胃癌组织原钙黏蛋白10 mRNA表达水平与预后的相关性
林 莹,闫 艳,吴 峥,葛潇潇,林凤娟,李 进,3
1.复旦大学附属肿瘤医院肿瘤内科,复旦大学上海医学院肿瘤学系,上海 200032;2.上海交通大学医学院附属瑞金医院肿瘤科,上海 200025;3.同济大学附属天佑医院肿瘤科,上海 200311
胃癌组织原钙黏蛋白10 mRNA表达水平与预后的相关性
林 莹1,闫 艳1,吴 峥2,葛潇潇1,林凤娟1,李 进1,3
1.复旦大学附属肿瘤医院肿瘤内科,复旦大学上海医学院肿瘤学系,上海 200032;2.上海交通大学医学院附属瑞金医院肿瘤科,上海 200025;3.同济大学附属天佑医院肿瘤科,上海 200311
背景与目的:编码原钙黏蛋白10(protocadherin-10,PCDH10)的PCDH10基因启动子甲基化与胃癌患者不良预后相关。但PCDH10表达水平与胃癌预后的关系不明确。该研究旨在分析PCDH10表达水平与胃癌预后及临床病理因素间的关系,寻找预测胃癌患者复发及死亡风险的指标。方法:采用实时荧光定量聚合酶链反应(real-time fluorescent quantitative polymerase chain reaction,RTFQ-PCR)方法检测115对胃癌组织与相应癌旁组织PCDH10 mRNA的表达水平,分析PCDH10 mRNA的表达水平与预后及临床病理因素的关系。采用Logistic回归分析建立预测患者5年内复发或死亡风险的模型。结果:PCDH10 mRNA低表达组与非低表达组相比,无进展生存时间(progression-free survival,PFS)与总生存时间(overall survival,OS)显著延长(P值分别为0.046与0.033),淋巴结转移较少(P=0.001),TNM分期较早(P=0.001)。Cox单因素分析发现,Lauren分型、T分期、N分期、M分期及PCDH10 mRNA表达水平与PFS及OS显著相关。包含PCDH10作为参数的Logistic回归模型对胃癌患者术后5年内复发或死亡风险的预测效率与仅包含传统临床病理学参数的Logistic回归模型的预测效率相当。结论:PCDH10低表达胃癌患者淋巴结转移较少,TNM分期较早,预后较好,可以作为预测胃癌患者预后的辅助指标。基于PCDH10表达水平的Logistic回归模型可以在淋巴结转移情况不明时起到辅助判断患者预后的作用。
胃癌;原钙黏蛋白10; 表达; 预后
胃癌是全球最常见的肿瘤之一。在中国,胃癌的发病率和死亡率在肿瘤性疾病中分别居于第2位和第3位[1]。尽管近年来胃癌的发病率和死亡率在逐渐下降,但胃癌的预后并没有显著改善[2]。寻找能指示胃癌预后的生物学标志物不仅有助于临床医生判断预后并制定个体化治疗方案,也有助于明确胃癌发生、发展的机制,对制定胃癌的治疗策略有重要意义。
近年来的研究发现,编码原钙黏蛋白10(protocadherin-10,PCDH10)的PCDH10基因启动子甲基化可能参与胃癌发生、发展的过程[3]。包括胃癌在内的多种肿瘤组织及肿瘤细胞系存在PCDH10基因甲基化现象,PCDH10表达量降低与肿瘤组织及肿瘤细胞中PCDH10基因甲基化水平相关[4-20]。在胃癌及其他数种肿瘤中,PCDH10基因的甲基化水平与临床不良预后相关[4,7-9,13,20],但是PCDH10的表达水平与胃癌预后及临床病理因素间的关系仍不明确。
为了进一步研究PCDH10与胃癌发生、发展的关系,本研究检测了115例胃癌及癌旁组织中PCDH10 mRNA表达水平,分析PCDH10的表达水平与预后及临床病理因素的关系,建立基于PCDH10表达水平的预测胃癌患者复发及死亡的Logistic回归模型。
1 资料和方法
1.1 临床资料
