3-甲基-4-乙基-5-[2(或4)-吡啶基]-1,2,4-三唑银配合物的合成、晶体结构和光学性质
唐辉郭艳红盛俊峰仝玉柱宋菲王作祥*,瞿志荣
(1东南大学化学化工学院,南京211189)
(2杭州师范大学有机硅化学及材料技术教育部重点实验室,杭州311121)
3-甲基-4-乙基-5-[2(或4)-吡啶基]-1,2,4-三唑银配合物的合成、晶体结构和光学性质
唐辉1郭艳红1盛俊峰1仝玉柱1宋菲1王作祥*,1瞿志荣2
(1东南大学化学化工学院,南京211189)
(2杭州师范大学有机硅化学及材料技术教育部重点实验室,杭州311121)
以3-甲基-4-乙基-5-(2-吡啶基)-1,2,4-三唑(L)和3-甲基-4-乙基-5-(4-吡啶基)-1,2,4-三唑(L′)为配体分别合成了2个银配合物[Ag2(μ-L)2L2(NO3)2](1)和{[Ag2(μ-L′)2(H2O)2](NO3)2}n(2),测定了X射线单晶结构,用红外光谱、荧光和热重等进行了表征。2个配合物都属于三斜晶系,空间群为P1。晶体结构分析表明2个配合物都具有畸变的四面体结构[AgN3O]。配合物1是一个双核银配合物,NO3-参与配位;而配合物2是一个配位聚合物,水分子参与配位。
晶体结构;合成;三唑;银配合物
0 Introduction
1,2,4-triazole and its derivatives are important part of heterocyclic compounds and have aroused much interest of people in the past decades due to theirspecificproperties,various structure[1-9]and broad applications as antifungal,antitumor,fungicidal, herbicide agents and so on[10-13].At the same time,1,2,4-triazole has many other important applications in different fields such as pharmaceutical synthesis[14]and materials[15-16].Moreover,much attention has also been paid to substituted 1,2,4-triazole because it displays versatile coordination modes[17-19]in coordination chemistry.By introducing different substituents at 3-(or 4-, 5-)positions[3,20-21],1,2,4-triazole can be functionalized and has more coordination modes.
Although many metal complexes with substituted 1,2,4-triazoles have been synthesized and characterized[4,7,22-24],the silvercomplexes with 3-methyl-4-ethyl-5-(2(or 4)-pyridyl)-1,2,4-triazoles have not been reported so far.As a continuation of our investigation of the asymmetrical substituted 1,2,4-triazole[4,20,25-26], herein we report two Agcomplexes,[Ag2(μ-L)2L2(NO3)2](1)and{[Ag2(μ-L′)2(H2O)2](NO3)2}n(2),where L (or L′)=3-methyl-4-ethyl-5-(2(or 4)-pyridyl)-1,2,4-triazole,and their crystal structures and spectroscopic properties are studied.
1 Experimental
1.1 Materials and measurements
All chemicals were analytical grade and used withoutfurtherpurification.Meltingpointswere determined using an X4digital microscopic melting point apparatus and uncorrected.The C,H,N elemental analyses were performed on a Perkin-Elmer 240analyzer.1H NMR spectra were measured with a Bruker Avance 300spectrometer at ambient temperature in CDCl3using TMS as an internal reference.IR spectra were recorded from 4000to 400cm-1using KBr pellets on a Vector22Bruker spectrophotometer.UV-Vis spectra were recorded on a Hitachi-4100UV-Vis absorption spectrophotometer at room temperature in thin acetonitrile solution.Fluorescence spectra data for 2and L′were measured on a Fluoromax-4spectrofluorometer at room temperature.Thermogravimetric analysis(TGA)measurememts were obtained with a NETZSCH STA49F3thermal analyzer in a nitrogen atmosphere at a heating rate of 10K·min-1.
1.2 Syntheses of L and L′
The ligand,3-methyl-4-ethyl-5-(2-pyridyl)-1,2,4-triazole(L)was synthesized by the following reaction: oxalylchloride(5.7mL,66mmol)was added to a solution of N-ethylacetamide(5.22g,60mmol)and 2, 6-lutidine(14.0mL,120mmol)in CH2Cl2(300mL)at 0℃under nitrogen atmosphere.The mixture was stirred for 40min,and 2-picolinic acid hydrazide (8.22g,60mmol)was added.The reaction mixture was stirred for 5h at room temperature,and then the volatiles were removed under reduced pressure.The residue was dissolved in saturated