBHQ (0.1, 0.3, 0.5, 1.0, 2.0 and 3.0 mg/L) was used as a positive control. An equal volume of distilled water and absolute ethanol was blended; mixture solution was used as a blank control. Antioxidant effects were expressed using IC50value, which was the concentration of PSO that scavenge 50% of DPPH free radicals.

Where Aiis the absorbance of the PSO sample solution and DPPH solution mixture; Ajis the sample solution absorbance; Acis the absorbance of the DPPH solution and different extraction reagent mixtures.
1.3.1.3 Determination of reducing power
The reducing power of PSO was examined using the Prussian blue method[23]. Briefly, 1 mL of each sample solution (0.01, 0.05, 0.10, 0.30 and 0.50 mg/mL TBHQ; 1.20, 2.40, 3.60, 4.80, 6.00, and 7.20 mg/mL PSO) was added to a solution containing 2.5 mL of 1% K3Fe(CN)6and phosphate buffer (pH 6.6). The buffered solutions were placed at 50 ℃, 20 min, then added to 2.5 mL of 10% trichloroacetic acid (TCA). The next, 2.5 mL of distilled water and 2.5 mL of 0.1% FeCl3were blended into 2.5 mL of the previous mixtures. After 10 min, at 700 nm, the absorbance value (A) was measured with a UV-visible spectrophotometer. The reference absorbance value (A0) was obtained from a blank control. Deionized water was used as the blank control, and TBHQ was used as the positive control. The total antioxidant capacity was expressed as WA.

1.3.2 Antiproliferative effect of PSO in vitro
The antitumor cell growth of PSO in vitro was tested by calculating MTT dyestuff absorbance values in living cells (HeLa, Eca-109, MCF-7) with Vero cell (normal) as controls. Briefly, cells were seeded in the 96-well, flat-bottomed plates having cell suspension (100 μL) in per well with known concentration and set at 37 ℃, 5% CO2incubator. Usually, 5×104cells were seeded per well. PSO was dissolved in DMSO and then leached using filter membranes (0.45 and 0.22 μm) to obtain filtrate. PSO (200 μL) at concentrations of 12.5, 50.0, 200.0, 800.0, 1 600.0 and 3 200.0 µg/mL were added to their respective wells. Then 96-well plates were placed in 37 ℃, 5% CO2incubator to cultivate 24, 48, or 72 h. In total, MTT solution (5 mg/mL, 20 μL) was joined in for dyeing, and then continued to train 4 h (37 ℃). After the incubation, DMSO (200 μL) was added to dissolve formazan crystals. Absorbance at 570 nm was measured by applying enzyme-linked immunosorbent assay (ELISA) reader. The IC50value was the concentration of PSO that inhibited the growth of cells by 50%[8].
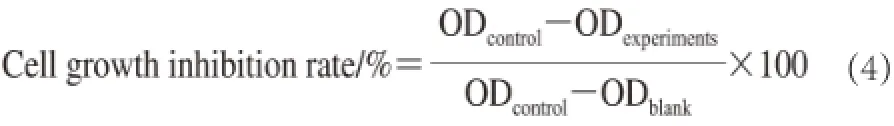
1.3.3 Effect of PSO on the oxidative stability of horse oil during storage
The peroxide value (POV) is an indicator of lipid oxidation. The POV was determined by the Schall Oven method[24]. Briefly, an oil sample was incubated in a digital electric heating blast oven at (63 ± 1) ℃ constant temperature, The lower the POV, the stronger the oxidative stability of the sample. The POV of the sample was computed by equation (5):
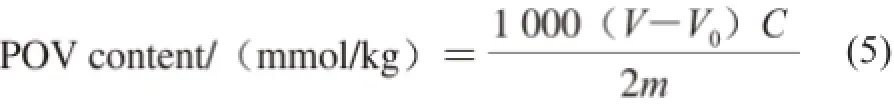
Where V is the volume of Na2S2O3for the measurement/mL; V0is the volume of Na2S2O3for the blank experiment/mL; C is the concentration of Na2S2O3solution /(mol/L); m is the mass of the oil sample/g.