本研究所使用的115例胃癌与相应癌旁组织标本均来自2008—2009年在复旦大学附属肿瘤医院行胃癌切除术的患者的手术切除标本,由复旦大学附属肿瘤医院组织库保存。所有患者术前均知情并签署复旦大学附属肿瘤医院组织库样本采集知情同意书。样本选取标准如下:① 病理诊断为胃腺癌;② 术前未接受放化疗;③ 无其他恶性肿瘤病史;④ 临床资料及5年随访资料完整。所有标本病理诊断明确,并统一根据美国癌症联合会(American Joint Committe on Cancer,AJCC)第七版TNM分期标准进行分期。术后辅助治疗及一线、二线治疗均以患者就诊当时最新的NCCN指南为统一的治疗原则进行。
1.2 RNA提取及实时荧光定量聚合酶链反应(real-time fluorescent quantitative polymeras chain reaction,RTFQ-PCR)检测
采用TRIzol法提取组织中的总RNA,反转录并进行RTFQ-PCR。RTFQ-PC所用的PCDH10的引物为:上游引物5’-ACTGCTATCAGGTATGCCTG-3’;下游引物5’-GTCTGTCAACTAGATAGCTG-3’。以18s核糖体RNA(18s rRNA)为RTFQPCR的内参,所用引物为:上游引物5’-CGGCTACCACATCCAAGGAAG-3’;下游引物 5’-GCTGGAATTACCGCGGCTGCT-3’。PCDH10 mRNA相对于18s rRNA的表达量的计算方法为2-ΔCt(ΔCt=癌或癌旁组织中PCDH10的Ct值-同一样本中18s rRNA的Ct值)。PCDH10 mRNA在胃癌组织与癌旁组织中的相对表达量的计算方法为:2-ΔΔCt(ΔΔCt=ΔCt癌组织-ΔCt相应癌旁组织)。根据胃癌组织相对癌旁组织的PCDH10表达水平将样本分成3组,小于等于0.5为低表达组,大于等于2为高表达组,介于两者之间为无显著差异组。
1.3 随访情况
本研究随访终点为患者发生死亡事件或随访时间已满5年。无进展生存期(progression-free survival,PFS)定义为从接受手术开始到患者出现肿瘤进展或死亡的时间。总生存时间(overall survival,OS)定义为患者自接受手术之日起至死亡之日或随访结束之日的时间。
1.4 统计学处理
采用SPSS 19.0软件进行统计学分析。采用Kaplan-Meier方法及Log-rank检验评估PCDH10 mRNA表达水平与PFS、OS的关系。采用配对t检验评估胃癌组织与癌旁组织PCDH10 mRNA表达差异,采用χ2检验评估PCDH10相对表达水平与临床病理因素的关系。双侧检验P<0.05为差异有统计学意义。用二元Logistic回归分析建立Logistic回归模型。采用Cox回归分析单因素与PFS、OS的关系,P<0.05为差异有统计学意义。
2 结果
2.1 胃癌及癌旁组织PCDH10 mRNA表达水平的差异
在115例样本中,41例样本(35.7%)存在PCDH10 mRNA表达水平的下调,39例样本(33.9%)存在PCDH10 mRNA表达水平的上调,其余35例样本(30.4%)未检测到PCDH10 mRNA水平明显变化。胃癌组织中的PCDH10 mRNA平均表达水平显著高于癌旁组织(P=0.039 7,图1)。
2.2 PCDH10 mRNA相对表达水平与胃癌预后的关系
根据PCDH10 mRNA相对表达水平将样本分为高表达组、等表达组和低表达组,进行Kaplan-Meier生存分析,成对比较结果显示,PCDH10 mRNA高表达组与PCDH10 mRNA等表达组的PFS和OS差异无统计学意义(P=0.718和P=0.466),故将改两组合并为非低表达组。再次分析结果表明,PCDH10 mRNA低表达组PFS及OS均较非低表达组显著延长(图2,P=0.046和P=0.033)。

