欧前胡素通过下调c-met的表达提高肺癌CD133+细胞亚群对吉非替尼的敏感性
吴乾波, 张 敏, 陶志华
(1浙江大学医学院附属第二医院检验科, 2浙江省血液中心献服二科, 3浙江省立同德医院检验科,浙江 杭州 310000)
欧前胡素通过下调c-met的表达提高肺癌CD133+细胞亚群对吉非替尼的敏感性
吴乾波1,2, 张 敏3▲, 陶志华1△
(1浙江大学医学院附属第二医院检验科,2浙江省血液中心献服二科,3浙江省立同德医院检验科,浙江 杭州 310000)
目的: 研究PC9 CD133+细胞亚群对吉非替尼的耐药性并探讨欧前胡素提高吉非替尼抗肺癌活性的机制。方法: MTT法检测PC9细胞在吉非替尼和欧前胡素处理下的细胞活力。Western blot实验检测吉非替尼和欧前胡素对PC9细胞c-met表达水平、caspases 活化水平及表皮生长因子受体(EGFR)、PI3K、AKT磷酸化水平的影响。流式细胞术检测欧前胡素和吉非替尼对PC9细胞系的CD133+细胞亚群种群比例的影响及PC9细胞在二者处理下的凋亡率。结果: PC9 CD133+细胞亚群对吉非替尼的敏感性显著低于PC9 CD133-细胞亚群。吉非替尼能显著抑制PC9 CD133-细胞亚群EGFR/PI3K/AKT的活化,但对PC9 CD133+细胞亚群该通路的影响不大。吉非替尼单独处理能提高PC9细胞系中CD133+细胞亚群的比例,然而联用欧前胡素后PC9 CD133+细胞亚群的种群比例显著下降。Western blot实验表明欧前胡素能显著降低PC9 CD133+细胞亚群的c-met蛋白表达水平,表明c-met是欧前胡素的治疗靶点。MTT、Western blot、流式细胞术实验结果表明在PC9 CD133+细胞亚群中,欧前胡素通过抑制c-met的表达提高吉非替尼对PI3K/AKT的抑制作用,从而诱导PC9 CD133+细胞亚群发生caspases活化和凋亡。结论: 欧前胡素通过下调c-met的表达提高肺癌CD133+细胞亚群对吉非替尼的敏感性,两者存在协同抗肿瘤效应。
欧前胡素; c-met; 吉非替尼; PC9 CD133+细胞亚群; PI3K/AKT信号通路
非小细胞肺癌(nonsmall-cell lung cancer,NSCLC)是一种发病率和死亡率都非常高的恶性肿瘤。尽管现在恶性肿瘤的治疗手段有了很大的发展,化疗在NSCLC的治疗过程中仍是不可替代的重要方法,然而随着化疗药物的反复使用,NSCLC细胞往往能产生获得性药物抵抗,从而降低化疗的效果[1-2]。近些年来的研究表明在肿瘤组织中存在着一组名为肿瘤干细胞的细胞群,与化疗的获得性抵抗密切相关[3]。肺癌组织细胞中的CD133+的肿瘤干细胞被报道能引起化疗的失败和肿瘤的复发[4],因此肿瘤干细胞已经成为肺癌治疗的靶点。
吉非替尼属于一种表皮生长因子受体(epidermal growth factor receptor, EGFR)酪氨酸激酶抑制剂,广泛应用于肺癌患者的治疗,由于它有很好的靶向性,因此吉非替尼目前已经成为治疗非小细胞肺癌的一线药物[5]。然而随着吉非替尼的持续使用,肺癌细胞对吉非替尼的敏感性会逐渐降低。鉴于肺癌干细胞的耐药性是造成吉非替尼抵抗的重要因素[6],因此靶向于肺癌干细胞的辅助治疗是提高吉非替尼疗效的有效方法。
欧前胡素是从白芷根中提取的香豆素类天然活性物质,据报道有一定的抗肿瘤效应,并且能提高化疗药物对肿瘤细胞的杀伤活性[7-8],然而其对肿瘤干细胞的生物作用至今却仍很少报道。本研究的目的在于探讨欧前胡素是否能调节非小细胞肺癌CD133+细胞亚群对吉非替尼的敏感性。
