人巨细胞病毒通过 STAT3上调胶质瘤中endocan表达
王士杰,范东瀛,罗乔荔,邢燕,王一松,安静
首都医科大学基础医学院微生物学教研室,北京 100069
·论著·
人巨细胞病毒通过 STAT3上调胶质瘤中endocan表达
王士杰,范东瀛,罗乔荔,邢燕,王一松,安静
首都医科大学基础医学院微生物学教研室,北京 100069
本研究旨在探讨人巨细胞病毒(human cytomegalovirus,HCMV)和内皮细胞特异性分子1(endocan)在胶质瘤发生发展中的作用及机制。利用HCMV感染人脑胶质瘤U87细胞,检测感染后第1、2、4天信号转导及转录激活蛋白 3(signal transducer and activator of transcription 3,STAT3)和endocan的表达变化,并分析HCMV、STAT3和endocan三者之间的可能关联。结果显示,HCMV感染的U87细胞中pSTAT3和endocan表达上调,并随感染时间延长而逐渐升高;用 RNAi干扰STAT3基因表达,endocan表达量下降;利用抗病毒药物更昔洛韦抑制 HCMV 复制后,pSTAT3表达量下降,endocan表达量随之下降。进一步利用免疫组织化学法检测79例脑胶质瘤患者和8例对照者脑组织中pSTAT3表达情况,发现pSTAT3在胶质瘤组织中表达上调;与低级别胶质瘤相比,高级别胶质瘤中pSTAT3染色强度较高。结果提示,HCMV感染可能通过STAT3信号通路上调endocan表达,从而参与胶质瘤的进展。
胶质瘤;人巨细胞病毒;内皮细胞特异性分子1;信号转导及转录激活蛋白3
胶质瘤是发病率最高的颅内肿瘤,具有高发病率、高复发率、高死亡率及低治愈率的特点。胶质瘤的病因未明,可能与遗传、环境及电离辐射等多种因素有关。近年来,病毒感染与肿瘤发生的关系成为研究热点。2002年,Cobbs 等首次报道在不同级别的胶质瘤组织中人巨细胞病毒(human cytomegalovirus,HCMV)IE1-72抗原和基因检出率高达100%[1]。随后一系列研究表明,HCMV基因序列和表达产物存在于大多数恶性胶质瘤中,但缺少HCMV参与胶质瘤进展的直接证据[2]。
HCMV属疱疹病毒科β亚科,是人疱疹病毒家族中基因组最大的病毒。HCMV在人群中的感染率高达60%~90%,且一旦感染终身携带。已有研究在多种肿瘤组织中检出HCMV相关组分;同时发现HCMV可通过与一些关键信号通路相互作用而参与调节胶质母细胞瘤的恶性表型[3-5]。已知信号转导及转录激活蛋白(signal transducer and activator of transcription, STAT)家族是一组可被多种细胞因子激活的转录因子。STAT3在人体正常组织及细胞中有少许表达,是胚胎发育、分化,特别是神经干细胞和星形胶质细胞发育的重要调节因子[6]。然而,在肿瘤组织及细胞中STAT3异常高表达[6],其组成性激活(constitutive activation)参与肿瘤细胞的恶性侵袭和转移[7]。HCMV是否通过激活STAT3信号通路参与胶质瘤的发生发展,尚待进一步研究。
丰富的新生血管是恶性胶质瘤生长、侵袭和转移的重要病理学基础之一,这一过程受血管生成因子调控。已知人内皮细胞特异性分子1(endocan)是一个相对分子质量为50 000的蛋白聚糖[8]。生理条件下,endocan由血管内皮细胞表达,在健康人血液中仅可微量检出。近年来研究表明,endocan与细胞黏附、炎症反应、肿瘤形成等过程有关[8-10]。炎性细胞因子如肿瘤坏死因子α(tumor necrosis factor α,TNF-α)、促血管生成因子〔如血管内皮生长因子(vascular endothelial growth factor,VEGF)、成纤维细胞生长因子2(fibroblast growth factor 2,FGF-2)、肝细胞生长因子/分裂因子(hepatocyte growth factor/scatter factor,HGF/SF)〕等可促进endocan分泌[11]。另有研究表明,endocan在肿瘤患者的肿瘤组织和血液中表达增加[12-13]。在裸鼠荷瘤模型中,已证明endocan异位表达可促进肿瘤生长[14]。但endocan在胶质瘤进展中的作用尚未见报道。
