Hyperprolactinemia contributes to reproductive deficit in male rats chronically administered PDE5 inhibitors (sildenafil and tadalafil) and opioid (tramadol)
V. U. Nna, U. P. Akpan, E. E. Osim
1Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, University of Calabar, P.M.B. 1115, Calabar, Cross River State, Nigeria
2Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, University of Uyo, Uyo, Akwa Ibom State, Nigeria
Hyperprolactinemia contributes to reproductive deficit in male rats chronically administered PDE5 inhibitors (sildenafil and tadalafil) and opioid (tramadol)
V. U. Nna1*, U. P. Akpan2, E. E. Osim1
1Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, University of Calabar, P.M.B. 1115, Calabar, Cross River State, Nigeria
2Department of Physiology, Faculty of Basic Medical Sciences, College of Health Sciences, University of Uyo, Uyo, Akwa Ibom State, Nigeria
ARTICLE INFO
Article history:
Received
Received in revised form
Accepted
Available online
Gonadotropins
Hyperprolactinemia
Phosphodiesterase-5
Testosterone
Tramadol
Objective:To examine the influence of prolonged use of PDE5 inhibitors and opioids on prolactin among other male reproductive hormones, on the background that hyperprolactinemia has recently been associated with male sterility.Methods:Seventy male Wistar rats with weight 180–200 g were utilized in this study. They were assigned 1 of 5 groups (n=14) as follows; control (0.2 mL normal salinep.o.), sildenafil (10mg/kgp.o.), tadalafil (10mg/kgp.o.), tramadol (20mg/kgp.o.) and sildenafil+tramadol group (10 mg/kg and 20mg/kgp.o., respectively). All the animals had ad libitum access to rat chow and water. Administration was done every 2 d, for 8 wk, after which 7 rats were killed/group while the remaining 7 rats/group were left untreated for additional 8 wk (recovery period). Serum total cholesterol, low density lipoprotein, testosterone, follicle stimulating hormone, luteinizing hormone and prolactin were assessed in all animals.Results:Serum LDL-c and testosterone concentrations increased significantly (P<0.001) in the groups administered sildenafil and tadalafil, but reduced (P<0.001) in tramadol and sildenafil+tramadol groups relative to the control. FSH and LH reduced significantly (P<0.001) in the treated groups relative to the control, while prolactin concentration was increased (P<0.001) in the treated groups relative to the control. Reversibility was poor following withdrawal of the various treatments.Conclusion:Serum prolactin concentration correlated negatively with male reproductive hormones and may play a major role in reproductive deficits associated with chronic use of PDE5 inhibitors and opioids.
1. Introduction
Recently, PDE5 inhibitors and opioid use has gained popularity among young men without erectile dysfunction (ED). Reports showed that 21.5% of men within the age of 18–30 years ingested sildenafil without having ED, with 73.3% of that population accepting to having used it repeatedly[1]. One reason for this development is possibly that of the improvement in sexual health recounted by 72.5% of men who used PDE5 inhibitors[1]. It is perceived that erectile and ejaculatory dysfunctions often occur together[2,3]. This perception may have informed the formulation of the new therapy (sildenafil+tramadol) in vogue in Nigeria today. Like the previously available medications for treating erectile and ejaculatory disorders, sildenafil+tramadol has become popular among Nigerians and its incidence of abuse is high[4,5].
Chronic uses of PDE5 inhibitors and tramadol have been reported to adversely affect reproductive and non–reproductive tissues. For example, Al-Fartosi[6] and Khalafet al.[7] reported that prolonged used of PDE5 inhibitors increased the percentage of spermatocytes with abnormal morphology and altered testicular histology. Studies on opioids have demonstrated serious reproductive toxicity, like, increased sperm DNA damage mediated by testicular oxidative damage[8-10]. On non–reproductive tissues, chronic exposure to PDE5 inhibitors have been reported to adversely affect basal metabolic rate, liver enzymes and serum lipid profile[11-13].