材 料 和 方 法
1 细胞
人非小细胞肺癌细胞系PC9购于ATCC。PC9 CD133+细胞亚群用流式细胞仪进行分选,文献报道CD133+的肺癌细胞即为肺癌肿瘤干细胞,而CD133-细胞则为肺癌非干细胞[9]。细胞用含10%胎牛血清的DMEM培养基在37 ℃恒温培养箱中通入5% CO2培养。
2 试剂
吉非替尼、欧前胡素、噻唑蓝(MTT)、凋亡检测试剂盒购于Sigma-Aldrich;DMEM培养基购于Gibco;蛋白提取液、抗c-met、磷酸化EGFR、磷酸化PI3K、磷酸化AKT、cleaved caspase-9、cleaved caspase-7、cleaved caspase-3和β-actin抗体购于CST;ECL试剂盒购于Pierce;抗CD133-FITC荧光抗体购于BD;pcDNA3.1、Lipofectamine 2000购于Invitrogen。
3 方法
3.1 吉非替尼半数有效浓度(IC50)的测定 将PC9 CD133+细胞亚群和PC9 CD133-细胞亚群按每孔5×103个接种在96孔板上孵育过夜,用浓度为0~0.3 μmol/L的吉非替尼处理肿瘤细胞48 h,之后加入20 μL MTT (5 g/L)于37 ℃恒温培养箱中培养4 h,移除孔内培养基,加入100 μL二甲基亚砜,于570 nm波长下测定吸光度(A)值。细胞活力结果用吉非替尼处理组与对照组的A值比值表示。绘制细胞活力-吉非替尼浓度曲线,根据曲线计算IC50。
3.2 PC9 CD133+细胞亚群所占比例测定 将PC9细胞系用0.04 μmol/L吉非替尼和10 μmol/L欧前胡素处理48 h,之后在细胞培养基中加入抗CD133抗体在暗处孵育20 min后将细胞用流式细胞仪进行分析,计算PC9 CD133+细胞所占总的PC9细胞的比例。
3.3 c-met重组质粒的构建和转染 将c-met基因的cDNA全长序列(Gene ID: NM_001324401)以分子克隆的方法与pcDNA3.1连接后构建成c-met重组真核表达质粒[9]。使用Lipofectamine 2000按照试剂操作说明书步骤将2 mg/L c-met质粒转染入PC9 CD133+细胞亚群中。
3.4 欧前胡素联合吉非替尼对PC9 CD133+细胞亚群的杀伤活性 将PC9 CD133+细胞亚群按每孔5×103个接种在96孔板上孵育过夜,用2 mg/L c-met质粒转染24 h后加入0.04 μmol/L吉非替尼和10 μmol/L欧前胡素处理48 h,之后再加入20 mL MTT(5 g/L)于37 ℃恒温培养箱中培养4 h,移除孔内培养基,加入100 μL二甲基亚砜,570 nm波长下测定A值。细胞活力结果用药物处理组与对照组的A值比值表示。
3.5 Western blot实验 PC9 CD133+细胞亚群用2 mg/L c-met质粒转染24 h后用0.04 μmol/L吉非替尼和10 μmol/L欧前胡素处理细胞48 h,之后用蛋白提取液提取肿瘤细胞中的总蛋白质。将等量的总蛋白质用12.5% SDS-PAGE分离。分离完毕后通过电转方法将蛋白质从分离胶转到PVDF膜上,用抗c-met、磷酸化EGFR、磷酸化PI3K、磷酸化AKT、cleaved caspase-9、cleaved caspase-7、cleaved caspase-3和β-actin 兔抗人抗体孵育过夜,之后再用带辣根过氧化物酶的 Ⅱ 抗孵育2 h,蛋白条带用ECL试剂盒显色发光。