本课题组前期研究发现,胶质瘤组织中HCMV pp65和endocan表达明显增加,且与胶质瘤的病理级别密切关联[15],但HCMV是否与endocan相互作用及机制不明。为此,本研究通过体外实验探讨HCMV感染对STAT3和endocan表达的影响及三者可能的调控关系,在此基础上,用免疫组织化学方法检测79例脑胶质瘤标本(肿瘤组织)及 8 例正常脑组织标本中pSTAT3的表达水平及其与胶质瘤病理级别的相关性。
1 材料与方法
1.1 临床标本
79 例胶质瘤标本来自首都医科大学附属北京天坛医院神经外科于2007年1月—2013年9月手术的患者,对照脑组织取自脑外伤手术患者。所有病例的苏木精-伊红(hematoxylin-eosin,HE)染色切片均由两位病理科医师读片确认为神经胶质瘤,并根据2007年世界卫生组织(World Health Organization,WHO)中枢神经系统肿瘤分类标准对所有标本核对病理分级,其中低级别胶质瘤(WHO Ⅰ~Ⅱ)36例、高级别胶质瘤(WHO Ⅲ~Ⅳ) 43例。所选病例包括男性48例、女性31例;平均年龄43.8岁(8~78岁)。患者术前未进行任何特殊治疗,也无其他系统肿瘤病史。本研究涉及个体均由本人或其监护人签署知情同意书,同时获得首都医科大学附属北京天坛医院伦理委员会的批准。手术切除的组织标本置于4%多聚甲醛中保存。
1.2 方法
1.2.1 主要试剂 抗-STAT3单克隆抗体(MAB1799)、抗pSTAT3多克隆抗体(AF4607)均购自美国R&D 公司,抗endocan抗体(LIA-1001)购自法国Lunginnov公司。STAT3-RNAi质粒(9077)由上海吉凯基因化学技术有限公司构建,载体为GV248,嘌呤霉素抗性,可在脊椎动物细胞内瞬时或稳定表达。
1.2.2 细胞株及病毒株 人胚肺成纤维细胞MRC-5购于协和细胞资源中心,人胶质瘤细胞系U87由首都医科大学附属北京天坛医院王群教授惠赠,HCMV AD169 株由中国医科大学盛京医院阮强教授惠赠。
1.2.3 细胞感染实验 用HCMV AD169株感染U87细胞〔感染复数(multiplicity of infection,MOI)为3,于37 ℃ 孵育2 h〕,分别于感染后第1、2、4天收取细胞培养上清液,提取RNA,实时定量聚合酶链反应(polymerase chain reaction,PCR)测定HCMV US17基因表达水平,以确定感染是否成功。同时,利用实时定量PCR检测endocan和STAT3,并以β-actin为内参(引物见表1)。同一时间点收取细胞内蛋白,用蛋白免疫印迹法检测endocan、STAT3及pSTAT3表达,并利用ImageJ2x软件进行半定量分析。
表1 PCR使用的引物序列
Tab.1 Primers used in PCR
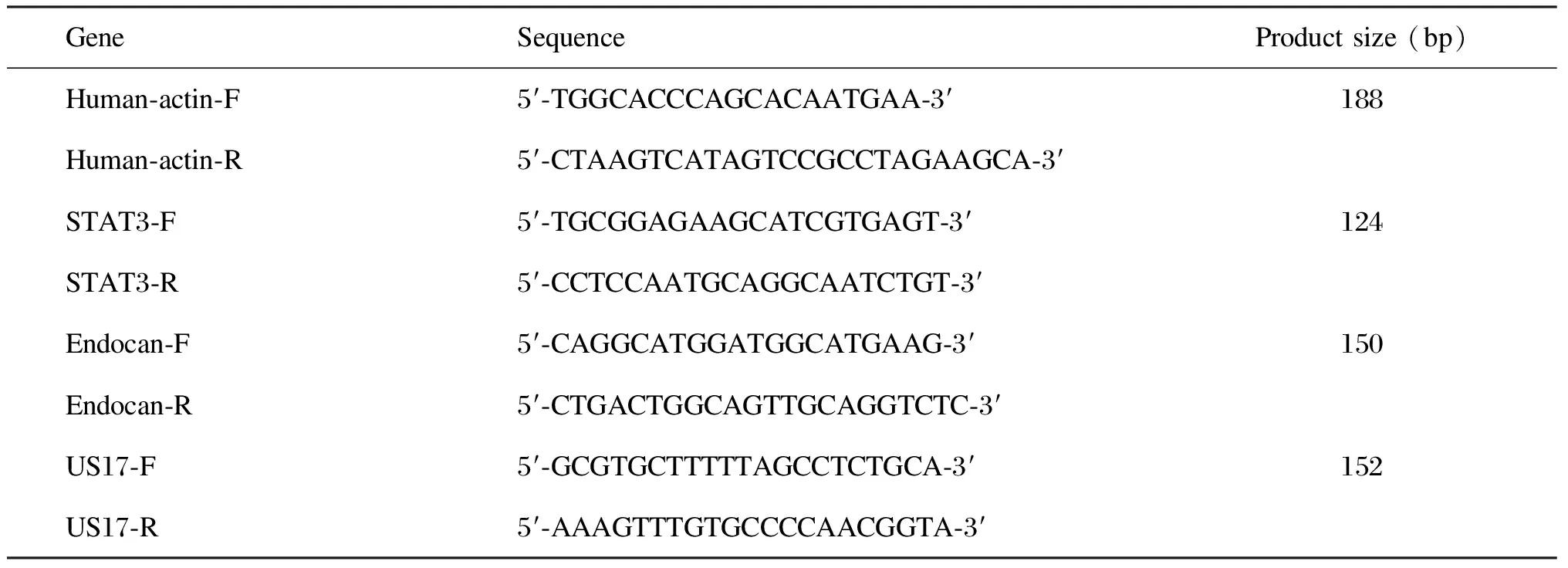
GeneSequenceProductsize(bp)Human⁃actin⁃F5′⁃TGGCACCCAGCACAATGAA⁃3′188Human⁃actin⁃R5′⁃CTAAGTCATAGTCCGCCTAGAAGCA⁃3′STAT3⁃F5′⁃TGCGGAGAAGCATCGTGAGT⁃3′124STAT3⁃R5′⁃CCTCCAATGCAGGCAATCTGT⁃3′Endocan⁃F5′⁃CAGGCATGGATGGCATGAAG⁃3′150Endocan⁃R5′⁃CTGACTGGCAGTTGCAGGTCTC⁃3′US17⁃F5′⁃GCGTGCTTTTTAGCCTCTGCA⁃3′152US17⁃R5′⁃AAAGTTTGTGCCCCAACGGTA⁃3′
1.2.4 STAT3-siRNA细胞株建立 STAT3-RNAi质粒由上海吉凯基因化学技术有限公司构建,包含针对STAT3 mRNA序列的shRNA编码克隆,序列为5′-TGACCAACAATCCCAAGAA-3′。分别提取STAT3-RNAi质粒和对照质粒,脂质体(LipofectamineTM2000)转染U87细胞。用0.5 g/mL嘌呤霉素对细胞进行筛选,获得阳性克隆细胞株,分别命名为U87-STAT3-down和U87-STAT3-control。 用蛋白免疫印迹法及定量PCR检测U87-STAT3-down 细胞株中STAT3的水平变化,并与U87-STAT3-control比较,以确定转染是否成功。