In males, serum concentration of prolactin usually correlates negatively with the concentration of testosterone, FSH and LH. Although the importance of prolactin in male reproductive function has not been clearly ascertained, it has been primarily linked with male sterility[14]. Several studies on the effect of chronicconsumption of PDE5 inhibitors and opioid on male reproductive system have revolved around testicular oxidative stress as the mediator of reproductive toxicity. Furthermore, there are no data on the new therapy (sildenafil+tramadol) in vogue in Nigeria today. Considering the role of prolactin and its interaction with gonadotropic hormones (FSH and LH) and testosterone in the aetiology of reproductive deficit, this study was embarked upon to ascertain the impact of long term administration and subsequent withdrawal of PDE5 inhibitors (particularly, sildenafil and tadalafil), opioid (tramadol) and sildenafil+tramadol on male reproductive hormones and prolactin.
2. Materials and methods
2.1. Laboratory animals
Seventy male Wistar rats weighing 180–200 g were used for this study. They were obtained from the Department of Agriculture, Faculty of Science, and kept in the Animal house of the Department of Physiology, University of Calabar, Nigeria. The rats were housed in standard animal cages, with wood dust as their bedding. The rats had ad libitum access to feed and water, and exposed to 12/12 h light/dark cycle. Acclimatization lasted for 7 d before drug administration. They were kept in line with the principles for animal care as prescribed in Helsinki’s 1964 declaration. This study protocol was approved by the University of Calabar animal ethics committee.
2.2. Experimental design
The rats were assigned 1 of 5 groups (n=14) as follows; group 1 (control), group 2 (sildenafil), group 3 (tadalafil), group 4 (tramadol) and group 5 (sildenafil+tramadol). The control group was gavaged 0.2 mL normal saline, sildenafil and tadalafil groups received 10 mg/ kg each of sildenafil and tadalafil, the tramadol group received 20 mg/kg tramadol, while the sildenafil+tramadol group received 10 mg/kg and 20 mg/kg sildenafil and tramadol, respectively. Sildenafil citrate (Maxheal Laboratories Pvt Ltd, India), tadalafil (Pfizer, India) and tramadol hydrochloride (Glow Pharma Pvt Ltd, India) were purchased from Unipervit Pharmacy, Ikot Omin, Calabar. The different drugs were administered per oral route, once, every two days as used in our previous studies[12,13], while the control group received normal saline. Drug administration lasted for 8 wk, after which 7 rats were killed/group and blood samples taken for analysis. The remaining 7 rats/group were allowed for additional 8 wk recovery period. After 8 wk of recovery, the rats were killed, and blood collected for analysis.
2.3. Determination of serum TC and LDL-c
Blood was taken through cardiac puncture under chloroform anaesthesia with the aid of a 5 mL syringe and needle after treatment with the different drugs. Blood samples were kept in plain capped sample bottles for 2 h, after which they were centrifuged at 1000 r/min for 5 min using a bucket centrifuge (B-Bran Scientific and Instrument Company, England). Serum settled on top and was utilized for TC and LDL-c assessment. Serum concentration of total cholesterol was assessed by method of Sieldelet al.[15], as used by[16-20]. LDL-c was obtained mathematically using Friedewaldet al.[21] formulai.e[TC – (HDL-c + VLDL-c)].
2.4. Measurement of serum concentration of reproductive hormones
Serum testosterone, FSH, LH and prolactin concentrations were determined using the ELISA kit method as used byUmoh et al.[22].
2.5. Data analysis
The data are expressed as mean±SEM. The one way analysis of variance (ANOVA) was utilized in comparing the difference between groups, followed by the least square difference (LSD) post hoc test. The difference between batch A and B animals was analysed using Student’s t–test. Computer software SPSS version 17.0 was utilized for the analysis. SignificantP–values were set atP<0.05.
3. Result
3.1.Comparison of the different parameters between the treated and control rats after 8 weeks treatment
Total cholesterol (TC) increased (P<0.001) in the tadalafil group, but reduced (P<0.001) in the groups treated with tramadol and sildenafil+tramadol relative to the control. Serum TC increased (P<0.001) in the tadalafil group, but reduced (P<0.001) in the tramadol and sildenafil+tramadol groups relative to the sildenafil group. Serum TC was reduced (P<0.001) in the tramadol and sildenafil+tramadol groups relative to the tadalafil group (Table 1). There was a significant increase in serum LDL-c (P<0.001) in both sildenafil and tadalafil groups, but a significant decrease (P<0.001) in tramadol and sildenafil+tramadol groups relative to the control. There was a significant increase (P<0.001) in serum LDL-c in the group treated with tadalafil, but a significant reduction (P<0.001) was observed in the groups treated with tramadol and sildenafil+tramadol relative to that treated with sildenafil. LDL-c significantly reduced (P<0.001) in tramadol and sildenafil+tramadol groups, relative to the tadalafil group (Table 1).