To investigate the effect of PSO on horse oil storage stability, we added 0.5% (m/m) PSO to 30 g of horse oil. After thoroughly mixing the solution, the subsequent operations were performed according to the Schall Oven method. A blank test without added PSO and TBHQ was necessary. The TBHQ-added group was used as a positive control.
To investigate the effect of the dose of PSO on horse oil storage stability, we added PSO with different proportions of 0.05%, 0.25% and 0.5% to horse oil. After thoroughly mixing the solution, the subsequent operations were performed according to the Schall Oven method. The group without PSO added was regarded as a control.
1.4 Statistical analysis
All experimental results were showed using±s. The data analyses were performed using analysis of variance (ANOVA) software. All the graphs were plotted using Graphpad Prism 5.0. P < 0.05 was deemed significantly different.
2 Results and Discussion
2.1 Evaluation of PSO antioxidant activity in vitro
2.1.1 ・OH scavenging activity of PSO
Among the tested free radicals, ・OH are the most active and toxic. Thus, ・OH scavenging capacity can be used as an indicator of antioxidant activity. As shown in Fig. 1, ・OH scavenging capacity of PSO was enhanced at higher concentrations in a nearly linear relationship until a concentration of 4 mg/mL. The IC50values of PSO and TBHQ were (1.388 ± 0.203) and (2.193 ± 0.101) mg/ mL, respectively. Moreover, at the same concentrations, the ranking of ・OH scavenging ability of PSO, TBHQ, almond oil, and grape seed oil was PSO > TBHQ > almond oil (IC502.53 mg/mL) > grape seed oil (IC506.66 mg/mL)[25]. Therefore, ・OH scavenging activity of PSO was the strongest one among the tested samples. In the literature, grape seed oil is reported to contain linoleic (65.0%), linolenic (1.5%), oleic (17.0%), palmitic (8.0%), stearic (4.4%) and arachidonic (0.6%) acids[26]; while oleic (63%-78%) and linoleic (12%-27%) acids are the major fatty acids in almond oil[27]. It is clear that the predominant fatty acid in PSO is linolenic ω-3 fatty acid. Therefore, we reasoned that the stronger antioxidant activity of PSO may be attributed to its higher content of linolenic acid. Indeed, previous research indicated that an increased amount of ω-3 PUFAs might enhance antioxidant activity[28-29]. However, whether the observed effect is due to the ω-3 or to the other fatty acids in PSO needs to be further evaluated.
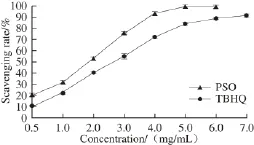
Fig.1 ・OH scavenging ability of PSO
2.1.2 DPPH free radical scavenging capacity of PSO
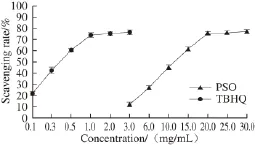
Fig.2 DPPH free radical scavenging ability of PSO
The DPPH free radical scavenging ability of PSO was enhanced when the oil concentration was increased (Fig. 2). A strong linear relationship is observed within the range of PSO concentrations from 3-20 mg/mL. Moreover, it is worth mentioning that PSO’s antioxidant activity could only be detected at concentrations at or above 3 mg/mL. The IC50values of DPPH free radicals scavenging of PSO and TBHQ were (11.160 ± 0.071) mg/mL and (0.378 ± 0.079) mg/mL, respectively, and the result indicated that the DPPH free radical scavenging capacity of PSO was weakerthan that of TBHQ. Additionally, the DPPH free radical scavenging capacity of PSO was higher than that of walnut oil (IC50147.0 mg/mL)[30]and weaker than that of flaxseed oil (IC503.31 mg/mL)[31]. It has been reported that the major fatty acids in walnut oil are linoleic (60.42%-65.77%), oleic (13.21%-19.94%) and linolenic (7.61%-13.00%) acids[30]. while the main fatty acid components of flaxseed oil are alpha-linolenic (41.22%), linoleic (15.44%), and oleic (28.2%) acids. Obviously, the stronger DPPH free radical scavenging ability of PSO may be due to its higher content of linolenic ω-3 fatty acid.