1.2.5 更昔洛韦毒性实验与更昔洛韦抗病毒实验 用四甲基偶氮唑盐(methylthiazolyl tetrazolium,MTT)法检测更昔洛韦对U87细胞的毒性作用,结果显示更昔洛韦在 800 μmol/L 以内无明显细胞毒性作用。同时检测更昔洛韦对HCMV的抑制作用,发现200 μmol/L即可明显抑制HCMV复制,因此选取200 μmol/L为本研究中更昔洛韦的工作浓度。
HCMV感染 2 h后加入更昔洛韦(200 μmol/L)维持 4 d。于感染后第1、2、4天收集细胞,提取 RNA,实时定量PCR检测 U87 细胞中endocan 和STAT3 mRNA表达水平;RIPA裂解法提取总蛋白,蛋白免疫印迹法检测endocan、STAT3及pSTAT3蛋白表达水平。
1.2.6 免疫组织化学染色 石蜡切片厚度为6 μm,采用常规方法进行脱蜡,随后用0.01 mol/L枸橼酸钠缓冲液进行抗原修复,常规一抗(anti-pSTAT3单克隆抗体)、二抗孵育及显色。显微镜下观察染色情况,每张切片随机取5个高倍视野,每个视野计数100个细胞,其中细胞质或细胞核染成黄色或棕色的细胞为阳性细胞。根据切片中阳性细胞的表达强度和阳性细胞数分别评分。染色强度评分如下:无色为0分、浅黄色为1分、黄或棕色为2分、深棕色为3分;阳性细胞数评分如下:<5%为0分、5%~25%为1分、26%~50%为2分、51%~75%为3分、>75%为4分,并将每份标本的两个分值相乘作为该胶质瘤中蛋白表达的相对量化指标,得分≤3分为阴性,3分<得分≤6分为阳性,得分>6分为强阳性。
1.3 统计学处理
采用SPSS 17.0统计软件进行处理。计量资料以mean±SD表示,采用单因素方差分析和t检验;计数资料采用卡方检验。
2 结果
2.1 HCMV感染对U87细胞中endocan和pSTAT3表达的影响
用实时定量PCR检测HCMV感染U87细胞中HCMV US17 mRNA表达情况。结果显示,病毒感染后第1天US17 mRNA表达,变化不明显,第2、4天表达水平明显增加,与第1天相比,分别上升160%和330%,表明HCMV成功感染U87细胞(图1)。
用实时定量PCR检测HCMV感染U87细胞中endocan mRNA表达水平。与对照细胞相比,HCMV 感染后第1 天即可见 endocan mRNA表达水平上升25%,感染后第 2 和 4 天 endocan mRNA表达水平显著升高,分别达270%和400%,与对照细胞相比有显著差异(P<0.05和P<0.01)(图 2A)。未感染HCMV的U87细胞在培养后第1、2和4天endocan mRNA水平无明显变化,与生理条件下endocan mRNA表达极低一致。用蛋白免疫印迹法检测endocan蛋白表达变化,结果与 mRNA 水平变化一致,在HCMV 感染后第 1天变化不显著,第2天达峰值(P<0.01),第4天仍维持在较高水平(P<0.05),分别为对照细胞的1.6倍和1.3倍(图2B)。
用蛋白免疫印迹法检测HCMV感染U87细胞中STAT3及pSTAT3的表达变化, 结果如图3所示,STAT3表达变化趋势与pSTAT3一致。对pSTAT3的分析显示,HCMV 感染 U87 细胞后第1天pSTAT3表达水平即升高1.7倍,与对照细胞相比有显著差异(P<0.01),且明显早于endocan表达上调的时间;感染后第2、4天pSTAT3表达水平仍维持在较高水平,与对照细胞相比分别上调1.5倍和1.3倍。
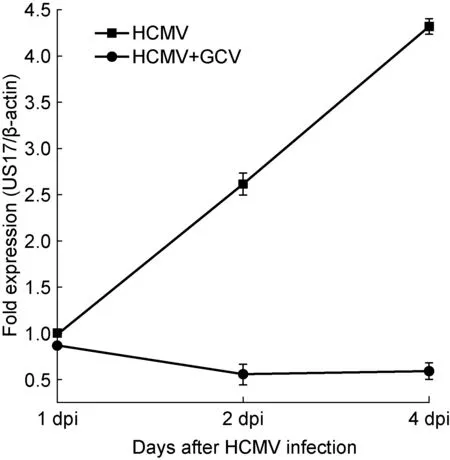
HCMV US17 mRNA expression level in U87 cells was analyzed by real-time PCR. US17 mRNA expression level gradually increased at 1, 2, and 4 d post-infection. The treatment with ganciclovir (GCV) markedly decreased US17 mRNA expression level. Values represent mean±SD from at least three independent experiments. dpi, days post-infection.