Serum testosterone increased markedly (P<0.001) in the groups treated with sildenafil and tadalafil, but decreased (P<0.001) in tramadol and sildenafil+tramadol groups, relative to the control. Serum testosterone concentration was markedly (P<0.001) decreased in tramadol and sildenafil+tramadol groups, relative to sildenafil and tadalafil groups. Serum testosterone was raised (P<0.001) in sildenafil+tramadol group relative to the tramadol group (Table 1).
Serum FSH was decreased (P<0.001) in sildenafil, tadalafil, tramadol and sildenafil+tramadol groups, relative to the control. FSH decreased (P<0.001) in tadalafil, tramadol and sildenafil+tramadol groups, relative to the sildenafil group. FSH was lower in tramadol group, relative to the groups treated with tadalafil (P <0.05) and sildenafil+tramadol (P<0.05) (Table 1).
Serum LH was markedly (P<0.001) lowered in sildenafil, tadalafil, tramadol and sildenafil+tramadol groups, relative to the control. Serum LH concentration was increased in tramadol group, compared with sildenafil (P<0.001) and sildenafil+tramadol (P<0.001) groups (Table 1).
Relative to the control, serum prolactin concentration increased (P<0.001) in sildenafil, tadalafil, tramadol and sildenafil+tramadol groups. Serum prolactin increased (P<0.001) in tadalafil, tramadol and sildenafil+tramadol groups, relative to the sildenafil group. It also increased (P<0.001) in tadalafil and tramadol groups relative to the sildenafil+tramadol group (Table 1).
3.2. Comparison of the different parameters between the different groups following recovery
Serum TC reduced (P<0.001) significantly in tramadol and sildenafil+tramadol recovery groups relative to the control. TC reduced (P<0.001) in tramadol and sildenafil+tramadol recovery groups relative to sildenafil and tadalafil recovery groups (Table 2).

Table 1Comparison of the different parameters between the different experimental groups after 8 weeks of drug administration (mmol/L).

Table 2Comparison of the different parameters between the different experimental groups after 8 weeks recovery period (mmol/L).
Serum LDL-c increased in sildenafil recovery group (P<0.05), but reduced in tramadol (P<0.01) and sildenafil+tramadol (P<0.001) recovery groups relative to the control. Serum LDL-c was significantly reduced in tadalafil, tramadol and sildenafil+tramadol (P<0.01) recovery groups relative to the sildenafil recovery group (Table 2).
Serum testosterone was higher (P<0.001) in sildenafil recovery group, but lower (P<0.01) in tramadol and sildenafil+tramadol recovery groups relative to the control. Serum testosterone reduced (P<0.001) in tadalafil, tramadol and sildenafil+tramadol recovery groups relative to the control. It also reduced in tadalafil and sildenafil+tramadol (P<0.05) recovery groups relative to the tramadol recovery group (Table 2).
Serum FSH concentration was markedly reduced (P<0.001) in all recovery groups relative to the control. Serum concentration of FSH was significantly reduced in tramadol recovery group relative to sildenafil (P<0.01), tadalafil (P<0.05) and sildenafil+tramadol recovery groups (Table 2).
Serum LH concentration was reduced (P<0.001) in all recovery groups relative to the control. Serum LH was higher in tadalafil (P<0.001), tramadol (P<0.01) and sildenafil+tramadol (P<0.05) recovery groups relative to the sildenafil recovery group (Table 2).
Tadalafil, tramadol and sildenafil+tramadol recovery groups had a significant increase (P<0.001) in prolactin concentration relative to the control and sildenafil recovery groups. Serum prolactin increased in tramadol (P<0.001) and sildenafil+tramadol (P<0.05) recovery groups relative to the tadalafil recovery group. Prolactin concentration reduced (P<0.05) in sildenafil+tramadol recovery group relative to the tramadol recovery group (Table 2).