2.1.3 Reducing power of PSO
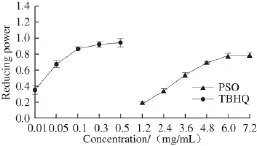
Fig.3 Reducing power of PSO
Many studies have shown that the antioxidant activity of substance is closely related to its reducing power: the greater the reducing power is, the better the antioxidant effects. Thus, it can well reflect the materials antioxidant activity[32]. The results in Fig. 3 showed that the reducing power was increased as the concentration of PSO was increased, and there was a strongly and positively linear relationship. However, the reducing power of PSO was weaker than that of TBHQ. In summary, we observed a strong antioxidant activity of PSO, suggesting that PSO is a likely candidate as a food and cosmetic ingredient.
2.2 In vitro inhibitory effect of PSO on tumor cell proliferation
2.2.1 MTT assay for proliferation inhibition of tumor cells
The proliferation inhibition of PSO on MCF-7, Eca-109 and HeLa cells was assessed using MTT assay, and the IC50values were calculated through the dose-response curves (Fig. 4). PSO induced a significant dose- and time-dependent reduction in the growth rates of HeLa, MCF-7 and Eca-109 cells. The IC50values of PSO against MCF-7, HeLa, and E c a-1 0 9 c e l l s w e r e (1 5 6 6.1 3 ± 0.0 2), (1 8 4 4.4 7 ± 0.0 2) μ g/m L and (5 366.29 ± 0.04) μg/mL, respectively. PSO showed a stronger inhibition on the growth rate of MCF-7 cells.
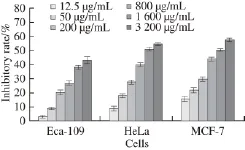
Fig.4 Inhibitory curves of PSO against the proliferation of MCF-7, HeLa and Eca-109 cells after treatment for 48 h
2.2.2 Proliferation inhibition of HeLa cells

Fig.5 Proliferation inhibition of HeLa cells as a function of different concentrations of PSO after treatment for different periods
It can be seen from Fig. 5 that antiproliferation effects of HeLa cells of PSO were presented in a dose- and timedependent manner. IC50values of (11 634.10 ± 0.03), (1 844.96 ± 0.02) and (1 179.35 ± 0.02) μg/mL, respectively after 24, 48 and 72 h. The same double dependence was found by Zhang Lumin et al.[33]related essential oil from Artemisia lavandulaefolia. Thus, PSO may obviously inhibit the growth of HeLa cells.
2.2.3 Effect on proliferation of Eca-109 cells
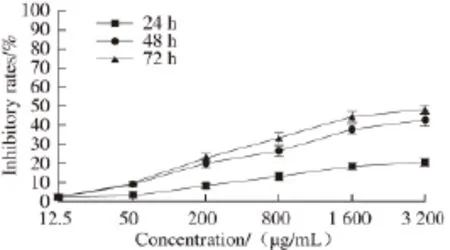
Fig.6 Proliferation inhibition of Eca-109 cells as a function of different concentrations of PSO after treatment for different periods
From Fig. 6, antiproliferation effects of Eca-109 cells of PSO were also presented in a dose- and time-dependent manner. IC50values of (65 540.09 ± 0.04), (5 366.76 ± 0.04) and (3 048.45 ± 0.04) μg/mL, respectively after 24, 48 and 72 h. Therefore, PSO may obviously inhibit the growth of Eca-109 cells.
2.2.4 Effect on proliferation of MCF-7 cells

Fig.7 Proliferation inhibition of MCF-7 cells proliferation as a function of PSO concentration after treatment for different periods
It can be seen from Fig. 7, antiproliferation effects of PSO on MCF-7 cells were presented in a dose- and timedependent manner. IC50values of (3 179.23 ± 0.02), (1 566.11 ± 0.02) and (1 064.24 ± 0.01) μg/mL, respectively after 24, 48 and 72 h. PSO obviously inhibit the growth of MCF-7 cells.