图 1 胃癌与癌旁组织中PCDH10 mRNA表达水平的差异Fig. 1 PCDH10 mRNA expression in gastric cancer tissues and adjacent normal gastric tissues

图 2 PCDH10低表达组与非低表达组患者的无进展生存曲线及总生存曲线Fig. 2 Kaplan-Meier analysis of PFS and OS in gastric cancer patients with different expressions of PCDH10
2.3 PCDH10 mRNA相对表达水平与胃癌患者临床病理特征的关系
PCDH10 mRNA相对表达水平与胃癌患者临床病理特征的关系见表1。PCDH10表达下调与TNM分期较早及淋巴结转移较少显著相关(P=0.001和P=0.001),且PCDH10表达下调在女性患者中比在男性患者中更常见(P=0.040)。PCDH10表达与年龄、Lauren分型、T分期、神经浸润及脉管浸润等临床病理特征之间无明显相关关系。
2.4 临床病理特征及PCDH10 mRNA表达水平与胃癌患者预后的Cox单因素分析
Cox单因素分析发现Lauren分型、T分期、N分期、M分期及PCDH10 mRNA表达水平与胃癌患者的PFS及OS均显著相关,而年龄和性别与胃癌患者的预后无显著相关性(表2、3)。
2.5 Logistic回归模型
根据Cox单因素分析结果,将与胃癌患者PFS及OS有显著相关性的Lauren分型、T分期、N分期、M分期及PCDH10 mRNA相对表达水平纳入Logistic回归模型。基于传统临床病理学参数的预测胃癌5年内复发或死亡风险的Logistic回归模型纳入了Lauren分型、T分期、N分期和M分期作为参数。基于PCDH10表达水平的预测胃癌5年内复发或死亡风险的Logistic回归模型纳入了Lauren分型、T分期、M分期及PCDH10 mRNA表达水平作为参数,但未包括N分期为参数。基于传统临床病理学参数预测胃癌5年内复发风险模型受试者工作特征(receiver operating characteristic,ROC)曲线的曲线下面积(0.744,95%CI:0.651 ~0.837)与基于PCDH10 mRNA表达水平预测胃癌5年内复发风险模型的曲线下面积(0.717,95%CI:0.623 ~0.811)差异无统计学意义(P>0.05,图3)。基于传统临床病理学参数预测胃癌5年内死亡风险模型ROC曲线的曲线下面积(0.749,95%CI:0.658 ~0.840)与基于PCDH10 mRNA表达水平预测胃癌5年内死亡风险模型的曲线下面积(0.729,95%CI:0.637 ~0.820)差异无统计学意义(P>0.05,图3)。提示两类模型效率相当。由于基于PCDH10表达水平的Logistic回归模型不需要N分期作为参数,它可以在临床N分期资料不全的情况下起到替代N分期,辅助判断患者预后、指导临床治疗与随访的作用。
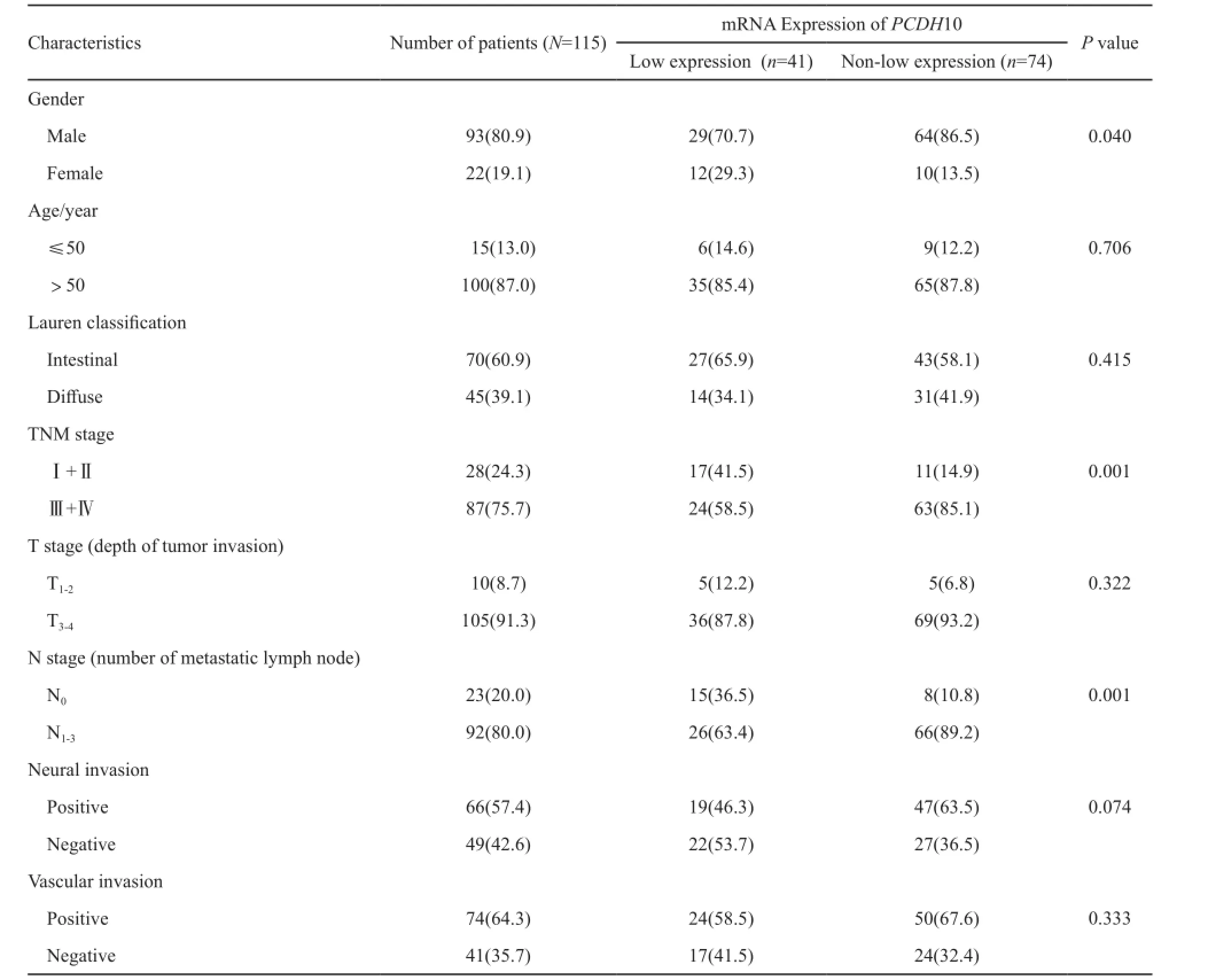
表 1 PCDH10 mRNA表达水平与胃癌患者临床病理特征的关系Tab. 1 Correlation between the expression of PCDH10 mRNA and clinicopathological features of gastric cancer[n(%)]

表 2 临床病理特征及PCDH10 mRNA表达水平与胃癌患者PFS的Cox单因素分析Tab. 2 The correlation between clinicopathological characteristics and expression of PCDH10 mRNA and PFS in gastric cancer patients: univariate Cox’s regression analysis