3.3. Comparison between the treated and recovery groups
Comparison between the treated and recovery groups showed that serum TC was reduced (P<0.001) in tadalafil recovery group relative to the treated group, while sildenafil recovery group recorded an increase (P<0.05) in serum TC relative to its treated group. Tramadol and sildenafil+tramadol recovery groups had insignificant (P>0.05) increase in serum TC relative to their treated counterparts (Figure 1A). LDL-c concentration reduced (P<0.001) in the tadalafil recovery group relative to the treated group (Figure 1B).
Serum testosterone was reduced in sildenafil (P<0.01) and tadalafil (P<0.001) recovery groups, but increased (P<0.001) in tramadol and sildenafil+tramadol recovery groups relative to their respective treated groups (Figure 1C). Serum FSH concentration was markedly increased (P<0.001) in the sildenafil, tadalafil, tramadol and sildenafil+tramadol recovery groups relative to their respective treated groups (Figure 1D). Serum LH was higher (P<0.05) in sildenafil, tadalafil, tramadol and sildenafil+tramadol recovery groups relative to their respective treated groups (Figure 1E), while serum prolactin was markedly lowered (P<0.001) in sildenafil, tadalafil, tramadol and sildenafil+tramadol recovery groups relative to their respective treated groups (Figure 1F).
4. Discussion
Steroid hormones (notably testosterone) depend to a great extent on cholesterol for their production, though acetyl Co–enzyme A equally adds to the substrates pool[23]. Serum total cholesterol partly regulates steroid hormones concentration. Androgens (synthesized from cholesterol), primarily testosterone, have peripheral and central effects that modulate erection of the penis[24,25]. Androgens contributes majorly in the maintenance of libido and sexual desire in men and contributes majorly in regulating erectile ability[24-27]. The serum concentration of androgens and other reproductive hormones (like, FSH and LH), and recently, prolactin[14], are important regulators of male reproductive function. Testosterone and PDE5 inhibitor combination therapy is being used to treat patients whose ED is accompanied by hypogonadism. However, Spitzeret al.[28] in their study maintained that testosterone therapy did not significantly influence the outcome of sexual performance in patients who suffered ED alone. After eight weeks of treatment with the different experimental drugs, there were significant alterations in male reproductive hormone concentrations in serum. Testosterone (a steroid hormone) was raised in sildenafil and tadalafil groups, compared with the control, while tramadol and sildenafil+tramadol groups had reduced values of testosterone concentration, compared to control. This contradicts the report of Ceccarelliet al.[29] who previously documented unaltered testosterone concentration following tramadol administration. However, their report was based on an assessment done after 4 hours of administration. Our findings therefore, may be attributed to the longer duration of administration,consistent with the findings of Abdellatiefet al.[30] and Ahmed and Kurkar[31], who both separately reported reduced serum testosterone concentration following administration of tramadol. The reduction in testosterone concentration in tramadol and sildenafil+tramadol groups could be attributed to low levels TC and LDL-c (the substrates for testosterone synthesis) recorded in these groups. Furthermore, the reduction in testosterone concentration in the tramadol and sildenafil+tramadol groups can be attributed to the low levels of TC and LDL-c (the substrates for testosterone synthesis) recorded in these groups. Tramadol may have negatively affected cholesterol synthesis. However, further studies in this direction are necessary to fully establish the mechanism.