3.6 细胞凋亡实验 PC9 CD133+细胞亚群用2 mg/L c-met质粒转染24 h后用0.04 μmol/L吉非替尼和10 μmol/L欧前胡素处理细胞48 h,之后按照凋亡试剂盒说明书步骤将碘化丙啶和Annexin V加入细胞中孵育20 min,采用流式细胞术检测肿瘤细胞的凋亡。
4 统计学处理
用SPSS 14.0统计分析软件进行数据处理。所有实验重复3次,实验数据用均数±标准差(mean±SD)表示。多组间均数的比较采用单因素方差分析(one-way ANOVA),两组间均数的比较采用 Student’st检验,以P<0.05为差异有统计学意义。
结 果
1 PC9 CD133+细胞亚群对吉非替尼的敏感性显著低于PC9 CD133-细胞亚群
MTT实验结果显示吉非替尼对PC9 CD133+细胞亚群的杀伤力显著低于PC9 CD133-细胞亚群,并且PC9 CD133+细胞亚群的吉非替尼IC50显著高于PC9 CD133-细胞亚群,这些结果表明PC9 CD133+细胞亚群对吉非替尼存在抵抗性。Western blot实验结果显示吉非替尼能显著抑制PC9 CD133-细胞亚群的EGFR、PI3K、AKT的磷酸化,然而吉非替尼仅能抑制PC9 CD133+细胞亚群EGFR的活化,而对其下游PI3K和AKT磷酸化的抑制效果不明显,表明PC9 CD133+细胞亚群PI3K/AKT的活化不依赖于EGFR的磷酸化,这可能是PC9 CD133+细胞亚群对吉非替尼不敏感的分子机制。另外,流式细胞实验结果显示在吉非替尼处理下,PC9 CD133+细胞亚群的凋亡率显著低于PC9 CD133-细胞亚群,表明PC9 CD133+细胞亚群对吉非替尼诱导的凋亡信号不敏感,见图1。
2 欧前胡素增强PC9 CD133+细胞亚群对吉非替尼的敏感性
流式细胞实验结果显示吉非替尼单独治疗能显著提高PC9细胞系中CD133+细胞亚群的比例,然而联用欧前胡素后,PC9 CD133+细胞亚群的比例显著下降。另外,MTT实验结果显示联用欧前胡素能显著提高吉非替尼对PC9 CD133+细胞亚群的杀伤力,这些结果表明联用欧前胡素能显著提高PC9 CD133+细胞亚群对吉非替尼诱导的细胞死亡的敏感性,两者存在协同效应,见图2。
3 欧前胡素通过下调c-met的表达提高PC9 CD133+细胞亚群对吉非替尼的敏感性
Western blot实验结果显示PC9 CD133+细胞亚群中的c-met表达水平显著高于PC9 CD133-细胞亚群,提示c-met可能在PC9 CD133+细胞亚群中发挥重要作用。另外,欧前胡素能显著降低PC9 CD133+细胞亚群中c-met的表达水平,而吉非替尼对c-met的表达无明显影响,提示欧前胡素可能以c-met为靶点增强PC9 CD133+细胞亚群对吉非替尼的敏感性。MTT实验结果显示在PC9 CD133+细胞亚群中转染c-met表达质粒后,欧前胡素联合吉非替尼对PC9 CD133+细胞亚群的杀伤力受到显著抑制,表明欧前胡素与吉非替尼的联合杀伤效应依赖于c-met的下调。Western blot实验结果显示欧前胡素联合吉非替尼能显著抑制PC9 CD133+细胞亚群的EGFR、PI3K、AKT的磷酸化水平,然而转染c-met质粒后,两者联合对PI3K和AKT磷酸化的抑制作用丧失,表明c-met是PI3K/AKT分子的上游信号,且PC9 CD133+细胞亚群通过过表达c-met使PI3K/AKT分子持续活化。流式细胞实验和Western blot实验结果显示转染c-met质粒能显著抑制欧前胡素对吉非替尼诱导PC9 CD133+细胞亚群的凋亡和caspase活化的促进作用,表明欧前胡素通过调控c-met提高PC9 CD133+细胞亚群对吉非替尼依赖的凋亡信号通路的敏感性,见图3。
