Quire Interaction of Certain Nitramine Type Explosives with Proton
——A DFT Study
Lemi Türker
(Department of Chemistry,Middle East Technical University,Ankara Turkey 06231)
Quire Interaction of Certain Nitramine Type Explosives with Proton
——A DFT Study
Lemi Türker
(Department of Chemistry,Middle East Technical University,Ankara Turkey 06231)
The composites of certain nitramine type explosives,TETRYL,RDX and EDNA,with proton in vacuum have been considered within the constraints of density functional theory at the level of B3LYP/6-31++G(d,p) (restricted and unrestricted).The results indicate that unexpectedly hydrogen molecule production occurs by the interaction of proton and a hydrogen of CH3(TETRYL ) and CH2(RDX and EDNA) groups.As a result,a carbocation is generated on the explosive molecules.Thereafter,TETRYL which potentially has many protonation sites were investigated in more detail in vacuum and aqueous conditions.The data reveals that the composite system (TETRYL+proton) is less stable than TETRYL protonated on nitramine NH or oxygen of the nitro groups.
nitramines;TETRYL;RDX;EDNA;proton;DFT
Introduction
A molecule in an electrical field,has distorted electron distribution and the molecular geometry[1-3].The polarizability is a measure of the ease of this process.The atomic,electronic and the orientation polarizabilities are the contributors to the overall polarizability of a molecule in an electrical field[1].The applied field orients the molecules having a permanent dipole moment and the entire sample acquires net polarization.Note that highly polarizable molecules respond strongly to the application of the field and they become highly polarized,thus the centroid of negative electronic charge is displaced[1,4].As for the molecular level,the applied electric field,E,disturbs the charge distribution of the system to produce a force (F=q·Ewhereqis the charge) that causes displacement of the electron density away from the nuclear framework.The result is a separation of the positive and negative charge and consequently an induced dipole,μindarises[4].Energy of polarizable molecule in an electric fieldEis given by[1-4]
(1)
whereμ0stands for the permanent dipole moment,αis the polarizability andβis the first hyperpolarizability.The dipole moment in a fieldEis[1]
(2)
If the electric field increases sufficiently,polarization results in ionization of the molecule as a limiting case.In the literature there exist various studies about the charged forms of energetic materials[5-10].The effect of static electricity on explosive materials was discussed[11-12].
Electrostatic discharge is a serious problem in general in the chemical industry and especially important related to explosive materials.Several types of discharges are possible with static electricity such as brush discharge,propagating brush discharge,corona discharge etc.[13].Skinner et al.proposed that ignition occurs when a spark raises the temperature of the explosive particles to the point,where thermal runaway occurs[14].Gao et al.,considered that the electric spark initiation is primarily of thermal nature[15].However,physically there should be high polarization of the material prior to spark formation (spark breakdown).When a charged-particle approaches a molecule,the electric field generated by it disturbs the electric field around the neutral molecule.This is a time-dependent dynamic process.As the charged particle gets slower and slower its ionization density (ionization along the particle′s path,the Bragg′s curve) increases[16].However,prior to or just after the spark breakdown (very smallΔtseconds earlier or later) the whole picture can be simplified as the molecule and an charged-particle (composite system) at the equilibrium geometry.Obviously,due to the polarization effects,bond lengths and angles of the molecule adapt themselves to minimize the energy of the composite system.It is known that any form of nuclear radiation (charged particles,neutrons or electromagnetic radiation) directly or indirectly produces ionization and certain chemical changes in its passage through matter[16-17].Positively charged particles,engender rather strong electric field around them to initiate ionization of nearby molecules.
In the present study,the composite systems of certain nitramine type explosives and proton have been considered in vacuum within the constraints of density functional theory (DFT).
1 Method
The MM2 method followed by semi-empirical PM3 SCF-MO method[18-19]at the restricted level was employed for the initial structure optimizations of all the systems leading to energy minima[20].Then,structure optimizations were achieved by using various RHF methods successively and finally optimizing within the framework of DFT,B3LYP[21-25]at the levels of 6-31G(d,p) and 6-31++G(d,p) (restricted closed-shell).For some systems (eg.,the charged forms),unrestricted DFT calculations,UB3LYP/6-31++G(d,p),were performed.
For each set of calculations,vibrational analyses were achieved (using the same basis set employed in the corresponding structure optimizations).The normal mode analysis for each structure yielded no imaginary frequencies for the 3N-6 vibrational degrees of freedom,whereNis the number of atoms in the system.This indicates that the structure of each molecule corresponds to at least a local minimum on the
potential energy surface.All these computations were performed by using the Spartan 06 program software[26].