All treated groups recorded significant (P<0.001) reduction in FSH and LH concentrations, compared with control. Aloisiet al.[32] previously reported that opiate administration induced hypogonadism by subduing the hypothalamic–pituitary–gonadal–axis, thus suppressing the hypothalamic secretion of GnRH, and anterior pituitary’s LH and FSH secretions. This may be the possible cause of the significant reduction in serum concentrations of FSH and LH in tramadol and sildenafil+tramadol groups in this study. It is known that the levels of LH positively correlates with testosterone concentration, since LH (also called interstitial cell stimulating hormone–ICSH) triggers the Leydig cells located in the testis to secrete testosterone[23]. Decreased levels of LH concentration in tramadol and sildenafil+tramadol groups may contribute to low levels of testosterone recorded in these groups. The high concentration of testosterone in sildenafil and tadalafil groups despite having low levels of LH may be consequent upon the increased total cholesterol available for testosterone synthesis. Although the sildenafil treated group had increased serum TC, LDL and testosterone, the tramadol treated group had lower values for serum TC, LDL and testosterone relative to the control group. Therefore, the observed decrease in serum TC, LDL and testosterone in the sildenafil+tramadol group relative to the control group can be attributable to the effect of tramadol. Furthermore, LH is known to trigger the Leydig cells to secrete testosterone, and when testosterone concentration increases, it usually inhibits LH secretion via a negative feedback mechanism[23]. This feedback inhibition of LH by testosterone may have contributed to the decreased LH concentration in sildenafil and tadalafil groups. However, the upregulation of testosterone by LH was not observed in the tramadol and sildenafil+tramadol groups in this study. This shows that tramadol negatively affected the interplay between testosterone and LH, since sildenafil administered alone did not disrupt this feedback mechanism (Table 1). Previous studies reported reduced Leydig cell expression in the testis of rats treated with tramadol[31,33,34]. We observed that tramadol and sildenafil+tramadol administration caused more significant histological distortions (including reduced Leydig cell density) and decreased testicular levels of antioxidant enzymes compared with sildenafil (data not shown). This may be responsible for the disruption of the LH – testosterone co-operation observed in sildenafil+tramadol group in this study. FSH was reduced along with LH in the tramadol and sildenafil+tramadol groups. It is likely that tramadol may have suppressed serum GnRH, as previously reported[32] which is responsible for triggering FSH and LH secretions. The role of prolactin (a hormone synthesized by lactotrophs of the anterior pituitary gland) in male reproductive function has not been clearly established. Nevertheless, the presence of receptors for prolactin on hypothalamus and choroid plexuses presumes its fundamental effect in the control of fertility in males[14]. Though the importance of prolactin to male reproductive function has not been explicitly recognized, it has been principally linked with male sterility[14]. Increased prolactin concentration suppresses the synthesis of testosterone as well as fertility in males via prolactin–induced exaggerated secretion of adrenal corticoids or by impeding GnRH secretion via prolactin receptors on hypothalamic dopaminergic neurons. Serum prolactin concentration usually correlates negatively with the concentration of testosterone, LH and FSH in males. In this study, serum prolactin concentration was markedly raised in all treated groups, compared to control, with tramadol treated group recording the highest concentration. The observed hyperprolactinemia in the treated groups may contribute to the possibility of developing fertility problems in males who indulge in recreational use of PDE5 inhibitors and opioids.
After 8 weeks of treatment withdrawal, the alterations in serum concentrations of reproductive hormones were not completely reversed, as values for testosterone, FSH, LH and prolactin were still significantly different from control. This probably shows that recovery from the effects of these drugs was rather slow.
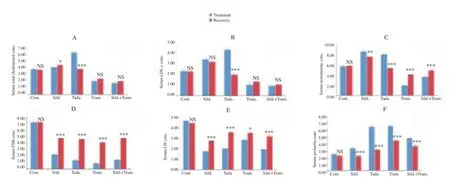
Figure 1. Comparison of serum parameters between the treated and recovery groups (mmol/L).Values are mean ± SEM,n= 7.*P<0.05,**P< 0.01,***P<0.001vs. treated, NS = not significant. Cont. = Control, Sild. = Sildenafil, Tada. = Tadalafil, Tram. = Tramadol.
We conclude that chronic exposure to high doses ofsildenafil, tadalafil, tramadol or the new combination therapy (sildenafil+tramadol) negatively affects reproductive hormones in male rats, with poor reversibility following withdrawal. Apart from testicular oxidative stress previously reported by other researchers, this study has shown that up–regulation of prolactin [a hormone known to correlate negatively with serum concentrations of testosterone, FSH and LH (hormones that support spermatogenesis)] in males may play a major role in the etiology of reproductive deficit when chronically exposed to PDE5 inhibitors and opioids.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Bechara A, Casabe A, De Bonis W, Helen A, Bertolino MW. Recreational use of phosphodiesterase type 5 inhibitors by healthy young men.J Sex Med2010;7(11): 3736-3742.