图1 HCMV感染U87细胞后HCMV US17基因mRNA水平变化及更昔洛韦处理对其影响
Fig.1 The changes in HCMV US17 mRNA level in HCMV-infected U87 cells treated with or without ganciclovir
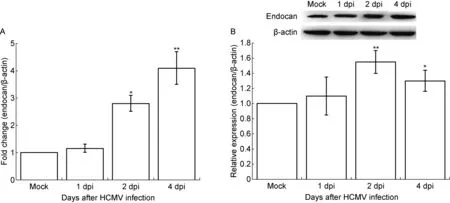
A. Endocan mRNA level in HCMV-infected U87 cells analyzed by real-time PCR. B. Endocan protein level in HCMV-infected U87 cells analyzed by Western blotting. Upper panel: Representative images of endocan expression in HCMV-infected U87 cells. β-actin was used as a loading control. Lower panel: A densitometric analysis of Western blotting results (n=3). Values represent mean±SD from at least three independent experiments.*P<0.05,**P<0.01versusmock group as determined by the one-way analysis of variance. dpi, days post-infection.
图2 HCMV感染U87细胞后endocan mRNA和蛋白表达水平上调
Fig.2 Upregulation of endocan expression level in HCMV-infected U87 cells
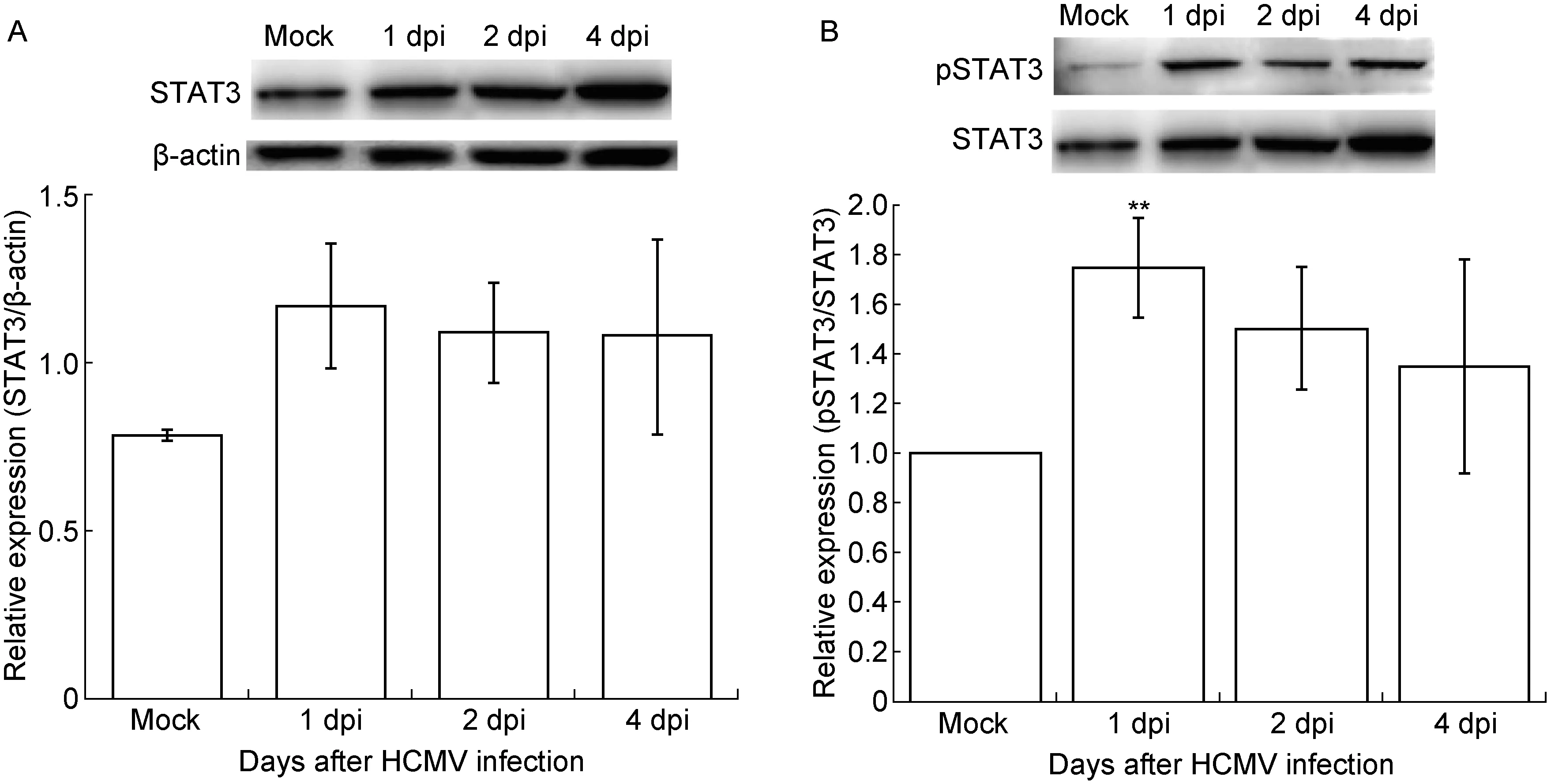
A. STAT3 expression level. Upper panel: Representative images of STAT3 and β-actin expression in HCMV-infected U87 cells. Lower panel: A densitometric analysis of Western blotting results. B. pSTAT3 expression level. Upper panel: Representative images of pSTAT3 and STAT3 expression in HCMV-infected U87 cells. Lower panel: A densitometric analysis of Western blotting results. Values represent mean±SD from at least three independent experiments.*P<0.05,**P<0.01versusmock group as determined by the one-way analysis of variance. dpi, days post-infection.