NaHCO3solution (300mL)and refluxed for 3h at 100℃.After cooling to room temperature,the water phase was extracted three times with CHCl3.The organic phase was dried over MgSO4,and concentrated.Recrystallization from EtOAc gave a colorless crystals(dried at 80℃,3.6g, Yield:31.9%),m.p.90~91℃.Anal.Calcd.for C10H12N4(%):C 63.81,H 6.43,N 29.77.Found(%):C 63.94,H 6.35,N 29.80.1H NMR(300MHz,CDCl3):δ 1.316~1.351(t,3H),2.506(s,3H),4.478~4.530(m,2H), 7.260~7.291(t,1H),7.739~7.777(t,1H),8.218~8.238(d,1H).8.582~8.591(d,1H).UV-Vis(λmax/nm):297.5. IR(KBr,cm-1):3056,2989,2933,1587,1504,1438, 1355,1149,1064,993,798,730,707.
3-Methyl-4-ethyl-5-(4-pyridyl)-1,2,4-triazole(L′) was synthesized according the same process as L. Colorless(dried at 80℃,4.2g,Yield:37.2%),m.p. 128~129℃.Anal.Calcd.for C10H12N4(%):C 63.81,H 6.43,N 29.77.Found(%):C 63.87,H 6.31,N 29.64.1H NMR(300MHz,CDCl3):δ 1.333~1.369(t,3H) 2.547(s,3H),4.013~4.056(m,2H),7.543~7.563(d, 2H),8.762~8.769(d,2H).UV-Vis(λmax/nm):289.0.IR (KBr,cm-1):3039,2983,2935,1600,1525,1483, 1430,1359,1414,835,721,679.
1.3 Synthesis of complex 1
A solution of AgNO3(0.170g,1mmol)was added to a warm solution of L(0.376g,2mmol)in 30mL acetonitrile.A colorless mixture was formed and filtered. The filtrate was left to stand at room temperature for evaporation.Several days later a colorless crystals was collected(0.447g,Yield:81.8%).The crystals are stable in air and a single crystal suitable for X-ray was picked.Anal.Calcd.for C40H48Ag2N18O6(%):C 43.97,H 4.43,N 23.07.Found(%):C 44.12,H 4.57, N 22.68.UV-Vis(λmax/nm):294.5.IR(KBr,cm-1):3056,2979,2939,1631,1539,1519,1489,1452, 1384,1329,1282,1092,995,966,800,731,621.
1.4 Synthesis of complex 2
A solution of AgNO3(0.170g,1mmol)was added to a warm solution of L′(0.376g,2mmol)in 30mL acetonitrile.The mixture turned turbidity slightly.The mixture gradually become clear after 2.5mL of water was added dropwise.A colorless mixture was formed and filtered.The filtrate was left to stand at room temperature for evaporation.Several days later a colorless crystals was collected(0.337g,Yield:89.6%), and a single crystal suitable for X-ray was picked. Anal.Calcd.for C20H28Ag2N10O8(%):C 31.93,H 3.75, N 18.62.Found(%):C 31.47,H 3.92,N 18.24.UVVis(λmax/nm):290.5.IR(KBr,cm-1):3430,2993, 2960,1641,1612,1537,1498,1384,1066, 1003,964,836,740,700,632.
1.5 Crystal structure determination
Well-shaped single crystals of L′,1and 2were selected for X-ray diffraction studies.The data were collected at 296(2)K on a Bruker Smart APEXⅡCCD diffractometer with a detector distance of 5cm and frame exposure time of 10s using graphitemonochromated Mo Kα(λ=0.071073nm)radiation. The structures were solved by direct methods and refined on F2by full-matrix least squares procedures using SHELXTL software[27].All non-hydrogen atoms were anisotropically refined,and all hydrogens on carbon atoms were generated geometrically and allowed to ride on their parent atoms,but not refined.The hydrogen atoms in water were found from the Fourier difference map,but not refined anisotropically.Crystal data and structure refinement for L′,1and 2are listed in Table 1.Selected bond lengths and angles for L′,1and 2are listed in Table 2.The molecular graphics were made by using the DIAMOND 3.1program.
CCDC:1046706,L′;1046707,1;1046708,2.

