Sheep oocyte expresses leptin and functional leptin receptor mRNA
Seyyed Jalil Taheri, Abbas Parham,2*
1Division of Physiology, Department of Basic Sciences, Veterinary Faculty, Ferdowsi University of Mashhad, Mashhad, Iran
2Embryonic and Stem Cell Biology and Biotechnology Research Group, Institute of Biotechnology, Ferdowsi University of Mashhad, Mashhad, Iran
Sheep oocyte expresses leptin and functional leptin receptor mRNA
Seyyed Jalil Taheri1, Abbas Parham1,2*
1Division of Physiology, Department of Basic Sciences, Veterinary Faculty, Ferdowsi University of Mashhad, Mashhad, Iran
2Embryonic and Stem Cell Biology and Biotechnology Research Group, Institute of Biotechnology, Ferdowsi University of Mashhad, Mashhad, Iran
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Leptin
Leptin receptor
Sheep
Oocyte
Objective:To investigate the expression of leptin and its functional receptor mRNA by reverse transcription-polymerase chain reaction (RT-PCR) analysis in sheep oocytes.Methods:Sheep ovaries were collected in reproductive season from abattoir and immediately were transported to the lab. Cumulusoocyte complexes were aspirated from antral follicles and were denuded to obtain denuded oocytes. Total RNA of denuded oocytes was extracted, cDNA synthesis was carried out subsequently and cDNA was subjected to PCR amplification using specific designed primers.Results:Gel electrophoresis of PCR products showed the amplification of 162 and 121-bp amplicons for leptin and functional leptin receptor respectively. Sequencing the amplified fragments also confirmed the results.Conclusions:The result of present study reveals that leptin and its functional receptor (Ob-Rb) mRNA are expressed in sheep oocyte and further studies should investigate the role(s) of leptin on sheep oocyte physiology and embryo development.
1. Introduction
Leptin, a 167-amino acid hormone, is secreted mainly by fat tissue[1]. Leptin belongs to helical cytokines class 1 which affect food intake, energy expenditure and reproductive functions [2]. Physiological effects of leptin on reproduction including puberty, estrous cycle, pregnancy, lactation, and even the early stages of embryo development have been proven[3].
It is known that leptin and its receptor in addition to adipose tissue, express in other tissues which confirm role of leptin in various cells, organs and systems [4]. Several forms of the leptin receptor have been identified including several short isoforms (Ob-Ra, c, d and e) and a long functional isoform, Ob-Rb. It is thought that only the long form of the receptor (Ob-Rb) is able to activate the JAK-STAT signaling pathway and is responsible for most of the biological effects of leptin[5]. Leptin and Ob-Rb are expressed in the goat oocyte and follicular cells[6], sheep granulosa and theca cells[7], human follicles[8], bovine testis[9] and bovine ovary[10]. Nevertheless, the expression of leptin and Ob-Rb in ovine oocyte has not yet been reported.
It seems that leptin is essential for the maturation of the reproductive axis, because it has the ability to induce puberty and restores fertility in ob/ob mice[11]. Leptin stimulates endocrine system of reproduction in both sexes and is a signal to the reproductive system in normal animals[12]. In addition to secretion control of gonadotropins through the axis hypothalamus–pituitary, leptin has a direct effect on the function of gonads. Source of leptin for this effect could be blood or their organs and tissues. Due to presence of leptin receptors on the surface of reproductive organs,autocrine/paracrine effects of leptin is probable [2].
Leptin participates in the regulation of ovarian folliculogenesis by influencing the proliferation of granulosa cells, steroidogenesis and apoptosis [13]. In human, leptin secretion caused monthly period and is required at certain amount for maintenance of natural cycle and the appearance of oocyte in ovaries [14]. Moreover, the beneficial effects of leptin on oocyte maturation and embryo development have also been observed [15,16]. As a first step towards understanding the effects of leptin in sheep oocyte physiology, it is necessary to demonstrate the expression of leptin and its functional receptor in oocyte. The objective of this study was to determine whether leptin and Ob-Rb mRNA is expressed in the sheep oocyte.