讨 论
欧前胡素是一种天然药物,具有毒性低,副作用小的优点,文献表明欧前胡素的辅助治疗能有效提高化疗药物对肿瘤细胞的杀伤力,因此有良好的应用前景[10]。然而,欧前胡素是否能在肿瘤干细胞中发挥良好的辅助治疗作用,至今还未有充分研究。近期的研究表明肿瘤干细胞对药物治疗的低敏感性是造成肿瘤治疗失败的重要原因[11]。研究报道在肺癌中,CD133可作为肿瘤干细胞的表面标志[9]。而在本研究中,当用吉非替尼直接处理非小细胞肺癌细胞后发现,PC9细胞系中CD133+细胞亚群比例显著提高,这可能是因为PC9 CD133+细胞亚群对吉非替尼的敏感性显著低于PC9 CD133-细胞亚群,从而导致PC9 CD133+细胞亚群在吉非替尼的处理下存活下来从而引起所占比例的升高。当用欧前胡素进行联合治疗后,由于PC9 CD133+细胞亚群在欧前胡素的作用下对吉非替尼的敏感性显著提高,从而导致CD133+细胞亚群的占比显著下降。本研究结果表明欧前胡素能以CD133+肺癌细胞为靶点提高吉非替尼对非小细胞肺癌的治疗效果。

Figure 1.The sensitivity of the PC9 CD133+cell subsets to gefitinib was significantly lower than the PC9 CD133-cell subsets. A: the cytotoxicity of gefitinib to PC9 CD133+cell subsets was significantly lower than the PC9 CD133-cell subsets; B: the IC50of gefitinib to PC9 CD133+cell subsets was significantly higher than the PC9 CD133-cell subsets; C: gefitinib failed to inhibit the phosphorylation of PI3K/AKT in the PC9 CD133+cell subsets; D: PC9 CD133+cell subsets showed low sensitivity to the gefitinib-induced apoptosis. Mean±SD.n=3.*P<0.05vsPC9 CD133-;#P<0.05vsPC9 CD133--control;△P<0.05 PC9 CD133+-control.
图1 PC9 CD133+细胞亚群对吉非替尼的敏感性显著低于PC9 CD133-细胞亚群
研究表明,众多肿瘤类型(特别是非小细胞肺癌)细胞表面的EGFR会发生过表达或突变。这些EGFR突变型的肺癌细胞(如PC9)对吉非替尼等表皮生长因子受体酪氨酸激酶抑制剂表现为高度敏感,因此吉非替尼目前是治疗非小细胞肺癌的一线药物[12-13]。在EGFR信号通路中,活化的EGFR能导致PI3K及其下游分子AKT的磷酸化使之发生活化。肿瘤细胞中AKT的持续活化又能通过激活下游分子促进细胞的增殖和存活,同时抑制凋亡的发生[14-15]。然而在本研究中,实验结果显示吉非替尼虽然能抑制PC9 CD133+细胞亚群的EGFR信号,但却不能抑制这些细胞中PI3K/AKT的活化,从而导致肺癌CD133+细胞亚群对吉非替尼诱导的凋亡途径产生抵抗。因此本研究的结果表明非小细胞肺癌CD133+细胞可能通过另外一种信号通路维持细胞中PI3K/AKT的活化。