Table 1Crystal data and structure refinement for L′,1and 2

Continued Table 1

Table 2Selected bond lengths(nm)and angles(°)for L′,1and 2
2 Results and discussion
2.1 Description of crystal structure
X-ray diffraction structure analyses reveals that L′crystallizes in the monoclinic system with space group P21/c,and it comprises one 1,2,4-triazole molecule and three lattice water molecules(Fig.1).Both of the triazole ring and pyridyl ring of L′are planar.The dihedral angle between the triazole ring and pyridyl ring is 37.18°.Three hydrogen bond interactions are found among the water molecules:O1W-H1WA…O3W,O3W-H3WB…O1W and O3W-H3WA…O2W. There also exist three hydrogen bonds among water molecules and nitrogen atoms:O1W-H1WB…N1, O2W-H2WB…N2and O2W-H2WA…N4(Table 3and Fig.2).In addition,two weak face-to-face π…π interactions exist between the triazole ring and pyridyl ring(Fig.2).All theseinteractions link theL′molecules into a two-dimensional structure(Fig.3).
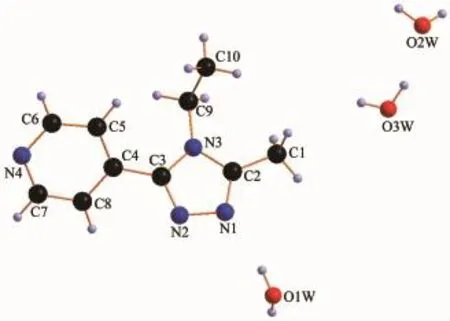
Fig.1Structure of L′with atomic labeling
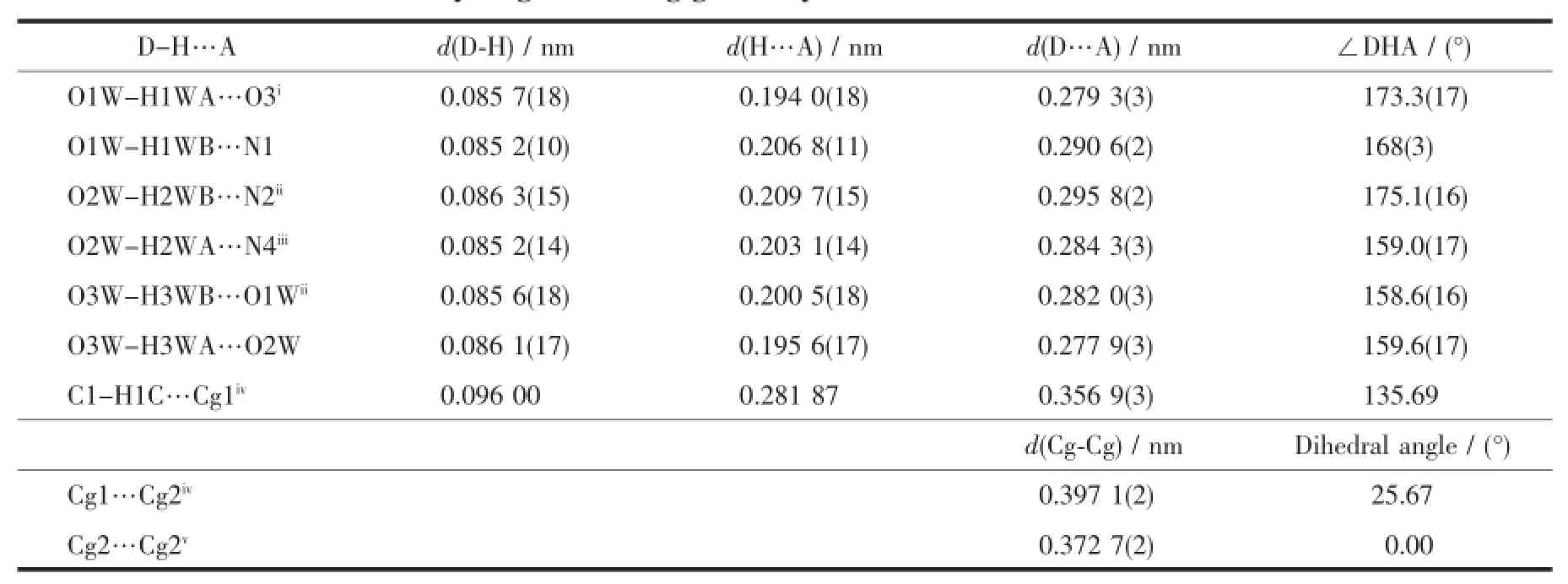
Table 3Hydrogen bonding geometry and π…π interactions for L′

Fig.2H-bonding and π…π interactions in L′

Fig.32D structure of L′
In complex 1,there exist two intramolecular hydrogen bonds(C11-H11A…O2and C19-H19B… N8)and several intermolecular hydrogen bonds(Table 4).There are also three face-to-face π…π interactions and one edge-to-face C-H…π interaction of C17-H17…Cg1(Table 4and Fig.5).All these interactions link complex 1into a three-dimensional structure (Fig.6).