2. Materials and methods
2.1. Collection of oocytes
Ovaries from adult sheep (Kurdish breed) were obtained from a local slaughterhouse (Mashhad, Iran) in reproductive season and transported to the laboratory in saline solution at 37.5 ℃. Ovaries were used to obtain cumulus-oocyte complexes (COCs) by aspiration of antral follicles. After collecting the COCs, the cumulus cells were separated by treating COCs with sodium citrate 3% for 5 min, vortex and pipetting. Then denuded oocytes (DOs) were observed under a stereomicroscope to confirm deletion of cumulus cells. DOs were immediately subjected to total RNA extraction.
2.2. RNA extraction
Total RNA was extracted from oocyte pool using High Pure RNA Isolation Kit (Roche, Germany) according to the manufacturer’s instructions. To delete any probable genomic DNA contamination, DNase treatment was done during RNA extraction protocol based on manufacturer’s instructions. Total extracted RNA was quantified by a Nanodrop Spectrophotometer and quality of RNA was determined using gel electrophoresis. Only appropriate samples were selected for next stages.
2.3. Reverse transcription - polymerase chain reaction (RTPCR)
For each sample, 1 µg of total RNA was reverse transcribed (RT) into complementary DNA (cDNA) using the AccuPower RT-PCR PreMix (Bioneer, South Korea) according to the manufacturer’s instructions.
PCR reactions were performed using Taq DNA Polymerase 2x Master Mix RED (Amplicon, Denmark) and specific designed primers of beta-actin, leptin and functional leptin receptor (Table 1). The forward and reverse functional leptin receptor primer pairs were designed to span the junction of two exons to be RNA specific. So, the amplification of the cDNA and DNA resulted in the different PCR products in length. One microliter of each RT reaction product was used as template for PCR reactions in a final volume of 25 µL with 12.5 µL Master Mix of 'Amplicon', 10.5 µL distilled water, 0.5 µL of forward and 0.5 µL of reverse primer. The following amplification conditions were utilized: 1 cycle 94 ℃ for 5 min, followed by 40 cycles of denaturing at 94 ℃ for 45 s, annealing at 58 ℃ (for beta-actin), 50 ℃ (for leptin), 57.4 ℃ (for leptin receptor), and extension at 72 ℃ for 45 s. The program was terminated with a final extension step at 72 ℃ for 10 min. Beta-actin (a housekeeping gene) was used as positive internal controls for all samples to verify accuracy of RT-PCR reactions.
As the expression of leptin and functional leptin receptor has been previously shown in ovine fat tissue [17], fat tissue was used as positive control. Negative control reactions were also performed similarly with addition of 1 µL distilled water instead of template. All PCR products were run on a 1.5% agarose gel in Tris-acetate EDTA buffer, stained with ethidium bromide and visualized under UV transillumination. The size of the predicted products was confirmed using a 100 bp standard molecular ladder. In addition, PCR products of amplified fragments of three genes were sequenced (Macrogen, South Korea), and the results were edited by CLC bio, and finally checked in NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to verify the results.
3. Results
The sheep beta actin was selected as an internal positive control. So, the amplification of beta-actin was first assessed in all samples. Amplification of beta-actin from cDNA resulted in only 277-bpproduct, while amplification of bet-actin DNA produced 274 and 366-bp products. Detection of only 277-bp fragment at samples showed proper RNA purification and RT-PCR conditions along with no genomic contamination (Figure 1).