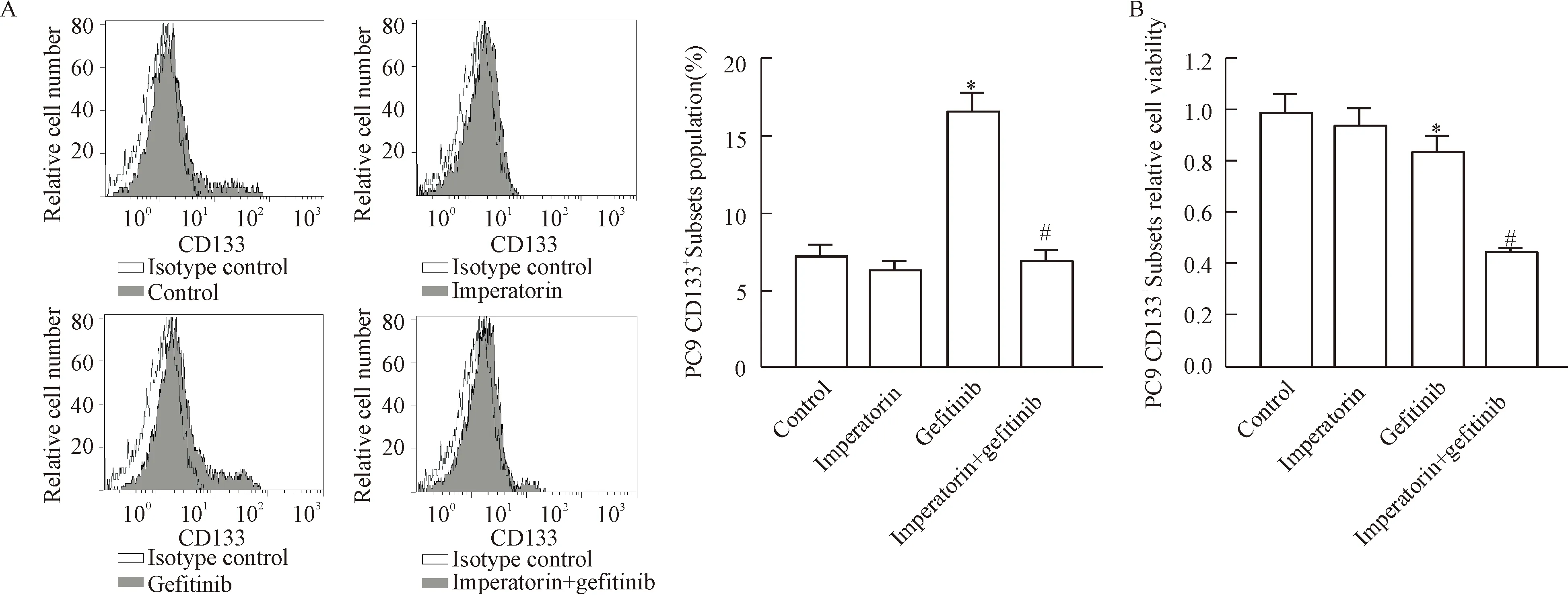
Figure 2.Imperatorin enhanced the sensitivity of the PC9 CD133+cell subsets to gefitinib. A: imperatorin significantly inhibited the enrichment of the PC9 CD133+cell subsets population induced by gefitinib; B: imperatorin enhanced the cytotoxicity of gefitinib to the PC9 CD133+cell subsets. Mean±SD.n=3.*P<0.05vscontrol;#P<0.05vsgefitinib.
图2 欧前胡素增强PC9 CD133+细胞亚群对吉非替尼的敏感性


Figure 3. Imperatorin increased the sensitivity of the lung cancer CD133+cell subsets to gefitinib by down-regulating the expression of c-met. A: the expression of c-met was up-regulated in the PC9 CD133+cell subsets; B: imperatorin inhibited the expression of c-met in the PC9 CD133+cell subsets; C: transfection of c-met plasmid inhibited the cell death of PC9 CD133+cell subsets induced by the combination of imperatorin with gefitinib; D: transfection of c-met plasmid inhibited the phosphorylation of PI3K and AKT in the PC9 CD133+cell subsets induced by the combination of imperatorin with gefitinib; E: transfection of c-met plasmid inhibited the apoptosis of PC9 CD133+cell subsets induced by the combination of imperatorin with gefitinib; F: transfection of c-met plasmid inhibited the activation of caspases in the PC9 CD133+cell subsets induced by the combination of imperatorin with gefitinib. Mean±SD.n=3.△P<0.05vsPC9 CD133-;*P<0.05vscontrol;#P<0.05vsimperatorin+gefitinib.