Fig.4Structure of 1with atomic labeling

Fig.5H-bonding and π…π interactions in 1
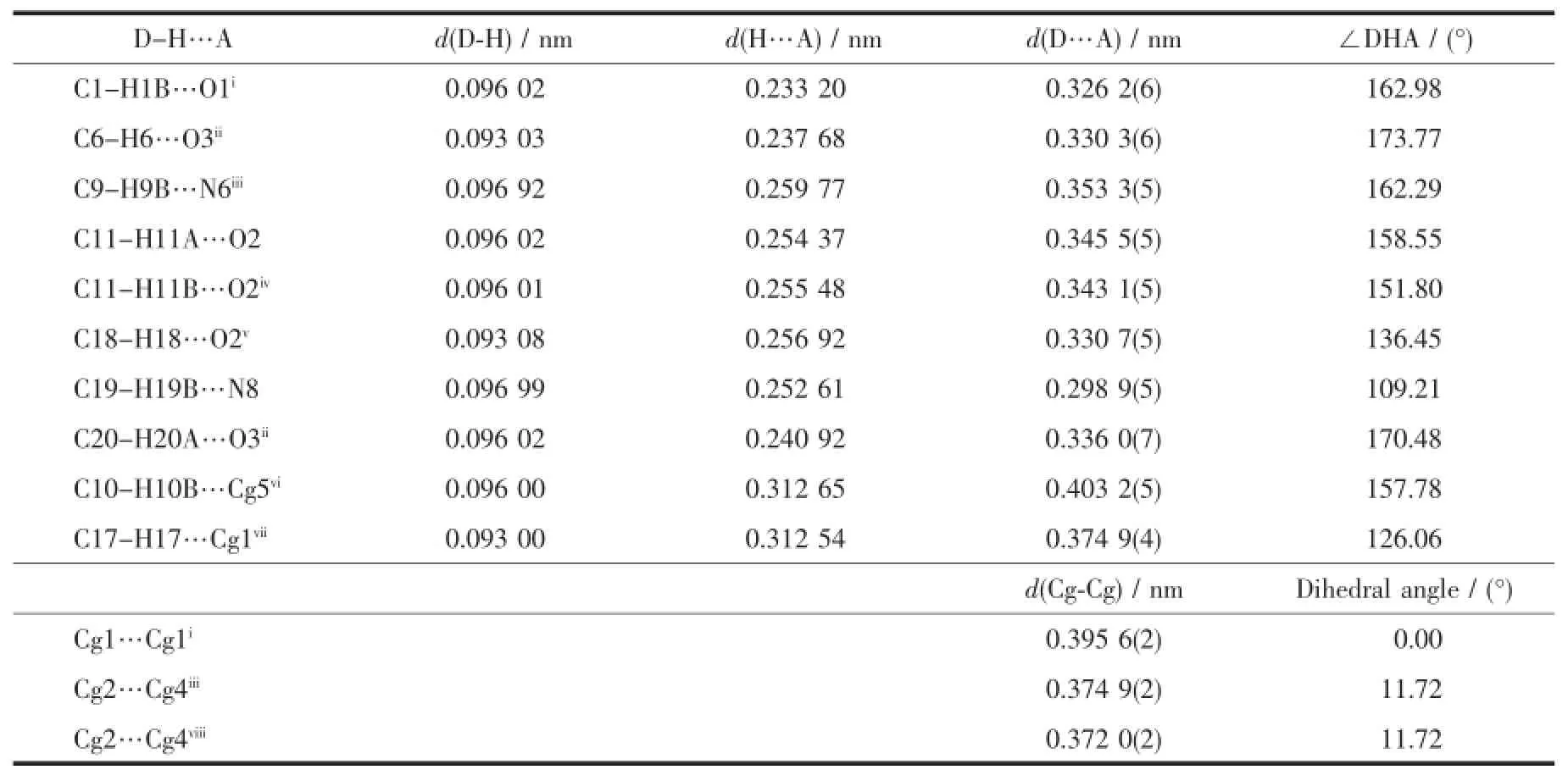
Table 4Hydrogen bonding geometry and π…π interactions for 1