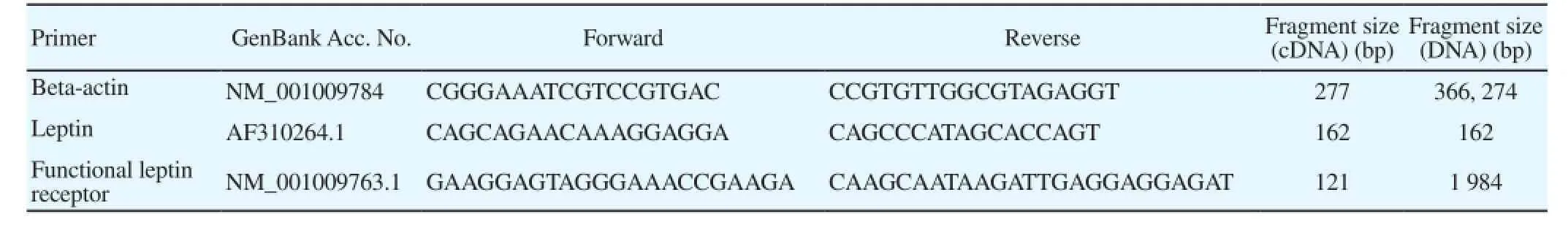
Table 1Primers sets which were used in PCR reactions.
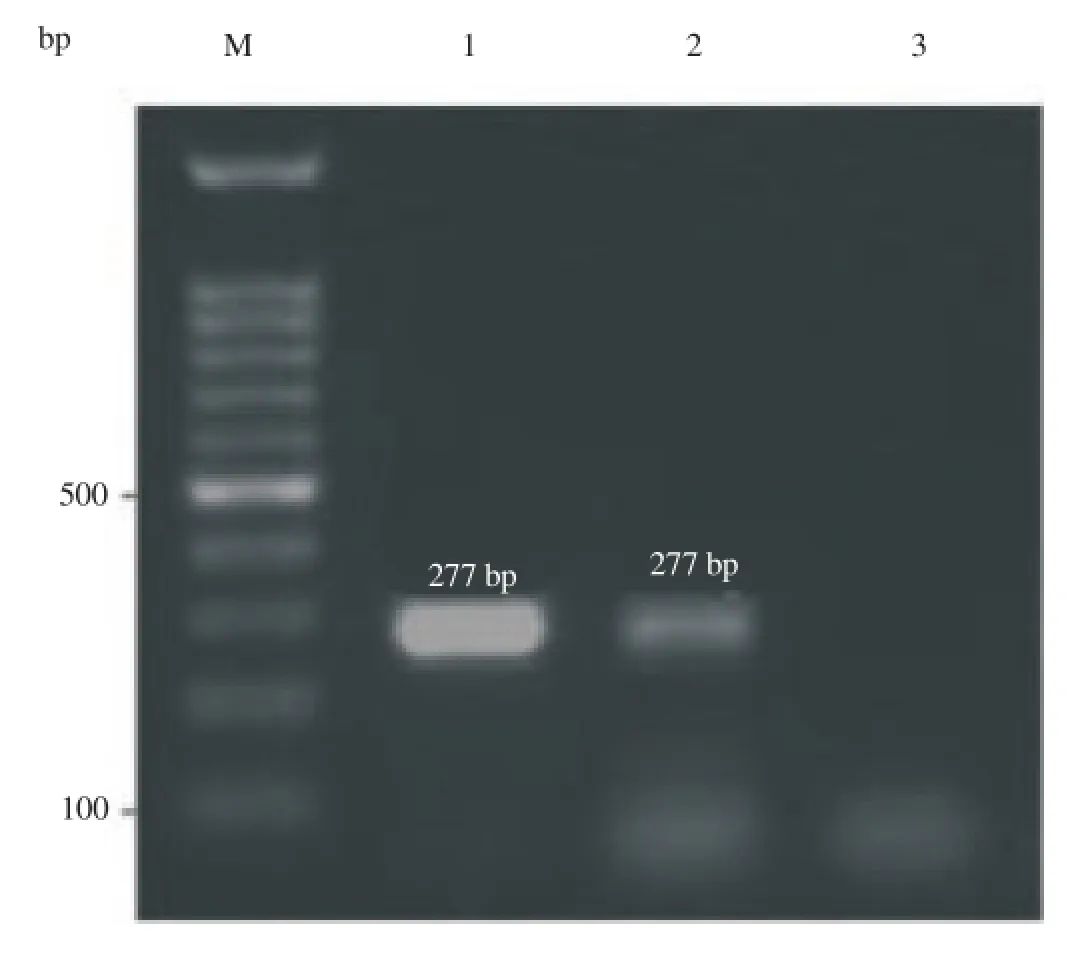
Figure 1.Beta actin was used as positive internal controls for all samples to verify that the RT-PCR reactions were successful, and representative agarose gel demonstrated amplification of only 277-bp sheep beta actin cDNA from fat (lane 1) and oocyte (lane 2). Lane 3 was negative control, and M was 100-bp DNA marker.