图3 HCMV感染U87细胞后STAT3和pSTAT3的表达水平
Fig.3 The changes in STAT3 and pSTAT3 expression levels in HCMV-infected U87 cells
2.2 HCMV感染对STAT3下调细胞株中pSTAT3和endocan表达的影响
用STAT3-RNAi质粒和对照质粒分别转染U87细胞,经嘌呤霉素筛选,定量PCR发现STAT3 mRNA 水平与对照细胞(U87-STAT3-control)相比降低17%(P<0.05)(图 4A)。与此相一致,STAT3蛋白表达量降低60%,与对照细胞相比有显著差异(P<0.01)(图 4B)。表明STAT3下调的U87细胞株构建成功,命名为 U87-STAT3-down。
用实时定量PCR检测U87-STAT3-down细胞中endocan mRNA表达水平变化,结果如图 4A所示。与U87-STAT3-control相比,在U87-STAT3-down细胞中STAT3表达下降的同时,endocan mRNA表达水平降低至43%(图4A),蛋白表达水平显示一定的下降趋势(图4B),提示STAT3可能调控endocan的表达。
进一步用蛋白免疫印迹法检测HCMV感染后第2天U87-STAT3-down和U87-STAT3-control细胞中endocan和STAT3蛋白表达水平,结果显示两种蛋白表达水平明显低于感染对照组,分别为对照组的80% 和43%,与感染的STAT3-control细胞相比具有显著差异(P<0.05)(图5)。感染并未引起U87-STAT3-down细胞中STAT3和endocan蛋白上调,提示STAT3下调可能阻断HCMV感染引起的endocan上调。
2.3 更昔洛韦对U87细胞中endocan和pSTAT3表达的影响
在HCMV感染后2 h,加入200 μmol/L更昔洛韦处理感染细胞,并维持至所观察的时间点。结果显示,处理后细胞培养上清液中US17 mRNA表达水平较HCMV感染组明显下降,表明更昔洛韦能有效抑制 HCMV 复制(图 1)。利用实时定量PCR检测STAT3和endocan mRNA转录水平,结果显示,在HCMV感染后第1、2、4天,更昔洛韦处理组STAT3和endocan mRNA表达水平均下调(P<0.05,P<0.01)(图6A)。与此相一致,pSTAT3和endocan蛋白表达水平也呈现不同程度下降。更昔洛韦处理组endocan蛋白表达量在感染后第1天降低至HCMV感染组的85%,在感染第2、4天下降更显著,分别降至40%和50%(P<0.01);pSTAT3蛋白表达量明显下降出现在HCMV感染后第1、2天(P<0.05),第4天略有恢复,降至HCMV感染组的85%(图6B)。
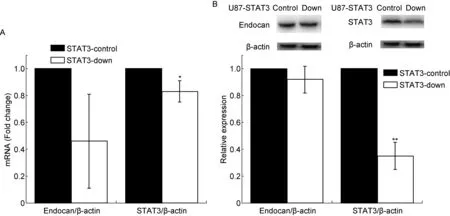
A: Expression levels of endocan and STAT3 mRNA in U87-STAT3-down cells analyzed by real-time PCR. RNAi-STAT3 downregulated endocan mRNA level. B: Expression levels of endocan and STAT3 proteins in U87-STAT3-down cells analyzed by Western blotting. Downregulated endocan and STAT3 were observed in U87-STAT3-down cells. Upper panel: Representative images of endocan and STAT3 expression in U87-STAT3-down cells. β-actin was used as a loading control. Lower panel: A densitometric analysis of Western blotting results (n=3). Values represent mean±SD from at least three independent experiments.*P<0.05,**P<0.01versusSTAT3-control group as determined by the one-way analysis of variance. dpi, days post-infection.