[2] Blanker MH, Bohnen AM, Groeneveld FP, Bernsen R, Prins A, Thomas S, et al. Correlates for erectile and ejaculatory dysfunction in older Dutch men: A community-based study.J Am Geriatr Soc2001;49(4): 436-442.
[3] Blanker MH, Bosch JR, Groeneveld FP, Bohnen AM, Prins AD, Thomas S, et al. Erectile and ejaculatory dysfunction in a community-based sample of men 50 to 78 years old: prevalence, concern, and relation to sexual activity.Urology2001;57(4): 763-768.
[4] Nna VU, Ani EJ, Ofutet EO, Ofem OE, Iroh CE, Osim EE. Recurrent side effects following chronic recreational use of sexual stimulants among male subjects in Calabar, Cross River State, Nigeria.Der Pharmacia Lettre2014;6(6): 56-61.
[5] Nna VU, Ofem OE, Osim EE. Prevalence of sex stimulants abuse among male subjects in Calabar, Cross River State, Nigeria, following perceived beneficial effect in increasing genital size.J Sex Med2016;13(5): S45.
[6] Al-Fartosi KG. Effect of long term administration of sildenafil citrate (Viagra) on some sperm characteristics and testis architecture of male rats.Basic J Veter Res2009;8(2): 91-103.
[7] Khalaf MA, Abbas MF, El-Fakahany HM. Effects of chronic tadalafil use on the testes and sperm parameters of old albino rats.Andrologia2012;1: 370-375.
[8] Pasqualotto FF, Sharma RK, Nelson DR, Thomas A, Agarwal A. Relationship between oxidative stress, semen characteristics, and clinical diagnosis in men undergoing infertility investigation.Fertil Steril2000;73: 459-464.
[9] Barenys M, Macia N, Camps L, de Lapuente J, Gomez-Catalan J, Gonzalez-Linares J. Chronic exposure to MDMA (ecstasy) increases DNA damage in sperm and alters testes histopathology in male rats.Toxicol Lett2009;191: 40-46.
[10] Safarinejad MR, Asgari SA, Farshi A, Ghaedi G, Kolahi AA, Iravani S, et al. The effects of opiate consumption on serum reproductive hormone levels, sperm parameters, seminal plasma antioxidant capacity and sperm DNA integrity.Reprod Toxicol2013;36: 18-23.
[11] Oka VO, Udefa AL, Nna VU, Owu DU. Sildenafil citrate and tramadol administered separately and in combination affects basal metabolic rate, triiodothyronine (t3) and cortisol levels in albino wistar rats.Trends Med Res2015;10(3): 51-62.
[12] Nna VU, Akpan UP, Osim EE. Separately administered phosphodiesterase-5 inhibitors (sildenafil and tadalafil) and opioid (tramadol), reversibly alter serum lipid profile in male albino wistar rats.J Biochem2015;10(4): 132-144.
[13] Nna VU, Akpan UP, Okon VE, Atangwho IJ. Hepatotoxicity following separate administration of two phosphodiesterase-5 inhibitors (sildenafil & tadalafil) and opioid (tramadol); evaluation of possible reversal following their withdrawal.J Pharm Sci2015;5(8): 105-113.
[14] Grattan DR. The actions of prolactin in the brain during pregnancy and lactation.Prog Brain Res2001;133: 153-171.
[15] Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency.Clin Chem1985;29: 1075-1080.
[16] Ofem OE, Nna VU, Archibong AN, Bassey SC. Alteration in serum lipid profile following separate administration of anti-malarial drugs (Coartem and Chloroquine): a comparative study.Der Pharma Chemica2014;6(4): 415-421.
[17] Ofem OE, Nna VU, Bassey SC, Archibong AN. Serum lipid lowering effect of vitamin C and E supplementation in high salt loaded rats.Der Pharmacia Lettre2014;6(4): 151-158.
[18] Nku CO, Ikpi DE, Nna VU, Agiande GU. Altered serum lipid profile in albino wistar rats following the consumption of Cola nitida rubra (kola nut).Aus Basic Applied Sci2014;8(13): 82-89.