2 Results and discussion
2.1 Geometries
Figure 1 shows the optimized geometries of proton composites of TETRYL,RDX and EDNA.All these nitramines show a common feature that a hydrogen of CH3or CH2group interacts with proton.The C-H bond elongates so much that it is ruptured.Figure 2 shows the bond lengths or distances.The distances between the hydrogens undergoing interaction are 0.0745,0.0744,0.0745nm in TETRYL,RDX and EDNA,respectively.The typical H-H bond length is 0.0741nm[1].
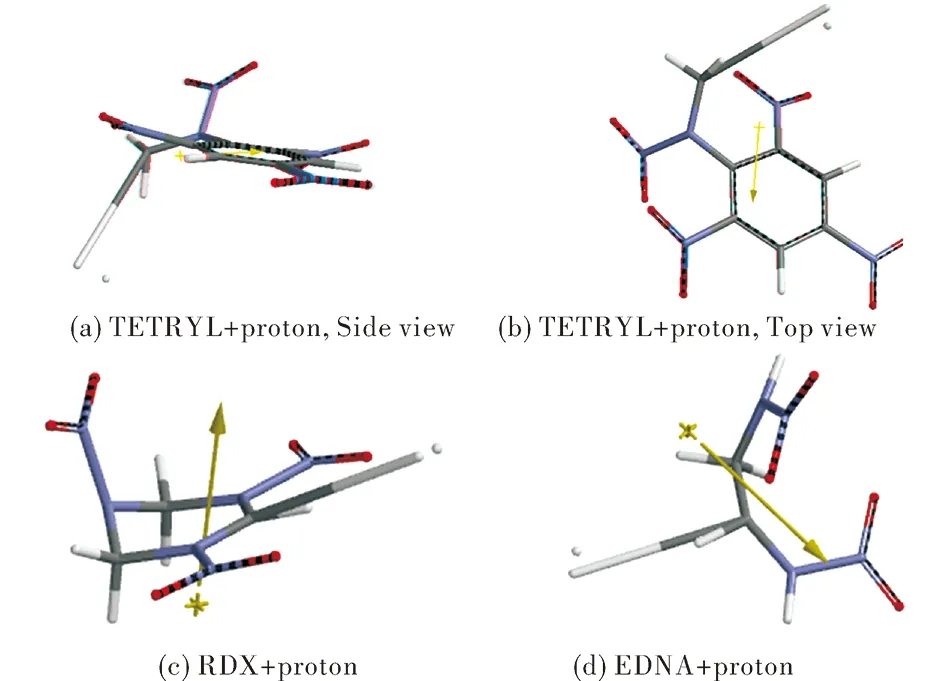
Fig.1 The optimized geometries of the hydrogen composites of the nitramines considered (B3LYP/6-31++G(d,p))
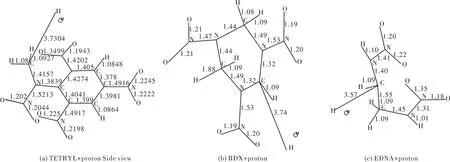
Fig.2 The bond lengths or distances (Å ) in the nitramine systems presently considered (B3LYP/6-31++G(d,p))
2.2 Energies
Table 1 tabulates various energies of the systems presently considered.Since,TETRYL possesses many sites (both the nitramine and aromatic nitro groups) to be protonated it is alone worth studying furthermore.For that purpose protonated forms of it at nitramino-NH,nitramine-NO2,o-NO2,p-NO2and π-complexed TETRYL have been considered at the level of UB3LYP/6-31++G(d,p).Table 2 shows the various energies of these TETRYL containing systems.In the table all the systems are isomeric ( have the same brutto Formula).The data reveal that the protonation of nitramine NO2group is more favorable than the nitramine amino group.The order is as follows: nitramine -NO2>o-NO2> nitramine-NH>p-NO2.The π-complex formation between the aromatic system and the proton is less favorable than the TETRYL+psystem.TETRYL protonated on NH group has very elongated N-NO2bond in the optimized form indicating bond cleavage (like TETRYL monocation).

Table 1 Various energies of the structures considered (B3LYP/6-31++G(d,p))

Table 2 Some energies of TETRYL-proton systems
On the other hand,the interaction energy is defined as
Eint=E(M+p)-E(M)-E(p)
(3)
and an approximate interaction energy as
Eint app=E(M+p)-E(M)
(4)
whereE(M+p),E(M)andE(p)stand forEcorrvalues for the composite explosive system considered (pin the equations stand for proton),the explosive nitramine system and proton,respectively.Since proton does not have any electrons,itsEvalue cannot be calculated.Then,meaningful relative interaction energy is defined as
Erel=E(M+p)-E(M)-E(RDX+p)-E(RDX)
(5)
which does not includeE(p).