表 3 临床病理特征及PCDH10 mRNA表达水平与胃癌患者OS的Cox单因素分析Tab. 3 The correlation between clinicopathological characteristics and expression of PCDH10 mRNA and OS in gastric cancer patients: univariate Cox’s regression analysis
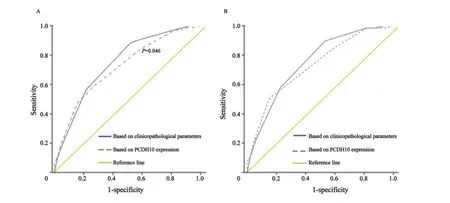
图 3 两类预测胃癌5年内复发或死亡风险模型的ROC曲线Fig. 3 ROC curves of two kinds of prediction models of recurrence or death in 5 years for gastric cancer
3 讨论
在胃癌的综合治疗中,传统的TNM分期对预测患者预后有一定的辅助作用,但临床上存在因病理资料不全而影响预后判断及治疗决策的情况,如术中淋巴结清扫不当导致对淋巴结转移情况的误判等,因此寻找客观的生物学标志物辅助临床判断与决策是十分重要的。
本研究发现,PCDH10 mRNA低表达组胃癌患者TNM分期较早,淋巴结转移较少,预后较好,可以作为胃癌患者预后的指标之一。基于PCDH10表达水平的Logistic回归模型(包括Lauren分型、T分期、M分期及PCDH10 mRNA表达水平为参数)对5年内复发或死亡的预测效率与基于传统临床病理学参数(包括Lauren分型、T分期、N分期及M分期)的Logistic回归模型的预测效率相当。由于基于PCDH10表达水平的Logistic回归模型不需要将N分期作为预测的参数之一,在临床应用中,可作为淋巴结转移情况不明或因各种原因术中没有进行充分的淋巴结清扫的胃癌患者判断预后、指导治疗和随访的辅助指标。
但本研究发现,PCDH10低表达胃癌患者预后较好的结论与文献报道的PCDH10基因是抑癌基因的结论不符。原因可能包括:① 各个研究的样本量较少,而且目前只有关于PCDH10基因甲基化水平与胃癌预后的研究,而PCDH10表达水平与胃癌预后的关系仍不明确;② 关于PCDH10基因在胃癌细胞中的功能研究较少,且机制仍不清楚。Yu等[7]及Li等[19]分别在胃癌细胞中进行了PCDH10的功能研究,两项研究均发现,PCDH10过表达或再表达可抑制肿瘤生长和迁移[7,19]。Yu等[7]研究发现,PCDH10过表达可以诱导凋亡,但是Li等[19]研究发现,PCDH10对细胞凋亡的影响不明显。两研究结果间不一致提示了PCDH10在胃癌发生、发展中的作用及其机制仍不清楚。Zhao等[5]研究发现,PCDH10在子宫内膜癌中是作为Wnt通路的负调控因子,在胃癌中是否也通过同样的通路起作用仍需进一步研究。虽然目前认为原钙黏蛋白基因大多为抑癌基因,但是编码原钙黏蛋白7(protocadherin-7,PCDH7)的PCDH7基因在乳腺癌中存在表达水平升高的现象,可能与乳腺癌的转移有关[21-22]。提示原钙黏蛋白家族的每个基因可能作用不同。关于PCDH10在胃癌发生、发展中的作用及其机制及其与预后的关系还有待进一步研究。
[1] FERLAY J, SOERJOMATARAM I, DIKSHIT R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012[J]. Int J Cancer, 2015, 136(5): 359-386.