图4 U87-STAT3-down细胞的鉴定及endocan和STAT3的表达变化
Fig.4 Identification of U87-STAT3-down cell line and changes in endocan and STAT3 expression levels
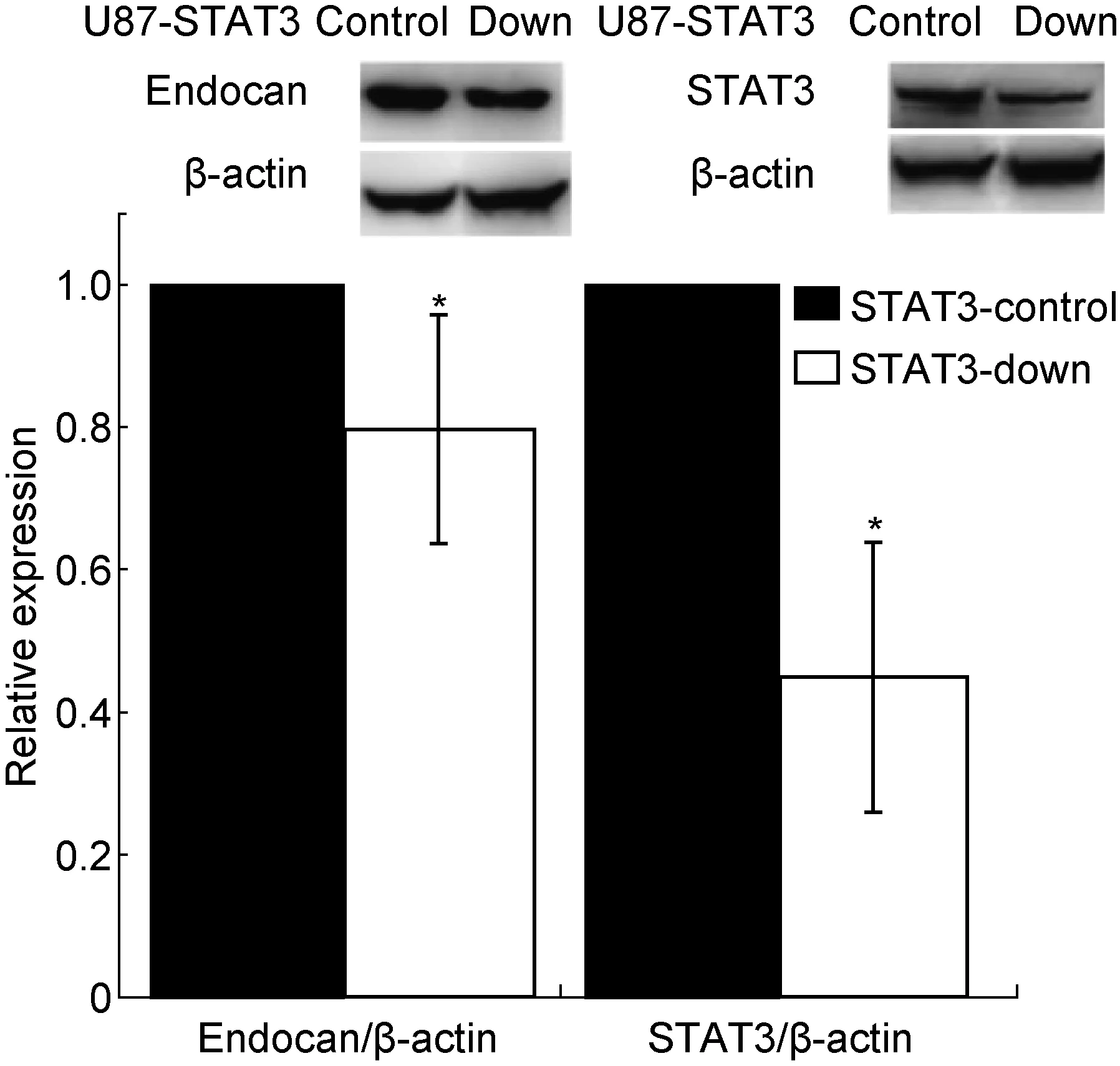
The expression levels of endocan and STAT3 in U87-STAT3-down cells at 2 d post-infection were analyzed by Western blotting. The expression levels of endocan and STAT3 were downregulated in U87-STAT3-down cells. Upper panel: Representative images of endocan and STAT3 expression in U87-STAT3-down cells. β-actin was used as a loading control. Lower panel: A densitometric analysis of Western blotting results (n=3). Values represent mean±SD from at least three independent experiments.*P<0.05,**P<0.01versusSTAT3-control group as determined by the one-way analysis of variance. dpi, days post-infection.
图5 HCMV感染后第2天U87-STAT3-down细胞中endocan和STAT3的表达变化
Fig.5 The changes in endocan and STAT3 expression levels in U87-STAT3-down cells at 2 d post-infection
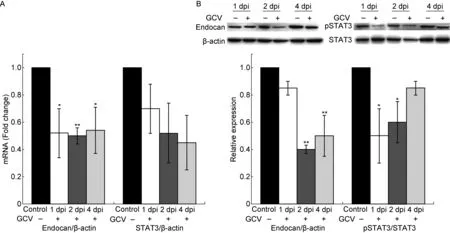
A: Endocan and STAT3 mRNA expression levels determined at 1, 2 and 4 dpi in HCMV-infected U87 cells treated with GCV (+) or DMSO as a control (-). B: Western blotting analysis of pSTAT3, STAT3 and endocan in HCMV-infected U87 cells treated with GCV (+) or DMSO as a control (-) at 1, 2 and 4 dpi. Upper panel: Representative images of pSTAT3, STAT3 and endocan expression in HCMV-infected U87 cells with or without GCV treatment. β-actin was used as a loading control. Lower panel: A densitometric analysis of Western blotting results. Values represent mean±SD from at least three independent experiments.*P<0.05,**P<0.01versuscontrol group as determined by the one-way analysis of variance. dpi, days post-infection.
图6 更昔洛韦处理对HCMV感染的U87细胞中endocan 和 STAT3表达水平的影响
Fig.6 The changes in endocan and pSTAT3 expression levels in HCMV-infected U87 cell after treatment with ganciclovir
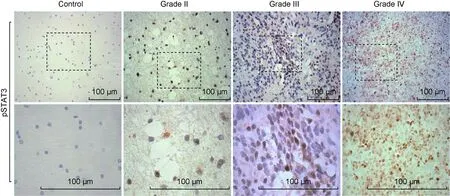
The expression level of pSTAT3 protein in gliomas was detected by IHC. Representative images were shown at low (upper panel) or high (lower panel) magnification. The positive staining of pSTAT3 was predominantly observed in the cytoplasm of tumor cells. The strong intensity of the staining was closely associated with high grade of glioma (grades III and IV). No expression of pSTAT3 was detected in the control brain tissues. Scale bar, 100 μm.