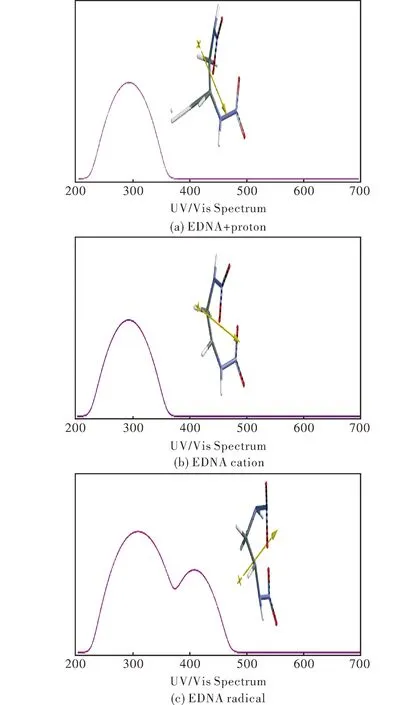
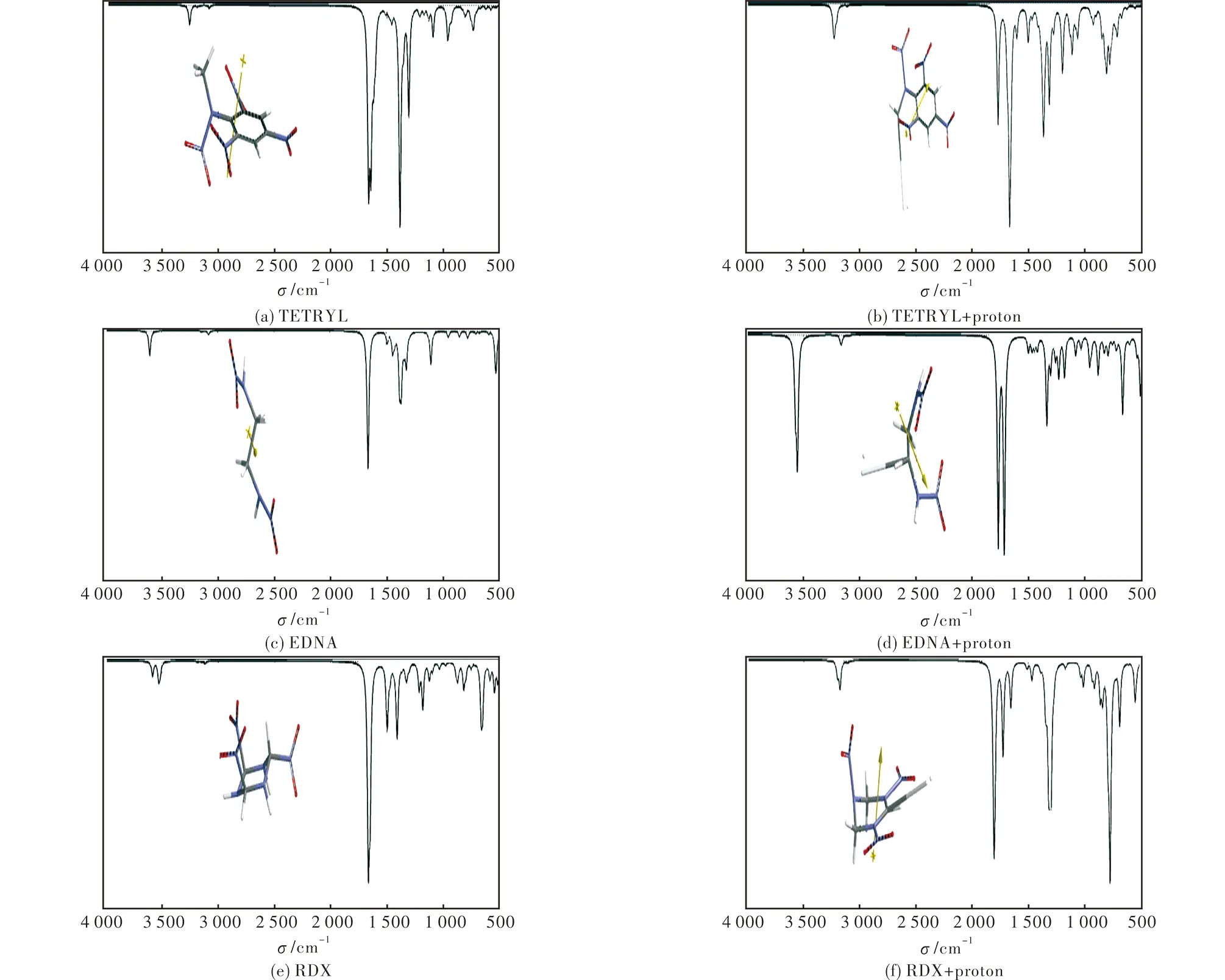
Table 3 displays the relative interaction energies of the considered nitramines with proton.The relative stability order of the composites is RDX Table 3 Relative interaction energy with proton (B3LYP/6-31++G(d,p)) in the composites 2.3 Molecular orbital energies and UV-VIS spectra Table 4 shows the HOMO,LUMO and the frontier molecular orbital (FMO) energy gaps (ΔE=ELUMO-EHOMO) of these composite systems.TheΔεvalues for them follow the order of TETRYL< RDX < EDNA.Figure 3 displays the UV-VIS spectra of the composites.The TETRYL composite has the smallestΔεvalue,thus TETRYL composite absorbs in the visible region in contrast to the others.It might be because of its aromatic ring system it has but the others do not have. Table 4 The HOMO,LUMO and interfrontier molecular orbital energies (Δε) .(B3LYP/6-31++G(d,p)) Cosmic rays contain abundant amounts of protons either fast or slow,thus comprising a large spectrum of velocities and energies[27-28].In the cosmic environment,the hydrogen formed could have single electron or two electron bond.To put some light on this,EDNA +pcomposite and EDNA remnant after deleting the proton from the composite have been subjected to optimization process at the same level of calculation (UB3LYP/6-31++G(d,p)).The remnant would be a cation (mono cation) or a radical depending on two or one electron transferred to proton together with a hydrogen atom (initially in the structure of EDNA) during the formation of H2molecule.The calculated UV-VIS spectrum indicates that if single electron would have been transferred,the remnant radical should exhibit pronounced bathochromic effect but the spectrum resembles the cation spectrum (Figure 4).So,two electrons should have been removed from EDNA molecule and most probably they are involved in the H-H bond.Note that the carbocation generated on the nitramine explosive is partly stabilized by the oxygen atom of NO2( the nitramine NO2in RDX and EDNA and NO2group on the aromatic ring in the case of TETRYL) group through space (see Figures 1 and 2). Fig.3 UV-VIS spectra of the nitramine composites considered (B3LYP/6-31++G(d,p)) Fig.4 UV-VIS spectra of EDNA+p composite and the EDNA remnants (UB3LYP/6-31++G(d,p)) 2.4 IR spectra The calculated (UB3LYP/6-31++G(d,p)) IR spectra of the systems considered are shown in Figure 5.TETRYL spectrum has a huge band at 1664-1660cm-1which is the aromatic skeletal breathing.The nitramino nitro group vibrations are at 1642cm-1.Another band to be mentioned occurs at 1382-1386cm-1standing for thep-nitro group.The other peaks are weak and broad.The TETRYL+pcomposite system has a moderately strong peak at 1775cm-1which is the stretching of nitramino nitro group.As compared to TETYRYL,this band is