3.1. Expression of leptin mRNA in sheep oocyte
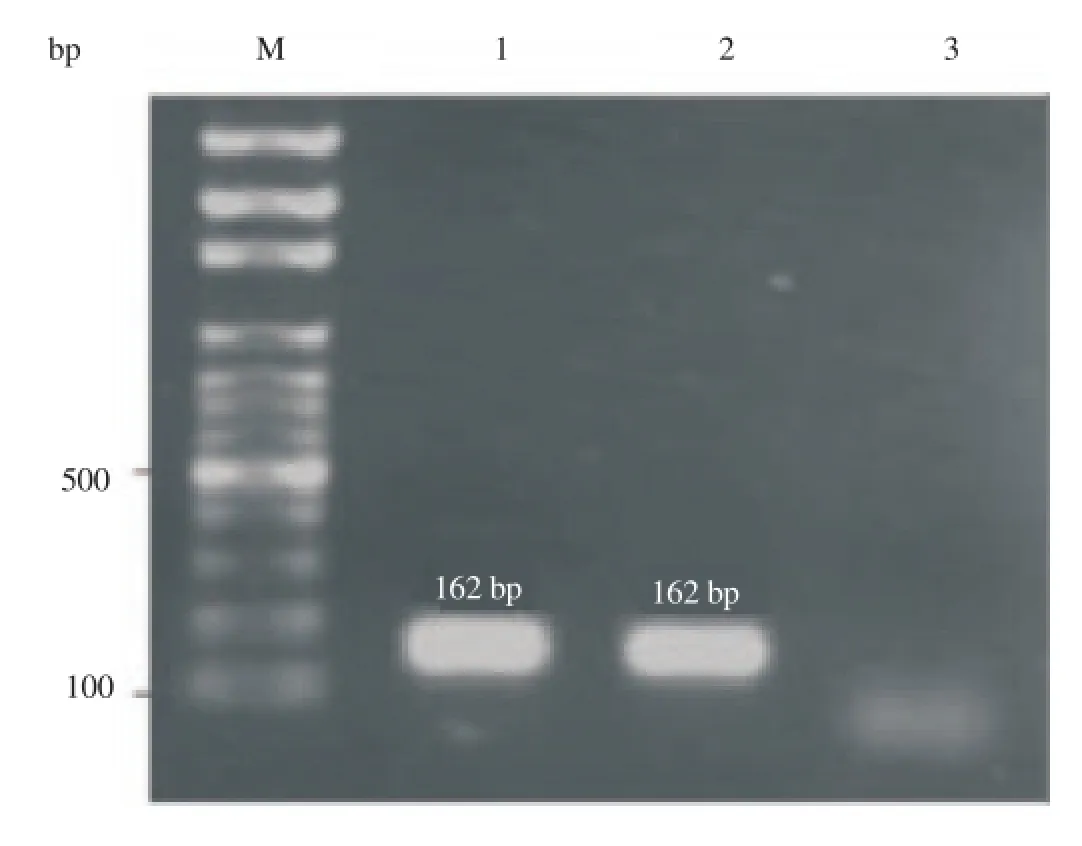
Figure 2.Expression of sheep leptin mRNA was analyzed by reverse
Gel electrophoresis results showed that the predicted PCR product fragment (162 bp) of leptin has been amplified in both oocyte and adipose tissue (positive control) (Figure 2). Thus, the RT-PCR analysis confirmed that leptin mRNA is expressed in the sheep oocyte. transcription PCR (RT-PCR) of RNA isolated from oocytes. Specified fragments (162 bp) were amplified by RT-PCR using leptin primer pairs from fat (lane 1) and oocyte (lane 2). Lane 3 was negative control. The sizes of the generated products were calculated by comparison with the mobility of 100 bp DNA step ladder (M).