2.2.5 Growth inhibition of Vero cells
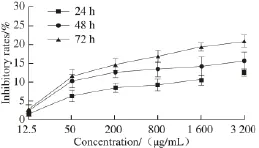
Fig.8 Proliferation inhibition of Vero cells as a function of different concentrations of PSO after treatment for different periods
From Fig. 8, the inhibition rate of PSO on Vero cells did not exhibited an obvious trend with the increase of time and concentration. When Vero cells were incubated 72 h with 1 600 µg/mL of PSO, the inhibitory rate was only (19.25 ± 1.22)%. The inhibitory rate against MCF-7 cells was up to (54.51 ± 1.17)% (P < 0.05), while against HeLa cells, the rate was up to (54.42 ± 1.75)% (P < 0.05). For Eca-109 cells, the rate was up to (44.33 ± 2.34)% (P < 0.05) under the same concentration and for the same time. Thus, our results showed that PSO had little side-effects on the normal Vero cells. In general, the growth inhibition of Vero cells by PSO was much weaker than those of MCF-7, HeLa and Eca-109 cells.
In summary, the above antitumor studies in vitro obviously indicated that the inhibitory effect of PSO on MCF-7 cells was stronger than that those on HeLa and Eca-109 cells. This is the first report that demonstrates inhibition of the breast cancer cell growth in vitro by PSO. This report suggests a potential therapeutic role of PSO in the therapy of breast cancer. Therefore, which one as the major component for its antioxidant and anti-proliferative activity are warranted to to be conducted in the future, and elucidate the mechanism underlying PSO inhibition of MCF-7 cell proliferation. Some studies have been reported about mechanism underlying oil inhibition of tumor cells growth and proliferation. Ye Ke et al.[34]showed that garlic oil could inhibit the growth and induce apoptosis of B16 cells, which might be related to up-regulation of p16 while down-regulation of C-myc expression or also inhibited vascular endothelial growth factor (VEGF) expression in B16 cell line, preventing cells from growing and metastasis; Zhu Fuliang et al.[35]confirmed that Curcumae rhizoma L. oil could inhibit the proliferation and induce apoptosis of saos-2 cells, and its antitumor effect was associated with suppressing the expression of insulinlike growth factor-1 receptor (IGF-1R), protein kinase B (Akt) and B-cell lymphoma/leukemia-2 (Bcl-2); Song Yanli et al.[36]verified that Brucea oil inhibited the proliferation of H22 cells in vitro and in vivo, and the antitumor effect might be related to the regulation of spleen cells in tumor-bearing mice.
2.3 Application of PSO to horse oil storage
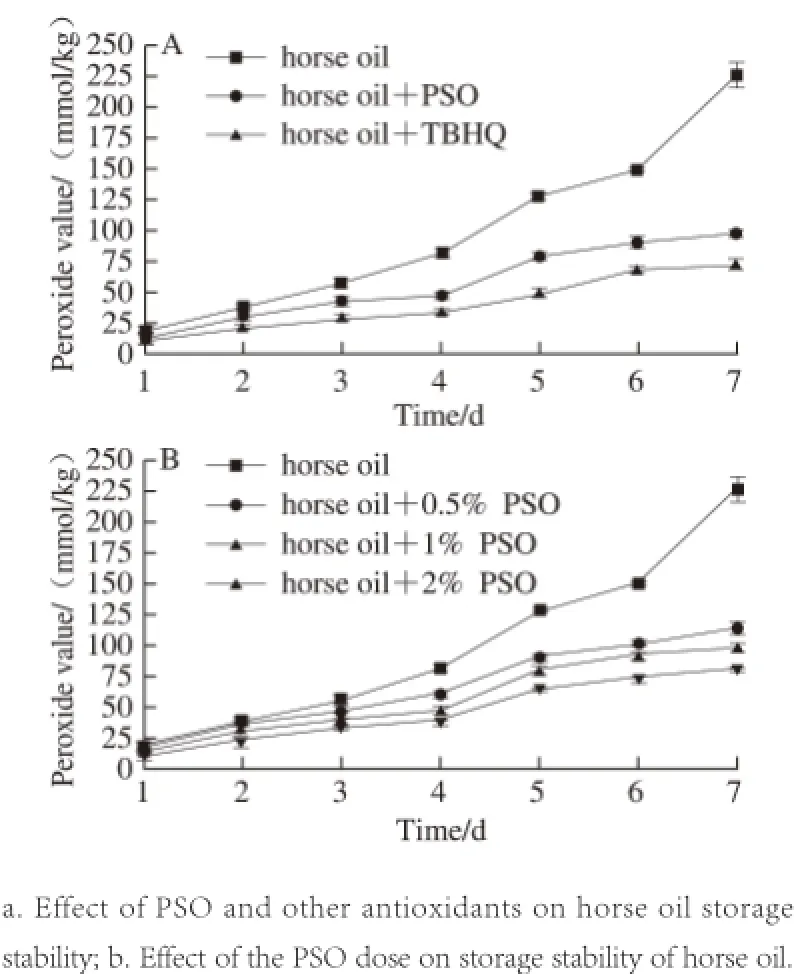
Fig.9 Effect of PSO on the oxidative stability of horse oil during storage