图3 欧前胡素通过下调c-met的表达提高肺癌CD133+细胞亚群对吉非替尼的敏感性
c-met是肝细胞生长因子(hepatocyte growth factor,HGF)的受体,有报道指出HGF/c-met途径能激活肿瘤细胞中包括PI3K/AKT在内的多种促进肿瘤存活和生长的信号通路[16-17]。此外,近期的研究表明c-met的过度活化是诱导肺癌细胞产生对吉非替尼获得性耐药的重要机制,肿瘤细胞中过表达的c-met同样能显著激活PI3K/AKT分子而不依赖于EGFR的活化[18]。因此针对c-met及其下游信号分子的靶向治疗已成为增强抗肿瘤药物疗效的重要策略。本研究的结果表明c-met蛋白在肺癌CD133+细胞亚群中发生过表达。当用欧前胡素对肺癌CD133+细胞亚群进行处理后,细胞中c-met的表达水平显著降低,使c-met/PI3K/AKT通路受到抑制,从而使肺癌CD133+细胞亚群中PI3K/AKT的活化恢复对EGFR信号的依赖性。因此,当用欧前胡素和吉非替尼对肺癌CD133+细胞亚群进行联合治疗时,肿瘤细胞的PI3K/AKT受到显著抑制,进而诱导CD133+肺癌细胞发生caspase的活化和凋亡的发生。
综上所述,本研究证明了欧前胡素通过抑制c-met的表达使肺癌CD133+细胞亚群中的PI3K/AKT恢复对EGFR信号的依赖性,从而提高肺癌CD133+细胞亚群对吉非替尼的敏感性。这些研究为吉非替尼等EGFR酪氨酸激酶抑制剂的化疗增效提供了新的策略和思路。
[1] Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013[J]. CA Cancer J Clin, 2013, 63(1):11-30.
[2] Wang S, Liu F, Zhu J, et al. DNA repair genes ERCC1 and BRCA1 expression in non-small cell lung cancer chemotherapy drug resistance[J]. Med Sci Monit, 2016, 22:1999-2005.
[3] Zhang GF, Li CX, Liu ZQ, et al. Cancer stem cell targets -a review[J]. Eur Rev Med Pharmacol Sci, 2016, 20(10):2045-2051.
[4] Sarvi S, Mackinnon AC, Avlonitis N, et al. CD133+cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist[J]. Cancer Res, 2014, 74(5):1554-1565.
[5] Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer-Recent advances and future perspectives[J]. Int J Cancer, 2016, 138(11):2549-2561.
[6] Kobayashi I, Takahashi F, Nurwidya F, et al. Oct4 plays a crucial role in the maintenance of gefitinib-resistant lung cancer stem cells[J]. Biochem Biophys Res Commun, 2016, 473(1):125-132.
[7] Hu J, Xu C, Cheng B, et al. Imperatorin acts as a cisplatin sensitizer via downregulating Mcl-1 expression in HCC chemotherapy[J]. Tumour Biol, 2016, 37(1):331-339.
[8] Jakubowicz-Gil J, Paduch R, Ulz Z, et al. Cell death in HeLa cells upon imperatorin and cisplatin treatment[J]. Folia Histochem Cytobiol, 2012, 50(3):381-391.
[9] Chen Y, Zhang F, Tsai Y, et al. IL-6 signaling promotes DNA repair and prevents apoptosis in CD133+stem-like cells of lung cancer after radiation[J]. Radiat Oncol, 2015, 10:227.
[10]郑 颖, 姜 凯. 欧前胡素增强多柔比星对HeLa细胞的抗肿瘤效应[J]. 中国病理生理杂志, 2015, 31(9):1578-1583.
[11]Codony-Servat J, Verlicchi A, Rosell R. Cancer stem cells in small cell lung cancer[J]. Transl Lung Cancer Res, 2016, 5(1):16-25.
[12]Tan CS, Gilligan D, Pacey S. Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer[J]. Lancet Oncol, 2015, 16(9):e447-e459.
[13]Han J, Zhao F, Zhang J, et al. miR-223 reverses the resistance of EGFR-TKIs through IGF1R/PI3K/Akt signaling pathway[J]. Int J Oncol, 2016, 48(5):1855-1867.