[19] Essien NM, Bassey SC, Nna VU, Ofem OE. Comparative effect of chronic consumption of some edible vegetable oils on lipid profile and some haematological parameters in rats.Ann Bio Res2014;5(7): 16-21.
[20] Ani EJ, Ofem OE, Nna VU, Jacob MU. Alteration in serum lipid profile following chronic consumption of thermally-oxidized palm oil and groundnut oil-modified diets in rats.Res J Pharm, Bio Chem Sci2015;6(2): 634-641.
[21] Friedewald WT, Levy LI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge.Clin Chem1972;18: 499-502.
[22] Umoh IO, Emmanuel AO, Nna VU. Aqueous seed extract of Cola nitida rubra reduces serum reproductive hormone concentrations and sperm count in adult male albino Wistar rats.Niger Med J2014;55(6): 456-459.
[23] Guyton AC, Hall JE.Textbook of medical physiology. 10th edition. Philadelphia: W. B. Saunders; 2012, p. 386-387.
[24] Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction.Eur Urol2007;52: 54-70.
[25] Buvat J, Maggi M, Gooren L, Guay AT, Kaufman J, Morgentaler A, et al. Endocrine aspects of male sexual dysfunctions.J Sex Med2010;7(4pt2): 1627-1656.
[26] Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men.J Clin Endocrinol Metab2005;90: 3838-3846.
[27] Gooren LJ, Saad F. Recent insights into androgen action on the anatomical and physiological substrate of penile erection.Asian J Androl2006;8: 3-9.
[28] Spitzer M, Basaria S, Travison TG, Davda MN, Paley A, Cohen B, et al. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial.Ann Intern Med2012;157(10): 681-691.
[29] Ceccarelli I, De Padova AM, Fiorenzani P, Massafra C, Aloisi AM. Single opioid administration modifies gonadal steroids in both the CNS and plasma of male rats.Neuroscience2006;140(3): 929-937.
[30] Abdellatief RB, Elgamal DA, Mohamed EEM. Effects of chronic tramadol administration on testicular tissue in rats: an experimental study. Andrologia 2014; 47(6):674-679.
[31] Ahmed MA, Kurkar A. Effects of opioid (tramadol) treatment on testicular functions in adult male rats: The role of nitric oxide and oxidative stress.Clin Exp Pharmacol Physiol2014;41(4): 317-323.
[32] Aloisi AM, Aurilio C, Bachiocco V, Biasi G, Fiorenzani P, Pace MC, et al. Endocrine consequences of opioid therapy.Psychoneuroendocrinology2009;34: 162-168.
[33] Ghoneim FM, Khalaf HA, Elsamanoudy AZ, Helaly AN. Effect of chronic usage of tramadol on motor cerebral cortex and testicular tissues of adult male albino rats and the effect of its withdrawal: histological, immunohistochemical and biochemical study.Int J Clin Exp Path2014;7(11):7323-7341.
[34] El Sawy MM, Malak HW. Effect of tramadol abuse on testicular tissue of adult albino rats: a light and electron microscopic study.Egypt J Hist2015;38(2):356-366.
ment heading
10.1016/j.apjr.2016.07.004
*Corresponding author: Mr. V. U. Nna, Department of Physiology, Faculty of Basic Medical Sciences, College of Medical Sciences, University of Calabar, P.M.B. 1115 Calabar, Cross River State, Nigeria
Tel: +2348062665814
E-mail:victor2nna@gmail.com
 Asian Pacific Journal of Reproduction2016年5期
Asian Pacific Journal of Reproduction2016年5期
- Asian Pacific Journal of Reproduction的其它文章
- Heparin binding proteins and their relationship with vital sperm function tests visà-vis fertility of buffalo bull semen
- Conception rate in Holstein dairy cows having both normal sized follicles and cystic follicles at estrus
- Polymyxin B changes the plasma membrane integrity of cryopreserved bull semen
- Freezability of buffalo semen with TRIS extender enriched with disaccharides (trehalose or sucrose) and different glycerol concentrations
- Gum arabic improves semen quality and oxidative stress capacity in alloxan induced diabetes rats
- Ovarian hyperstimulation syndrome followed by ovarian torsion in premenopausal patient using adjuvant tamoxifen treatment for breast cancer