Fig.63D structure of 1

Fig.7Structure of 2with atomic labeling

Fig.8Segment of the polymeric chain of 2
In complex 2there are five kinds of hydrogen bond interactions.A face-to-face π…π interaction was found between the pyridyl rings with a centroidcentroid distance of 0.38823nm(Table 5and Fig.9). There is also one edge-to-face C-H…π interaction of C1-H1B…Cg1involving the triazole ring.All these interactionslinkthecomplex2intoathreedimensional structure(Fig.10).

Fig.9H-bonding and π…π interactions in 2

Fig.103D structure of 2
2.2 Spectral characterization
The IR spectrum of the free L(or L′)shows medium-intensity bands at 1587,1504and 1438cm-1(or 1600,1525and 1430cm-1for L′),which are attributed to pyridine and triazole rings vibrations.As the nitrogen atoms coordinate to metal atoms,the bands for L(or L′)shift about 14cm-1(or 12cm-1)[24]. We can find a band at 1601,1519and 1452cm-1(or 1612,1537and 1445cm-1)in the spectrum of 1(or 2),which can be assigned to the coordinated triazole rings(or pyridyl rings and triazole rings),the bands at 1384cm-1is attributed to the NO3-in complex 1and 2.These features are in consistent with the results of X-ray analyses.
Thephotoluminescentpropertiesofthefree ligand L′and complex 2have been studied in the solid state at room temperature.The emission spectra for L′and complex 2showed a broad emission maximum at 491nm and 465nm with excitation at 413nm and 400nm,respectively.Their photoluminescent properties are attributed to the π-π*electronic transition.As shown in Fig.11,it can be observed that the fluorescence intensity of complex 2is weaker than the free ligand L′,and the emission peak of complex 2has blue-shift.As the nitrogen atoms of L′in complex 2coordinated to Agions,it makes the conjugation extent of L′in 2weaker than that of the free L′,and the weak interactions of mental atoms could exist due to the short distance of silver atoms, which leads to a higher energy under the excited status.

Fig.11Solid-state emission spectra of free ligand L′and complex 2at room temperature
2.3 Thermal analysis
Thermal gravimetric analyses(TGA)of complexes 1and 2were performed in the temperature range of 40~800℃in nitrogen atmosphere with a heating rate of 10K·min-1to investigate their thermal stabilities (Fig.12).In the TGA curve of complex 1,it is stable when the temperature is below 207℃.As the temperaturewasraised,complex1decomposed intensely between 207and 349℃,corresponding to the loss of the ligand L andThe total weight loss is about 76.82%at 490℃,which indicates the residual is Ag2O(Calcd.78.79%).In the curve of the complex 2,the first weight loss between 64and 102℃is 4.15%due to the removal of crystal water molecules(Calcd.4.79%).As the temperature was raised,complex 2decomposed intensely from 214to 271℃,corresponding to the loss of the ligand L and NO3-.The total weight loss is about 66.23%at 580℃, which indicates the residual is Ag2O(Calcd.69.19%). The results of thermal decompositions of 1and 2are consistent with their crystal structures.