3.2. Expression of functional leptin receptor (Ob-Rb) mRNA in sheep oocyte
Observation of predicted amplified fragment of 121 bp on gel electrophoresis using leptin receptor primer pairs confirmed that functional leptin receptor mRNA is expressed in the sheep oocyte (Figure 3). As mentioned before, the forward and reverse functional leptin receptor primer pairs were designed on two exons and no amplification from DNA was observed on gel, which confirms there was no DNA genomic contamination. Adipose tissue was used as positive control for the expression of Ob-Rb mRNA.
Finally, sequencing the amplified fragments of three genes and their blast in NCBI verified the results (Figure 4).

Figure 3.Expression of sheep functional leptin receptor (Ob-Rb) mRNA was analyzed by reverse transcription PCR (RT-PCR). Amplified specific fragments (121 bp) of leptin receptor cDNA by RT-PCR are seen from fat (lane 1) and oocyte (lane 2). Lane 3 was negative control. The sizes of the generated products were calculated by comparison with the mobility of 100 bp DNA step ladder (M). No amplification of leptin receptor DNA (1 984 bp) was observed on agarose gel which confirms the absence of DNA genomic contamination.
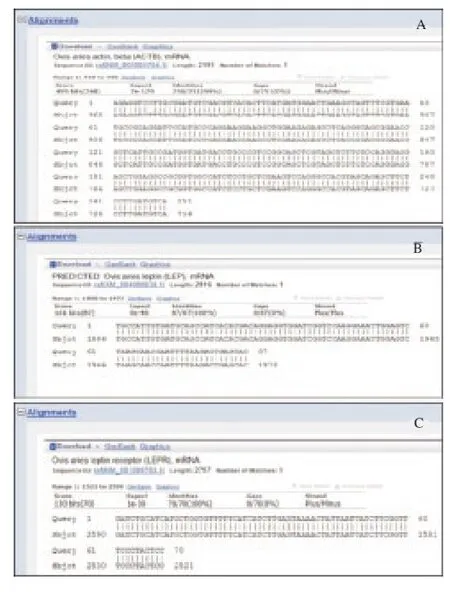
Figure 4.The amplified and sequenced fragments were aligned with their recognized sequences on NCBI using BLAST. "Query" is represented for questionable sequences (amplified fragment) and "sbjct" shows the gene sequence in NCBI. A: beta-actin; B; leptin; C: leptin receptor.
4. Discussion
The first step in understanding the physiology a cell such as oocyte is the identification of its expressed genes. To our knowledge, in this study for the first time we succeed to show that leptin and its functional receptor (Ob-Rb) mRNA is expressed in sheep oocyte, which is in agreement with results from other species such as mouse [18] and goat [6]. These findings show the presence of a leptin signaling system in sheep oocyte which may influence oocyte maturation in an autocrine/paracrine system.
Leptin plays an important role in reproduction and it is necessary for maintaining normal reproductive capacity [19]. Mice lacking leptin (ob/ob) are obese and infertile, but administration of exogenous leptin restores fertility in these animals [11]. The presence of leptin receptors on ovarian granulosa cells, theca cells and corpus luteum of some species have confirmed the direct effect of leptin on the ovaries [14,20].
Leptin have been detected in follicular fluid [21], in oviductal and uterine fluids during early pregnancy [22] which is available for the oocyte and embryo. Furthermore, intrafollicular leptin concentrations have been shown to be a good predictor of oocyte fertilization [23] and embryo quality [24]. The leptin system is also involved in embryo development and implantation [25]. A minimum level of leptin is required for maintenance of reproductive function in human [26]. Decrease in leptin concentration may have direct or indirect effects on oocyte or embryo quality, resulting in early embryonic mortality as a major reason of reproductive failure in cattle [27]. In contrast, high leptin concentrations have been associated with obesity and polycystic ovarian syndrome (PCOS) in women which reveals a link between metabolic disorders and low fertility [28].