more energetic in the composite system.The underlying reason is due to the formation of | Ar in which the lone-pair electrons of the amino nitrogen is no longer readily available for the NO2group (see above section for the cation formation).The composite system possesses bands at 1672cm-1(forp-nitro),1660cm-1(for o-nitro groups) overlapped with aromatic C=C stretchings.The bendings of nitramino nitro (1320cm-1)o-andp-nitro (1369cm-1) groups are moderately strong peaks. As for the EDNA,the calculated spectrum is characterized with a strong peak at 1660-1665cm-1for the nitramino nitro stretching and symmetrical and asymmetrical waggings of the CH2groups (1380-1339cm-1) .When EDNA is affected by the proton,a strong N-H peak emerges at 3553cm-1.At 1771cm-1and 1718cm-1nitramine nitro stretching bands appear as very strong peaks.There exist CH2bendings at 1340cm-1. Fig.5 IR spectra of the nitramines and nitramine + proton composite systems (UB3LYP/6-31++G(d,p)) The strongest peak in the RDX spectrum occurs at 1667cm-1and it is the stretching of equatorial nitramine bond.The other band at 1502cm-1and 1419cm-1belong to CH2group and the ring.The RDX+pcomposite system has a weak peak at 3179cm-1and it is the stretching of CH2nearby the elongated bond.The stretching of the equatorial nitramine bonds occur at 1806cm-1,whereas the axial one happens at 1725cm-1.The CH2waggings occur at 1318cm-1. As seen in the figure,the nitramines exposed to proton have highly affected IR spectra.Some weak or moderately strong bands of the parent system have become accentuated in the composites. (1) Obviously,as a proton approaches a nitramine,the attainability of the nitramine + proton composite should occur earlier than the protonation of the nitramines considered at different potential sites. (2) The composites have sufficiently deep local minima on the potential energy surface,namely have high activation barriers to glide over to global minimum where no H2production occurs. (3) Since H2production occurs prior to protonation of some potential sites,the process should be irreversible. (4) The remnant of the nitramine molecule is a corbocation which is stabilized partly by the nearby nitro oxygen atoms through space. [1] Atkins P W.Quanta,a Handbook of Concepts[M].London:Oxford University Press,1974. [2] Mundl F,Show G.Quantum Field Theory[M].New York:Wiley,1984. [3] Hinchliffe A,Munn R W.Molecular Electromagnetism[M].New York:Wiley,1985. [4] Interrante L V,Hampden-Smitt M J.Chemistry of Advanced Materials[M].New York:Wiley-VCH,1998. [5] Türker L.PM3 treatment of lead styphnate and its mono ionic forms[J].Theochem,2004,681: 143-147. [6] Türker L.Quantum chemical treatment of nitroguanidine and its mono ionic forms[J].Theochem,2004,681: 177-181. [7] Türker L.Quantum chemical treatment of