Fig.12TGA curves of complexes 1and 2
3 Conclusions
[1]Klingele M H,Boyd P D W,Moubaraki B,et al.Eur.J. Inorg.Chem.,2005,5:910-918
[2]Koomen-van Oudenniel W M E,de Graaff R A G,Haasnoot J G,et al.Inorg.Chem.,1989,28:1128-1133
[3]Lei C,Chao S T,Wu H K,et al.Z.Kristallogr.-New Cryst. Struct.,2016,231:405-406
[4]Li Y,Zhang C G,Cai L Y,et al.J.Coord.Chem.,2013,66: 3100-3112
[5]Liu K,Shi W,Cheng P.Dalton.Trans.,2011,40:8475-8490
[6]Nakabayashi K,Umeda A,Sato Y,et al.Polymer,2016,96: 81-93
[7]Qin J,Lei N,Ding H,et al.Synth.React.Inorg.Met.-Org. Chem.,2016,46:968-974
[8]Singh N K,Bharty M K,Dulare R,et al.Polyhedron,2009, 28:2443-2449
[9]Wajda-Hermanowicz K,Pieniaiczak D,Wrobel R,et al.J. Mol.Struct.,2016,1114:108-122
[10]Abdel-Aal M T,El-Sayed W A,El-Kosy S M,et al.Arch.Pharm.,2008,341:307-313
[11]Fang Y Q,Sun C L,Liu D C,et al.Iran.J.Pharm.Res., 2015,14:77-87
[12]Ledeti I,Bercean V,Alexa A,et al.J.Chem.,2015:879343(5pages)
[13]Romagnoli R,Baraldi P G,Cruz-Lopez O,et al.J.Med. Chem.,2010,53:4248-4258
[14]Al-Masoudi I A,Al-Souda Y A,Al-Salihi N J,et al.Chem. Heterocycl.Compd.,2006,42:1377-1403
[15]Kahn O,Martinez C J.Science,1998,279:44-48
[16]Zhu D R,Xu Y,Yu Z,et al.Chem.Mater.,2002,14:838-843
[17]Beckma nn U,Brooker S.Coord.Chem.Rev.,2003,245:17-29
[18]Haasnoot J G.Coord.Chem.Rev.,2000,200:131-185
[19]Klingele M H.Brooker S.Coord.Chem.Rev.,2003,241: 119-132
[20]Xie D J,Lu W,Wang Z X,et al.Acta Crystallogr.Sect.E, 2009,E65:O1177
[21]Karayel A,Ozbey S,Capan G.J.Mol.Struct.,2007,841:118-124
[22]QI Li(齐丽),ZHU Dun-Ru(朱敦如),XIE Da-Jing(谢大景), et al.Chinese J.Inorg.Chem.(无机化学学报),2008,24(6): 868-872
[23]Yang J,Bao W W,Ren X M,et al.J.Coord.Chem.,2009, 62:1809-1816
[24]Zhu D R,Xu Y,Mei Y,et al.J.Mol.Struct.,2001,559:119-125
[25]LU Wei(卢伟),XIE Da-Jing(谢大景),WANG Zuo-Xiang(王作祥),et al.Chinese J.Inorg.Chem.(无机化学学报),2010, 26(4):717-720
[26]WANG Zuo-Xiang(王作祥),XU Hai-Jun(徐海军),GONG Xiao-Ning(宫晓宁),et al.Chinese J.Inorg.Chem.(无机化学学报),2009,25(8):1492-1496
[27]Sheldrick G M.Acta Crystallogr.Sect.A,2008,64:112-122
Syntheses,Crystal Structures and Spectral Properties of Two SilverComplexes with 3-Methyl-4-ehtyl-5-(2(or 4)-pyridyl)-1,2,4-triazoles
TANG Hui1GUO Yan-Hong1SHENG Jun-Feng1TONG Yu-Zhu1SONG Fei1WANG Zuo-Xiang*,1QU Zhi-Rong2
(1College of Chemistry and Chemical Engineering,Southeast University,Nanjing 211189,China)
(2Key Laboratory of Organosilicon Chemistry and Material Technology of Ministry of Education, Hangzhou Normal University,Hangzhou 311121,China)
Two Agcomplexes of[Ag2(μ-L)2L2(NO3)2](1)and{[Ag2(μ-L′)2(H2O)2](NO3)2}n(2),where L(or L′)=3-methyl-4-ethyl-5-(2(or 4)-pyridyl)-1,2,4-triazole,have been synthesized and characterized by single-crystal X-ray diffraction,IR,fluorescence measurement and TGA.Complexes 1and 2crystallize in triclinic system with space group P1.Crystallographic studies show that both complex 1and 2have a distorted tetrahedral core [AgN3O]with one NO3-in 1but one coordinated H2O molecule in 2.Complex 1is a binuclear silvercomplex bridged through the nitrogen atoms of triazole rings.Complex 2is a polymer complex bridged by the nitrogen atoms of pyridine and triazole rings.CCDC:1046706,L′;1046707,1;1046708,2.
crystal structure;synthesis;triazole;silvercomplex
O614.122
A
1001-4861(2017)01-0134-09
10.11862/CJIC.2017.012
2016-06-23。收修改稿日期:2016-11-16。
*通信联系人。E-mail:wangzx0908@aliyun.com