Due to the secondary action of synthetic antioxidants to health, for instance TBHQ and butylated hydroxyanisole (BHA), there has been a significant increase in needs of separating naturally biologically active molecules used in the food and pharmaceutical industries[37]. In this test, we contrasted the antioxidant activity in vitro of PSO to the synthetic antioxidant TBHQ in horse oil storage.
As shown in Fig. 9A, the addition of both PSO and TBHQ showed improved protection against auto-oxidation of horse oil. The POV were much lower than those of horse oil alone, but PSO was weaker than TBHQ in inhibiting lipid peroxidation. This implies that we can further improve horse oil stabilization if PSO and TBHQ are combined, producing a synergistic effect[38]while minimizing the amount of synthetic antioxidant.
Additionally, we investigated the dose-dependent effect of PSO on storage stability of horse oil. We found that the lipid antioxidant capacity of PSO increased with the dose. This is significantly different from the control group, which showed a significant increasing trend the POV in the absence of PSO (Fig. 9B). This result suggested that PSO was a natural preservative used for the food and toiletry industries.
3 Conclusion
In recent years, natural products as an antioxidant and anti-cancer drugs were studied increasingly[39]. These natural antioxidants can promote health, prevent putrescence and maintain nutritional value[40]. The positive correlation of purslane oil antioxidant activity and its concentration was obtained through the research of purslane seed antioxidant activity and antitumor activity within a certain range. PSO was added to the horse oil to delay oxidation speed. We found that of purslane seed caused stronger inhibition on the growth of several tumor cells in dose and time-dependent manner in vitro study, but the specific underlying mechanism need to be further studied.
[1] QUAN Meiping, HAO Xiaoning. Research advances on medicinal value and health products of Portulaca oleracea L.[J]. Storage and Process, 2012, 12(5): 44-47. DOI:10.3969/ j.issn.1009-6221.2012.05.010.
[2] XIONG Min. Portulaca oleracea L. pharmacological effects and clinical application[J]. Nei Mongol Journal of Traditional Chinese Medicine, 2011, 30(4): 46-47. DOI:10.3969/j.issn.1006-0979.2011.04.058.
[3] ZHANG X, JI Y, QU Z, et al. Experimental studies on antibiotic functions of Portulaca oleracea L. in vitro[J]. Chinese Journal of Microecology, 2002, 5(12): 33-36. DOI:10.3969/j.issn.1005-376X.2002.05.012.
[4] DONG C X, HAYASHI K, LEE J B, et al. Characterization of structures and antiviral effects of polysaccharides from Portulaca oleracea L.[J]. Chemical and Pharmaceutical Bulletin, 2010, 58(4):507-510. DOI:10.1248/cpb.58.507.