[2] DANG Y, WANG Y C, HUANG Q J. Microarray and nextgeneration sequencing to analyse gastric cancer[J]. Asian Pac J Cancer Prev, 2014, 15(19): 8033-8039.
[3] VAN ROY F. Beyond E-cadherin: roles of other cadherin superfamily members in cancer [J]. Nat Rev Cancer, 2014, 14(2): 121-134.
[4] DENG J, LIANG H, YING G, et al. Clinical significance of the methylated cytosine-phosphate-guanine sites of protocadherin-10 promoter for evaluating the prognosis of gastric cancer [J]. J Am Coll Surg, 2014, 219(5): 904-913.
[5] ZHAO Y, YANG Y, TROVIK J, et al. A novel wnt regulatory axis in endometrioid endometrial cancer[J]. Cancer Res, 2014, 74(18): 5103-5117.
[6] YING J, LI H, SENG T J, et al. Functional epigenetics identifies a protocadherin PCDH10 as a candidate tumor suppressor for nasopharyngeal, esophageal and multiple other carcinomas with frequent methylation[J]. Oncogene, 2006, 25(7): 1070-1080.
[7] YU J, CHENG Y Y, TAO Q, et al. Methylation of protocadherin 10, a novel tumor suppressor, is associated with poor prognosis in patients with gastric cancer[J]. Gastroenterology, 2009, 136(2): 640-651 e641.
[8] LIN Y L, LI Z G, HE Z K, et al. Clinical and prognostic significance of protocadherin-10 (PCDH10) promoter methylation in bladder cancer[J]. J Int Med Res, 2012, 40(6): 2117-2123.
[9] ZHONG X, ZHU Y, MAO J, et al. Frequent epigenetic silencing of PCDH10 by methylation in human colorectal cancer[J]. J Cancer Res Clin Oncol, 2013, 139(3): 485-490.
[10] LI Y, YANG Z S, SONG J J, et al. Protocadherin-10 is involved in angiogenesis and methylation correlated with multiple myeloma[J]. Int J Mol Med, 2012, 29(4): 704-710.
[11] WANG K H, LIU H W, LIN S R, et al. Field methylation silencing of the protocadherin 10 gene in cervical carcinogenesis as a potential specific diagnostic test from cervical scrapings [J]. Cancer Sci, 2009, 100(11): 2175-2180.
[12] YING J, GAO Z, LI H, et al. Frequent epigenetic silencing of protocadherin 10 by methylation in multiple haematologic malignancies[J]. Br J haematol, 2007, 136(6): 829-832.
[13] WANG L, XIE P G, LIN Y L, et al. Aberrant methylation of PCDH10 predicts worse biochemical recurrence-free survival in patients with prostate cancer after radical prostatectomy[J]. Med Sci Monit, 2014, 201363-1368.
[14] YU B, YANG H, ZHANG C, et al. High-resolution melting analysis of PCDH10 methylation levels in gastric, colorectal and pancreatic cancers[J]. Neoplasma, 2010, 57(3): 247-252.
[15] NARAYAN G, SCOTTO L, NEELAKANTAN V, et al. Protocadherin PCDH10, involved in tumor progression, is a frequent and early target of promoter hypermethylation in cervical cancer[J]. Genes Chromosomes Cancer, 2009, 48(11): 983-992.
[16] BERTRAND K C, MACK S C, NORTHCOTT P A, et al. PCDH10 is a candidate tumour suppressor gene in medulloblastoma[J]. Childs Nerv Syst, 2011, 27(8): 1243-1249.