Expression of leptin and/or leptin receptor in bovine granulosa and cumulus cells [29], mouse oocytes [18] and bovine blastocysts [15], suggesting that both oocytes and preimplantation embryos could be sensitive to leptin. It is well known that oocyte developmental potential is a reflection of suitable nuclear and cytoplasmic maturation. There is evidence that leptin stimulates oocyte maturation and its competence for fertilization [16,29,30].
In conclusion, this study revealed that leptin and leptin functional receptor (Ob-Rb) mRNA is expressed in sheep oocyte. So, this finding along with evidence regarding regulatory effects of leptin on oocyte maturation in other species are guaranteed further studies on the role(s) of leptin on sheep oocyte maturation and its competence for fertilization.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgments
This work was financially supported by Ferdowsi University of Mashhad.
[1] Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue.Nature1994;372(6505): 425-432.
[2] Baratta M. Leptin--from a signal of adiposity to a hormonal mediator in peripheral tissues.Med Sci Monitor2002;8(12): RA282-RA292.
[3] Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: A review.Fertil Steril2002;77(3): 433-444.
[4] Chilliard Y, Bonnet M, Delavaud C, Faulconnier Y, Leroux C, Djiane J, etal. Leptin in ruminants. Gene expression in adipose tissue and mammary gland, and regulation of plasma concentration.Dom Anim Endocrinol2001;21(4): 271-295.
[5] Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor, OB-R.Cell1995;83(7): 1263-1271.
[6] Batista A, Silva D, Rêgo M, Silva F, Silva E, Beltrão E, et al. The expression and localization of leptin and its receptor in goat ovarian follicles.Anim Reprod Sci2013;141(3): 142-147.
[7] Munoz-Gutierrez M, Findlay P, Adam C, Wax G, Campbell B, Kendall N, et al. The ovarian expression of mRNAs for aromatase, IGF-I receptor, IGF-binding protein-2,-4 and-5, leptin and leptin receptor in cycling ewes after three days of leptin infusion.Reproduction2005;130(6): 869-881.
[8] Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR. The expression of leptin and its receptors in pre-ovulatory human follicles.Mol Hum Reprod1997;3(6): 467-472.
[9] Abavisani A, Baghbanzadeh A, Shayan P, Tajik P, Dehghani H, Mirtorabi M. Leptin mRNA expresses in the bull reproductive organ.Vet Res Commun2009;33(8): 823-830.
[10] Sarkar M, Schilffarth S, Schams D, Meyer HH, Berisha B. The expression of leptin and its receptor during different physiological stages in the bovine ovary.Mol reprod Dev2010;77(2): 174-181.
[11] Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin.Nat Genet1996;12(3): 318-320.
[12] Williams G, Amstalden M, Garcia M, Stanko R, Nizielski S, Morrison C, et al. Leptin and its role in the central regulation of reproduction in cattle.Dom Anim Endocrinol2002;23(1): 339-349.
[13] Spicer LJ. Leptin: A possible metabolic signal affecting reproduction.Dom anim Endocrinol2001;21(4): 251-270.
[14] Liefers S, Veerkamp R, Te Pas M, Delavaud C, Chilliard Y, Platje M, et al. Leptin promoter mutations affect leptin levels and performance traits in dairy cows.Anim Genet2005;36(2): 111-118.
[15] Boelhauve M, Sinowatz F, Wolf E, Paula-Lopes FF. Maturation of bovine oocytes in the presence of leptin improves development and reduces apoptosis of in vitro-produced blastocysts.Biol Reprod2005;73(4): 737-744.
[16] Craig J, Zhu H, Dyce PW, Petrik J, Li J. Leptin enhances oocyte nuclear and cytoplasmic maturation via the mitogen-activated protein kinase pathway.Endocrinology2004;145(11): 5355-5363.
[17] Abavisani A, Dehghani H, Zahedi M. Expression of functional leptin receptor (OB-Rb) mRNA in the sheep tissues.Res Opin Anim Vet Sci2012;2: 449-453.