[5] GU J, ZHENG Z, YUAN J, et al. Comparison on hypoglycemic and antioxidant activities of the fresh and dried Portulaca oleracea L. in insulin-resistant HepG2 cells and streptozotocin-induced C57BL/6J diabetic mice[J]. Journal of Ethnopharmacology, 2014, 161: 214-223. DOI:10.1016/j.jep.2014.12.002.
[6] WANG W Y, DONG L W, JIA L, et al. Ethanol extract of Portulaca oleracea L. protects against hypoxia-induced neuro damage through modulating endogenous erythropoietin expression[J]. Journal of Nutritional Biochemistry, 2012, 23(4): 385-391. DOI:10.1016/ j.jnutbio.2010.12.015.
[7] SILVA R, CARVALHO I S. In vitro antioxidant activity phenolic compounds and protective effect against DNA damage provided by leaves stems and flowers of Portulaca oleracea (Purslane)[J]. Natural Product Communications, 2014, 9(1): 45-50. DOI:10.1016/ j.carbpol.2013.04.023.
[8] ZHAO R, GAO X, CAI Y, et al. Antitumor activity of Portulaca oleracea L. polysaccharides against cervical carcinoma in vitro and in vivo[J]. Carbohydrate Polymers, 2013, 96(2): 376-383. DOI:10.1016/ j.carbpol.2013.04.023.
[9] ABDEL MONEIM A E. The neuroprotective effects of purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat[J]. CNS & Neurological Disorders Drug Targets, 2013, 12(6): 830-841. DOI:10.2174/18715273113129990081.
[10] ZHU H, WANG Y, LIU Y, et al. Analysis of fl avonoids in Portulaca oleracea L. by UV-vis spectrophotometry with comparative study on different extraction technologies[J]. Food Analytical Methods, 2010, 3(2): 90-97. DOI:10.1007/s12161-009-9091-2.
[11] XIANG L, XING D, WANG W, et al. Alkaloids from Portulaca oleracea L.[J]. Phytochemistry, 2005, 66(21): 2595-2601.
[12] El-SAYED M. Effects of Portulaca oleracea L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy[J]. Journal of Ethnopharmacology, 137(1): 643-651. DOI:10.1016/j.jep.2011.06.020.
[13] LIU L, XIE C, YUE L, et al. Preparation of Portulaca oleracea L. seed oil by ultrasound-assisted enzyme hydrolysis combined with Soxhlet extraction method and the analysis of its fatty acids[J]. Food and Fermentation Industries, 2014, 7(18): 218-222.
[14] AI F F, BIN J, ZHONG D, et al. Comparison and analysis of fatty acids between oil-tea camellia seed oil and other vegetable oils[J]. China Oils Fats, 2013, 38(3): 77-80. DOI:10.3969/ j.issn.1003-7969.2013.03.020.
[15] ZHANG L F, MOU D H, DU Y S. Technology of extracting grape seed oil via supercritical fluid[J]. Journal of the Chinese Cereals and Oils Association, 2007, 22(2): 60-62; 65. DOI:10.3321/ j.issn:1003-0174.2007.02.014.
[16] DUNBAR B S, BOSIRE R V, DECKELBAUM R J. Omega 3 and omega 6 fatty acids in human and animal health: an African perspective[J]. Molecular and Cellular Biochemistry, 2014, 398(1/2):69-77. DOI:10.1016/j.mce.2014.10.009.
[17] ASIF M. Health effects of omega-3, 6, 9 fatty acids: Perilla frutescens is a good example of plant oils[J]. Oriental Pharmacy and Experimental Medicine, 2011, 11(1): 51-59. DOI:10.1007/s13596-011-0002-x.
[18] MA Chengjin, WU Zhuqing, HUANG Wei, et al. Optimization of supercritical carbon dioxide extraction process for tea seed oil by using response surface methodology[J]. Food Science, 2011, 32 (20): 108-113.
[19] LI Jiaxing, YU Jiao, HUANG Cheng, et al. In vitro antioxidant activity of kiwifruit seed oil[J]. Food Science, 2012, 33(23): 51-54.