[14]Freudlsperger C, Burnett JR, Friedman JA, et al. EGFR-PI3K-AKT-mTOR signaling in head and neck squamous cell carcinomas—attractive targets for molecular-oriented therapy[J]. Expert Opin Ther Targets, 2011, 15(1):63-74.
[15]Zhu X, Jiang H, Li J, et al. Anticancer effects of paris saponins by apoptosis and PI3K/AKT pathway in gefitinib-resistant non-small cell lung cancer[J]. Med Sci Monit, 2016, 22:1435-1441.
[16]Yao Y, Dou C, Lu Z, et al. MACC1 suppresses cell apoptosis in hepatocellular carcinoma by targeting the HGF/c-MET/AKT pathway[J]. Cell Physiol Biochem, 2015, 35(3):983-996.
[17]Trovato M, Torre ML, Ragonese M, et al. HGF/c-met system targeting PI3K/AKT and STAT3/phosphorylated-STAT3 pathways in pituitary adenomas: an immunohistochemical characterization in view of targeted therapies[J]. Endocrine, 2013, 44(3):735-743.
[18]Ozasa H, Oguri T, Maeno K, et al. Significance of c-MET overexpression in cytotoxic anticancer drug-resistant small-cell lung cancer cells[J]. Cancer Sci, 2014, 105(8):1032-1039.
(责任编辑: 林白霜, 余小慧)
Imperatorin increased sensitivity of lung cancer CD133+cell subsets to gefitinib by down-regulating c-met expression
WU Qian-bo1,2, ZHANG Min3, TAO Zhi-hua1
(1DepartmentofClinicalLaboratory,TheSecondAffiliatedHospital,MedicalCollegeofZhejiangUniversity,2DepartmentofBloodDonationService,BloodCenterofZhejiangProvince,3DepartmentofClinicalLaboratory,TongdeHospitalofZhejiangProvince,Hangzhou310000,China.E-mail:zhejiangwqb2@163.com)
AIM: To investigate the role of imperatorin in reversing the resistance of the PC9 CD133+cell subsets to gefitinib. METHODS: MTT assay was performed to evaluate the viability of PC9 cells treated with imperatorin and gefitinib. The expression of c-met, activation of caspases and phosphorylation of epidermal growth factor receptor (EGFR), PI3K and AKT in the PC9 cells treated with imperatorin and gefitinib were determined by Western blot. The percentage of CD133+cell subsets population and the apoptotic rate of the PC9 cells treated with imperatorin and gefitinib were analyzed by flow cytometry. RESULTS: The sensitivity of the PC9 CD133+cell subsets to gefitinib was significantly lower than that of the PC9 CD133-cell subsets. Treatment with gefitinib alone significantly inhibited the protein levels of EGFR/PI3K/AKT in the PC9 CD133-cell subsets but not the PC9 CD133+cell subsets. Treatment with gefitinib alone increased the percentage of CD133+cell subsets population in the PC9 cells. However, combination of gefitinib with imperatorin significantly inhibited the enrichment of CD133+cell subsets population. Imperatorin down-regulated c-met expression, suggesting the c-met was the target of imperatorin in the PC9 CD133+cell subsets. The results of MTT assay, Western blot analysis and flow cytometry indicated that imperatorin increased the gefitinib induced inhibition of PI3K/AKT protein levels by down-regulating the expression of c-met, which subsequently induced the cleavage of caspases and apoptosis in the PC9 CD133+cell subsets.CONCLUSION: Imperatorin increases the sensitivity of lung cancer CD133+cell subsets to gefitinib by down-regulating the expression of c-met, and the synergistic anti-tumor effect exists between imperatorin and gefitinib.
Imperatorin; c-met; Gefitinib; PC9 CD133+cell subsets; PI3K/AKT pathway
1000- 4718(2017)01- 0046- 07
2016- 09- 14
2016- 11- 12
R730.23; R735.7
A
10.3969/j.issn.1000- 4718.2017.01.008
杂志网址: http://www.cjpp.net
△通讯作者 Tel: 0571-89972218; E-mail: zhejiangwqb2@163.com
▲并列第1作者