[17] NARAYAN G, XIE D, FREDDY A J, et al. PCDH10 promoter hypermethylation is frequent in most histologic subtypes of mature lymphoid malignancies and occurs early in lymphomagenesis[J]. Genes Chromosomes Cancer, 2013, 52(11): 1030-1041.
[18] JAO T M, TSAI M H, LIO H Y, et al. Protocadherin 10 suppresses tumorigenesis and metastasis in colorectal cancer and its genetic loss predicts adverse prognosis [J]. Int J Cancer, 2014, 135(11): 2593-2603.
[19] LI Z, CHIM J C, YANG M, et al. Role of PCDH10 and its hypermethylation in human gastric cancer[J]. Biochim Biophys Acta, 2012, 1823(2): 298-305.
[20] LIN Y L, LI Z G, GUAN T Y. The clinical significance of PCDH10 promoter methylation in patients with bladder transitional cell carcinoma [J]. Urol Int, 2013, 90(2): 219-224.
[21] BOS P D, ZHANG X H, NADAL C, et al. Genes that mediate breast cancer metastasis to the brain[J]. Nature, 2009, 459(7249): 1005-1009.
[22] LI A M, TIAN A X, ZHANG R X, et al. Protocadherin-7 induces bone metastasis of breast cancer[J]. Biochem Biophys Res Commun, 2013, 436(3): 486-490.
Correlation between mRNA expression of protocadherin-10 and prognosis in gastric cancer
LINYing1, YAN Yan1, WU Zheng2, GE Xiaoxiao1, LIN Fengjuan1, LI Jin1,3
(1. Department of Medical Oncology, Fudan University Shanghai Cancer Center, Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 2. Department of Oncology, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China; 3. Department of Oncology, Tongji University Tianyou Hospital, Shanghai 200331, China)
LI Jin E-mail: tianyoulijin@163.com
Background and purpose:Promoter methylation of PCDH10, a gene encoding protocadherin 10, has been found to be correlated to poor prognosis in gastric cancer (GC) patients. However, the relationship between the expression of PCDH10 and prognosis in GC remained unknown. This study aimed to explore the relationship between the expression of PCDH10 and clinicopathological features and prognosis of GC, and to identify biomarker for predictions of recurrence and survival of GC.Methods:mRNA expressions of PCDH10 in 115 pairs of GC tissues and adjacent normal tissues were detected by real-time fluorescence quantitative polymerase chain reaction (RTFQ-PCR). The correlation between PCDH10 expression level and clinicopathological features and prognosis of GC was analyzed. Prediction models for 5-year recurrence and 5-year survival were established using logistic regression method.Results:Progression-free survival (PFS) and overall survival (OS) were significantly prolonged in patients with PCDH10 lowexpression compared to patients without PCDH10 low expression (P=0.046 and P=0.033 respectively). PCDH10 low expression significantly correlated with less lymph node metastasis (P=0.001) and earlier TNM staging (P=0.001), and was more common in female than in male (P=0.040). The mRNA expression of PCDH10 did not correlate with age, Lauren classification, T stage, neural invasion or vascular invasion. Univariate Cox analysis showed Lauren classification, T stage, N stage, M stage and PCDH10 expression significantly correlated with PFS and OS. Logistic regression models for the prediction of 5-year recurrence or 5-year survival based on clinicopathological features included Lauren classification, T stage, N stage and M stage as variables. Logistic regression models for the prediction of 5-year recurrence or 5-year survival based on PCDH10 expression included Lauren classification, T stage, M stage and PCDH10 expression level but not N stage as variables. The models based on PCDH10 expression had the same efficiencies as models based on clinical parameters in predicting 5-year recurrence or 5-year survival for GC patients.Conclusion:PCDH10 low expression correlated with better prognosis, less lymph node metastasis and earlier TNM stage in GC patients. Low expression of PCDH10 may be a biomarker of better survival for GC patients. Logistic regression model based on PCDH10 mRNA expression may serve as a prediction model when patients have unknown lymph node metastasis status.
Gastric cancer; Protocadherin-10; Expression; Prognosis
10.19401/j.cnki.1007-3639.2017.01.002
R735.2
A
1007-3639(2017)01-0007-07
2016-07-18
2016-09-01)
国家科技重大专项基金(2012ZX09303-018-002)。
李 进 E-mail: tianyoulijin@163.com