[20] JING S, AIBAILA R, LI Y. Study on process optimizing of ref i ning process and antibacterial effect of hurse oil[J]. Science and Technology of Food Industry, 2012, 33(8): 291-294; 298.
[21] XIAO J, SUN J, YAO L, et al. Physicochemical characteristics of ultrasonic extracted polysaccharides from cordyceps cephalosporium mycelia[J]. International Journal of Biological Macromolecules, 2012,51(1/2): 64-69. DOI:10.1016/j.ijbiomac.2012.04.029.
[22] TING H, HSU Y, TSAI C, et al. The in vitro and in vivo antioxidant properties of seabuckthorn (Hippophae rhamnoides L.) seed oil[J]. Food Chemistry, 2011, 125(2): 652-659. DOI:10.1016/ j.foodchem.2010.09.057.
[23] MIAO L, WU H, QIU N et al. Assessment on antioxidant activity of pomegranate seed oil in vivo[J]. China Oils Fats, 2010, 35(1): 37-40.
[24] KE H, CHUN C, MOUMING Z, et al. Comparative of Rancimat method and Schaal oven method for the determination of oxidation stability of peanut oil and peanut butter[J]. Food and Fermentation Industries, 2011, 37(10): 145-148.
[25] KONG H, ZHANG J. Study on the scavenging free radical capacity of almond oil and grape seed oil[J]. Gansu Science and Technology, 2008, 24(6): 21; 57-58. DOI:10.3969/j.issn.1000-0952.2008.06.020.
[26] KIKALISHVILI B, ZURABASHVILI D, TURABELIDZE D, et al. Fatty acids of grape seed oil and its biological activity as 1.0% and 2.5% food-additive[J]. Georgian Medical News, 2012, 207: 47-50.
[27] KODAD O, SOCIAS I, COMPANY R. Variability of oil content and of major fatty acid composition in almond (Prunus amygdalus Batsch) and its relationship with kernel quality[J]. Journal of Agricultural and Food Chemistry, 2008, 56(11): 4096-4101. DOI:10.1021/jf8001679.
[28] SIMOPOULOS A P. Omega-3 fatty acids and antioxidants in edible wild plants[J]. Biological Research, 2004, 37(2): 263-277. DOI:10.4067/S0716-97602004000200013.
[29] UDDIN M K, JURAIMI A S, HOSSAIN M S, et al. Purslane weed (Portulaca oleracea): a prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes[J]. Scientif i c World Journal, 2014(2):1661-1667. DOI:10.1155/2014/951019.
[30] BOUABDALLAH I, BOUALI I, MARTINEZ-FORCE E, et al. Composition of fatty acids, triacylglycerols and polar compounds of different walnut varieties (Juglans regia L.) from Tunisia[J]. Natural Product Research, 2014, 28(21): 1826-1833. DOI:10.1080/14786419.2 014.950573.
[31] LONG J, FU Y, ZU Y, et al. Ultrasound-assisted extraction of fl axseed oil using immobilized enzymes[J]. Bioresource Technology, 2011, 102(21): 9991-9996. DOI:10.1016/j.biortech.2011.07.104.
[32] ZHANG J, YUAN K, ZHOU W L, et al. Studies on the active components and antioxidant activities of the extracts of Mimosa pudica Linn. from southern China[J]. Pharmacognosy Magazine, 2011, 7(52):35-39. DOI:10.4103/0973-1296.75899.
[33] ZHANG L M, LÜ X W, SHAO L X, et al. Essential oil from Artemisia lavandulaefolia induces apoptosis and necrosis of HeLa cells[J]. Zhong Yao Cai, 2013, 36(12): 1988-1992.
[34] YE Ke, XU Aie. Effects of garlic oil on growth, apoptosis and expression of VEGF in melanoma B16 cells[J]. Chinese Journal of Dermatovenerology of Integrated Traditional and Western Medicine, 2015, 14(1): 4-7.
[35] ZHU Fuliang, LIU Jinyang, HUANG Fengxiang. Inhibition of IGF-1R, Akt and Bcl-2 by Curcumae Rhizoma oil on Human osteosarcoma saos-2 cell[J]. Chinese Journal of Experimental Traditional Medical Formulae, 2015, 21(17): 126-128.