图7 免疫组织化学法检测胶质瘤和对照脑组织中pSTAT3蛋白的表达
Fig.7 The expression level of pSTAT3 in gliomas and control brain tissues
2.4 pSTAT3蛋白在胶质瘤脑组织中的表达
用免疫组织化学法对79例胶质瘤石蜡标本中的pSTAT3表达情况进行检测。结果显示,对照脑组织中仅有微量pSTAT3表达,而胶质瘤标本中pSTAT3表达水平明显增加,主要分布于肿瘤细胞的细胞核内,细胞质中可见少许pSTAT3阳性反应。与低级别胶质瘤相比,高级别胶质瘤中pSTAT3染色强度较高(图7)。
3 讨论
恶性胶质瘤是神经系统肿瘤中发病率最高的颅内肿瘤,预后不良。其发病原因不明,可能与多种因素有关,如遗传、环境因素、病毒感染等。自2002年Cobbs首次报道HCMV在100%的胶质瘤组织中被检出后,HCMV在胶质瘤发生中的作用和机制倍受关注。本课题组在前期研究中发现,胶质瘤组织中HCMV pp65和endocan表达明显增加,且与胶质瘤的病理级别成正比[15],支持HCMV参与胶质瘤发生的观点,且可能与endocan密切关联,但分子机制尚不清楚。已知STAT3信号通路是调控细胞生长、分化等多种功能的关键分子,其磷酸化后转位入核,参与调控细胞存活、分化、增殖,血管生成和免疫功能的多种基因表达[16],与多种细胞生物学时间密切相关。有趣的是,Cobbs等研究发现HCMV US28蛋白在神经前细胞中可诱导STAT3活化,因而推测HCMV、STAT3、endocan三者之间可能存在某种关联。为验证此推论,本研究利用HCMV感染胶质瘤U87细胞,发现HCMV 感染可诱导U87细胞中endocan mRNA和蛋白表达水平上调;利用RNAi下调U87细胞中STAT3表达(U87-STAT3-down)后,endocan表达呈现明显下降,因此利用RNAi下调STAT3表达,可阻断HCMV感染引起的endocan水平上调。进一步加入更昔洛韦抑制HCMV复制,U87细胞中endocan mRNA和蛋白表达水平下降,同时pSTAT3表达下调。以上结果提示,HCMV感染可能通过STAT3上调endocan表达参与胶质瘤的进展。进一步利用胶质瘤组织标本检测pSTAT3(活性STAT3)的表达情况,发现pSTAT3在胶质瘤组织中高表达,且表达强度与肿瘤级别相关联。
新生血管生成是肿瘤生长与侵袭的关键步骤,受许多血管生成因子的调控。其中VEGF已被证实在胶质瘤等多种恶性肿瘤的发生发展中起重要作用,其单克隆抗体贝伐单抗已成为一些恶性肿瘤的主要辅助治疗手段,但仍有一定的局限性。Endocan于1996年首次被发现,由血管内皮细胞分泌,是血管生成的重要调控分子。近年来,国内外发表了诸多关于endocan作为肿瘤标记的研究,相继报道了人类不同组织中endocan表达及其与肿瘤恶性程度的关系,指出乳头状癌、肾透明细胞癌、肝癌、胃癌、大肠癌等肿瘤组织中endocan表达量明显高于正常对照组,且endocan在肿瘤组织中的高表达与预后不良密切相关[9,17-20]。国内外大量研究充分表明,endocan介导新生血管形成和癌症进展,可能是肿瘤发生发展的一个促进因素。HCMV参与胶质瘤发生发展的过程可能是复杂的,尚需进一步深入阐明。但更多的研究认为,病毒蛋白通过与宿主相互作用,引发炎症和激活关键的信号通路,可能在肿瘤发生中起重要作用。本研究发现, HCMV 感染能通过激活STAT3上调胶质瘤细胞中endocan表达,但有关HCMV参与胶质瘤发生的机制尚待进一步研究。
[1] Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, Nabors LB, Cobbs CG, Britt WJ. Human cytomegalovirus infection and expression in human malignant glioma [J]. Cancer Res, 2002, 62(12): 3347-3350.[2] Lawler SE. Cytomegalovirus and glioblastoma; controversies and opportunities [J]. J Neurooncol, 2015, 123(3): 465-471.
[3] Cobbs CS, Soroceanu L, Denham S, Zhang W, Britt WJ, Pieper R, Kraus MH. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness [J]. J Neurooncol, 2007, 85(3): 271-280.
[4] Cobbs CS, Soroceanu L, Denham S, Zhang W, Kraus MH. Modulation of oncogenic phenotype in human glioma cells by cytomegalovirus IE1-mediated mitogenicity [J]. Cancer Res, 2008, 68(3): 724-730.
[5] Slinger E, Maussang D, Schreiber A, Siderius M, Rahbar A, Fraile-Ramos A, Lira SA, Söderberg-Nauclér C, Smit MJ. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis [J]. Sci Signal, 2010, 3(133): ra58. doi: 10.1126/scisignal.2001180.
[6] Yoshimatsu T, Kawaguchi D, Oishi K, Takeda K, Akira S, Masuyama N, Gotoh Y. Non-cell-autonomous action of STAT3 in maintenance of neural precursor cells in the mouse neocortex [J]. Development, 2006, 133(13): 2553-2563.