[18] Matsuoka T, Tahara M, Yokoi T, Masumoto N, Takeda T, Yamaguchi M, et al. Tyrosine phosphorylation of STAT3 by leptin through leptin receptor in mouse metaphase 2 stage oocyte.Biochem biophys Res Commun1999;256(3): 480-484.
[19] Goumenou AG, Matalliotakis IM, Koumantakis GE, Panidis DK. The role of leptin in fertility.Eur J Obstet Gyneco Reprod Biol2003;106(2): 118-124.
[20] Cassy S, Metayer S, Crochet S, Rideau N, Collin A, Tesseraud S. Leptin receptor in the chicken ovary: potential involvement in ovarian dysfunction of ad libitum-fed broiler breeder hens.Reprod Biol Endocrinol2004;2: 72.
[21] Dayi A, Bediz C, Musal B, Yilmaz O, Comlekci A, Celiloglu M, et al. Comparison of leptin levels in serum and follicular fluid during the oestrous cycle in cows.Acta Vet Hunga2005;53(4): 457-467.
[22] González RR, Caballero-Campo P, Jasper M, Mercader A, Devoto L, Pellicer A, et al. Leptin and leptin receptor are expressed in the human endometrium and endometrial leptin secretion is regulated by the human blastocyst.J Clin Endocrinol Metab2000;85(12): 4883-4888.
[23] De Placido G, Alviggi C, Clarizia R, Mollo A, Alviggi E, Strina I, et al. Intra-follicular leptin concentration as a predictive factor for in vitro oocyte fertilization in assisted reproductive techniques.J Endocrinol Invest2006;29(8): 719-726.
[24] Asimakopoulos B, Abu-Hassan D, Metzen E, Al-Hasani S, Diedrich K, Nikolettos N. The levels of steroid hormones and cytokines in individual follicles are not associated with the fertilization outcome after intracytoplasmic sperm injection.Fertil Steril2008;90(1): 60-64.
[25] Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, et al. Leptin promotes the development of mouse preimplantation embryos in vitro.Endocrinology2002;143(5): 1922-1931.
[26] Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat.Endocrinology1997;138(2): 855-858.
[27] Diskin M, Morris D. Embryonic and early foetal losses in cattle and other ruminants.Reprod Dom Anim2008;43(s2): 260-267.
[28] Bellver J, Busso C, Pellicer A, Remohí J, Simón C. Obesity and assisted reproductive technology outcomes.Reprod Biomed online2006;12(5): 562-568.
[29] Paula-Lopes FF, Boelhauve M, Habermann FA, Sinowatz F, Wolf E. Leptin promotes meiotic progression and developmental capacity of bovine oocytes via cumulus cell-independent and-dependent mechanisms.Biol Reprod2007;76(3): 532-541.
[30] Joo J-K, Joo B-S, Kim S-C, Choi J-R, Park S-H, Lee K-S. Role of leptin in improvement of oocyte quality by regulation of ovarian angiogenesis.Anim Reprod Sci2010;119(3): 329-334.
ment heading
10.1016/j.apjr.2016.07.002
*Corresponding author: Abbas Parham, Division of physiology, Department of Basic Sciences, Faculty of Veterinary Medicine, Ferdowsi University of Mashhad, Mashhad, Iran.
Tel: +98 51 38805600
Fax: +98 51 38763852
E-mail: parham@um.ac.ir
Foundation project: This work was financially supported by Ferdowsi University of Mashhad.
 Asian Pacific Journal of Reproduction2016年5期
Asian Pacific Journal of Reproduction2016年5期
- Asian Pacific Journal of Reproduction的其它文章
- Heparin binding proteins and their relationship with vital sperm function tests visà-vis fertility of buffalo bull semen
- Conception rate in Holstein dairy cows having both normal sized follicles and cystic follicles at estrus
- Polymyxin B changes the plasma membrane integrity of cryopreserved bull semen
- Freezability of buffalo semen with TRIS extender enriched with disaccharides (trehalose or sucrose) and different glycerol concentrations
- Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats
- Ovarian hyperstimulation syndrome followed by ovarian torsion in premenopausal patient using adjuvant tamoxifen treatment for breast cancer