[36] SONG Yanli, XUE Rui, WU Qian,et al. Inhibition of proliferation of hepatoma cell line H22 cells by brucea oil in vitro and in vivo[J]. Journal of Practical Hepatology, 2014, 17(2): 180-183.
[37] ITO N, FUKUSHIMA S, HASEGAWA A, et al. Carcinogenicity of butylated hydroxyl anisole in F344 rats[J]. Journal of the National Cancer Institute, 1983, 70(2): 343-352.
[39] VATTEM D, GHAEDIAN R, SHETTY K. Enhancing health benef i ts of berries through phenolic antioxidant enrichment: focus on cranberry[J]. Asia Pacif i c Journal of Clinical Nutrition, 2005, 14(2):120-130.
[40] PILLAI P, RAMASWAMY K. Effect of naturally occuring antimicrobials and chemical preservatives on the growth of Aspercillus parasiticus[J]. Journal of Food Science and Technology, 2012, 49(2):228-233. DOI:10.1007/s13197-011-0275-6.
[41] RAZMKHAH Sara, TAN Chinping, LONG Kamariah, et al. Quality changes and antioxidant properties of microencapsulated kenaf (Hibiscus cannabinus L.) seed oil during accelerated storage[J]. Journal of the American Oil Chemists Society, 2013, 90(12): 1859-1867.
马齿苋籽油的抗氧化和抗肿瘤细胞增殖作用
郭 改1,岳 丽1,范少丽1,敬思群1,⋆,晏良军2
(1.新疆大学生命科学与技术学院,新疆 乌鲁木齐 830046;2.北德克萨斯大学健康科学中心药物科学系,美国 德克萨斯 沃斯堡 76107)
对马齿苋籽油(Portulaca oleracea L. seed oil,PSO)的体外抗氧化和抗增殖活性进行评价,同时对PSO在马油贮存过程中对其酸败的延缓效果进行考察。通过测定PSO的总还原力、·OH及1,1-二苯基-2-三硝基苯肼自由基的清除率来评价其抗氧化效果,并测定不同处理组马油的过氧化值。结果表明:PSO具有较强的抗氧化活性,且随着剂量的增加而增加,并且PSO能有效延缓马油脂质氧化。3-(4,5-二甲基噻唑-2)-2,5-二苯基四氮唑溴盐法检测PSO对宫颈癌细胞HeLa、食管癌细胞Eca-109、乳腺癌细胞MCF-7及非洲绿猴肾细胞Vero体外细胞增殖效果的影响。结果表明:PSO对肿瘤细胞增殖有较强的抑制作用,且抑制作用随着时间和剂量的增加而增强。因此,PSO有望成为市场上人工合成抗氧化剂的取代物用于食品保藏,同时也可作为食品和化妆品工业的主要成分。
马齿苋籽油;抗氧化;抗增殖;保藏
TS221
A
1002-6630(2017)03-0206-08
nces:
2016-06-30
新疆研究生科研创新项目(XJGRI2014037. [2014])
郭改(1989—),女,硕士研究生,研究方向为食品工程。E-mail:1126191238@qq.com
10.7506/spkx1002-6630-201703034
GUO Gai, YUE Li, FAN Shaoli, et al. Antioxidant and antiproliferative activities of Portulaca oleracea L. seed oil [J]. 食品科学, 2017, 38(3): 206-213. (in English with Chinese abstract)
10.7506/spkx1002-6630-201703034. http://www.spkx.net.cn
GUO Gai, YUE Li, FAN Shaoli, et al. Antioxidant and antiproliferative activities of Portulaca oleracea L. seed oil[J]. Food Science, 2017, 38(3): 206-213. (in English with Chinese abstract) DOI:10.7506/spkx1002-6630-201703034. http://www.spkx.net.cn
⋆通信作者:敬思群(1966—),女,教授,博士,研究方向为食品科学与工程。E-mail:jingsiqun@163.com
——走出萧条