[7] Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances [J]. Biomed Res Int, 2013, 2013: 421821. doi: 10.1155/2013/421821.
[8] Béchard D, Gentina T, Delehedde M, Scherpereel A, Lyon M, Aumercier M, Vazeux R, Richet C, Degand P, Jude B, Janin A, Fernig DG, Tonnel AB, Lassalle P. Endocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activity [J]. J Biol Chem, 2001, 276(51): 48341-48349.
[9] Chen LY, Liu X, Wang SL, Qin CY. Over-expression of the endocan gene in endothelial cells from hepatocellular carcinoma is associated with angiogenesis and tumour invasion [J]. J Int Med Res, 2010, 38(2): 498-510.
[10] Kang YH, Ji NY, Lee CI, Lee HG, Kim JW, Yeom YI, Kim DG, Yoon SK, Kim JW, Park PJ, Song EY. ESM-1 silencing decreased cell survival, migration, and invasion and modulated cell cycle progression in hepatocellular carcinoma [J]. Amino Acids, 2011, 40(3): 1003-1013.
[11] Gerritsen ME, Tomlinson JE, Zlot C, Ziman M, Hwang S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells [J]. Br J Pharmacol, 2003, 140(4): 595-610.
[12] Hatfield KJ, Lassalle P, Leiva RA, Lindås R, Wendelboe Ø, Bruserud Ø. Serum levels of endothelium-derived endocan are increased in patients with untreated acute myeloid leukemia [J]. Hematology, 2011, 16(6): 351-356.
[13] Roudnicky F, Poyet C, Wild P, Krampitz S, Negrini F, Huggenberger R, Rogler A, Stöhr R, Hartmann A, Provenzano M, Otto VI, Detmar M. Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis [J]. Cancer Res, 2013, 73(3): 1097-1106.
[14] Scherpereel A, Gentina T, Grigoriu B, Sénéchal S, Janin A, Tsicopoulos A, Plénat F, Béchard D, Tonnel AB, Lassalle P. Overexpression of endocan induces tumor formation [J]. Cancer Res, 2003, 63(18): 6084-6089.
[15] Xing Y, Wang Y, Wang S, Wang X, Fan D, Zhou D, An J. Human cytomegalovirus infection contributes to glioma disease progression via upregulating endocan expression [J]. Transl Res, 2016, 177: 113-126.
[16] Gray GK, McFarland BC, Nozell SE, Benveniste EN. NF-kappa B and STAT3 in glioblastoma: therapeutic targets coming of age [J]. Expert Rev Neurother, 2014, 14(11): 1293-1306.
[17] Soroceanu L, Cobbs CS. Is HCMV a tumor promoter? [J]. Virus Res, 2011, 157(2): 193-203.
[18] Ji NY, Kim YH, Jang YJ, Kang YH, Lee CI, Kim JW, Yeom YI, Chun HK, Choi YH, Kim JH, Kim JW, Lee HG, Song EY. Identification of endothelial cell-specific molecule-1 as a potential serum marker for colorectal cancer [J]. Cancer Sci, 2010, 101(10): 2248-2253.
[19] Liu N, Zhang LH, Du H, Hu Y, Zhang GG, Wang XH, Li JY, Ji JF. Overexpression of endothelial cell specific molecule-1 (ESM-1) in gastric cancer [J]. Ann Surg Oncol, 2010, 17(10): 2628-2639.
[20] Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, Califano A, Iavarone A. The transcriptional network for mesenchymal transformation of brain tumours [J]. Nature, 2010, 463(7279): 318-325.
s. AN Jing, E-mail: anjing@ccmu.edu.cn; WANG Yisong, E-mail: yswang@ccmu.edu.cn
Human cytomegalovirus infection is involved in STAT3-upregulated endocan expression in gliomas
WANG Shijie, FAN Dongying, LUO Qiaoli, XING Yan, WANG Yisong, AN Jing
DepartmentofMicrobiology,SchoolofBasicMedicalSciences,CapitalMedicalUniversity,Beijing100069,China
The aim of this study is to investigate the role of human cytomegalovirus (HCMV) infection in glioma pathogenesis and its possible mechanism. Based on the previous experiments, the expressions of signal transducer and activator of transcription 3 (STAT3) and endocan in HCMV-infected U87 cells, a human glioma cell line, were detected, and the relationship among HCMV, STAT3 and endocan was analyzed. The results showed that HCMV infection induced the upregulation of endocan mRNA and protein as well as the activated STAT3 in U87 cells. On the other hand, decreased levels of endocan mRNA and protein were observed in U87-STAT3-knockdown cells by RNAi. Furthermore, downregulated levels of endocan mRNA and protein were found in U87 cells treated by ganciclovir, an anti-viral drug. Then the expression of pSTAT3 in 79 glioma specimens and 8 control brain tissues was detected by immunohistochemistry (IHC) and the relationship between pSTAT3 level and glioma grade was analyzed. Compared with the control brain samples, increased level of pSTAT3 was found in glioma specimens and the staining intensity was related to glioma grade. The results suggest that HCMV infection is involved in glioma pathogenesis via upregulating endocan expression through STAT3 signaling pathway, and further provide some new clues for the treatment of glioma.
Glioma; Human cytomegalovirus; Endocan; Signal transducer and activator of transcription 3
国家自然科学基金(81271839、81471957),北京市教育委员会科技计划一般项目(KM201610025001),第48批教育部留学回国人员科研启动基金,首都医科大学自然基金(2015ZR11)
安静,王一松
2016-08-10)