EDNA[J].Theochem,2004,684: 95-98. [8] Türker L.Quantum chemical studies on EGDN and its monovalent ions[J].Theochem,2005,717: 9-14. [9] Türker L.An ab initio study on ethylenedinitramine and its monovalent ions[J].Journal of Hazardous Materials,A 2005,118: 67-73. [10] Türker L,Atalar T.Computational studies on nitratoethylnitramine (NENA),its tautomers and charged forms[J].Journal of Hazardous Materials,2009,162: 193-203. [11] Türker L.Contemplation on spark sensitivity of certain nitramine type explosives[J].Journal of Hazardous Materials,2009,169: 454-459. [12] Türker L.Tunneling effect and impact sensitivity of certain explosives[J].Journal of Hazardous Materials,2009,169: 819-823. [13] Crowl D A.Understanding Explosions[R].New York:CCPS Pub.Am.Inst.of Chemical Engineers,2003. [14] Skinner D,Olson D,Block-Bolten A.Electrostatic discharge ignition of energetic materials[J].Propellants,Explosives,Pyrotechnics,1998,23: 34-42. [15] Gao N,Yamawaki H,Tonokura K,Wakabayashi K,Yoshida M,Koshi M.Chemical reactions and other behaviors of high energetic materials under static ultrahigh pressures[J].Mater.Sci.Forum,2004,465 :189-195. [16] Harway B G.Introduction to nuclear physics and chemistry[J].Englewood Cliffs,New Jersey:Prentice-Hall,1969. [17] Friedlander G,Kennedy J W.Miller Miller J.M.,Nuclear and Radiochemistry[M].Tokyo:Wiley-Toppan,1964. [18] Stewart J J P.Optimization of parameters for semi empirical methods I,method[J].Journal of Computational Chemistry,1989,10: 209-220. [19] Stewart J J P.Optimization of parameters for semi empirical methods II[J].Journal of Computational Chemistry,1989,10: 221-264. [20] Leach A R.Molecular Modelling: Principles and Applications[M].London UK:Longman,1997. [21] Kohn W,Sham L J.Self-consistent equations including exchange and correlation effects[J].Physical Review,1965,140 A: 1133-1138. [22] Parr R G,Yang W.Density-Functional Theory of Atoms and Molecules[M].London UK:Oxford University Press,1989. [23] Becke A D.Density-functional exchange-energy approximation with correct asymptotic behavior[J].Physical Review A,1988,38: 3098-3100. [24] Vosko S H,Wilk L,Nusair M.Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis[J].Can J Phys,1980,58:1200-1211. [25] Lee C,Yang W,Parr R G.Development of the colle-salvetti correlation energy formula into a functional of the electron density[J].Physical Review B,1988,37: 785-789. [26] Spartan 06[M].US:Wavefunction Inc,2006. [27] Nikolaev L.Space Chemistry[M].Moscow:Mir Publication,1976. [28] Ohanian H C.Physics[M].New York:Norton,1989. 10.14077/j.issn.1007-7812.2017.01.001 Received date:2016-10-09; Revised date:2016-11-25 Biography:Lemi Türker(1950-),male,Prof.,Dr.,research field:organic chemistry.E-mail: lturker@metu.edu.tr TJ55;TQ560 Document Code:A Article ID:1007-7812(2017)01-0001-06



3 Conclusions

