稻米品质性状基因的克隆与功能研究进展
张昌泉,赵冬生,李钱峰,顾铭洪,刘巧泉
稻米品质性状基因的克隆与功能研究进展
张昌泉,赵冬生,李钱峰,顾铭洪,刘巧泉
(扬州大学农学院植物功能基因组学教育部重点实验室/江苏省粮食作物现代产业技术协同创新中心,江苏扬州225009)
水稻是中国重要的粮食作物之一,高产与优质一直是品种改良的主要目标。目前,中国稻米品质表现总体偏低,在一定程度上影响了其市场竞争力。稻米品质属综合性状,是指稻米或稻米相关产品满足消费者或生产加工需求的各种特性,主要涉及稻米的物理和化学特性,包括精米率、米粒形状、透明度、蒸煮时间、米饭质地与香味、冷饭质地以及营养成分等指标。通常用碾磨品质、外观品质、蒸煮与食味品质和营养品质4个方面来评价稻米品质。近10年来,在上述稻米品质性状相关基因的克隆与功能研究领域已取得了长足的进展。水稻粒形不仅是重要的产量性状也是碾磨和外观品质的重要决定因素,目前已克隆了多个粒形相关的QTL和基因。根据粒形相关基因的表型效应可将其分为3类,即伴随植株矮化的小粒控制基因(第一类,包括、、、和等)、粒形特异基因(第二类,如、、、、、、、、、、和等)和小圆粒基因(第三类,即),其中只有第二类基因具有较好的育种利用价值。垩白是决定稻米外观品质的首要性状,同时也会影响碾磨品质。目前尽管已经鉴定了大量QTL,但只有少数QTL被精细定位和克隆,如、、、、、和等主要通过调控胚乳灌浆和储藏物积累而影响稻米外观表现。淀粉占精米胚乳干重的90%以上,其组成与结构是决定稻米外观和蒸煮与食味品质的最重要因素。淀粉的合成是由多基因参与的复杂调控网络,直接参与淀粉合成的淀粉合成酶类基因的功能已经比较清楚;此外,参与胚乳淀粉代谢的一些转录因子如Dull、OsEBP89、OsEBP5、OsRSR1和OsbZIP58等也已被陆续鉴定和克隆。蛋白质是稻米的第二大成分,目前已克隆了众多的贮藏蛋白编码基因,并且已鉴定克隆了多个与蛋白质转运调控有关的基因如、、、、、、和等。赖氨酸是稻米中的第一限制必须氨基酸,通过过量表达富含赖氨酸蛋白(如RLRH1和RLRH2)或调控游离赖氨酸代谢等途径,均可显著提高稻米中的赖氨酸含量。稻米香味主要由2-AP决定,目前,已克隆了和等参与2-AP合成调控的基因。在与稻米贮藏有关的脂质代谢方面,已克隆了脂肪酸氧化酶基因、和以及脂质转运基因。此外,在稻米维生素、花青素和矿物质等合成调控方面也已鉴定克隆了多个重要基因。综上,稻米各品质性状都是由多基因控制,并且各性状间彼此交叉,其遗传调控非常复杂。本文重点就近年来控制稻米粒形与垩白、蒸煮与食味品质、储藏蛋白、脂类、维生素与矿质元素等合成与调控相关基因的克隆、等位变异和功能研究进行了综述,并对重要品质相关基因的育种利用进行了展望,期望为水稻优质育种提供参考。
稻米品质;基因克隆;QTL;等位变异;功能分析
水稻(L.)是中国乃至世界范围内的重要粮食作物,对保障粮食安全具有举足轻重的作用。近30年来,中国水稻生产经过高产育种、超高产育种、超级稻育种和绿色超级稻育种等计划的实施,水稻产量不断提高,其中,超级稻产量已达到了6 892.5 kg·hm-2的水平[1]。与此同时,对优质、多抗和广适应性等需求也不断提高,尤其是随着大众消费水平和生活品味的提高,对优质稻米的需求越来越多[2]。目前,中国在水稻品种审定中所涉及的品质指标主要包括碾磨品质中的整精米率、外观品质中的长宽比、垩白粒率和垩白度以及蒸煮与食味品质中的胶稠度与直链淀粉含量。从稻米的加工和商品价值来看,稻米籽粒形态与垩白是最重要的决定因素,从稻米的食用与营养角度来看,胚及胚乳中储藏物质的组成与比例又是最重要的影响因素。
水稻种子的发育是一个动态过程,主要以胚乳中淀粉和储藏蛋白的累积为主,同时也涉及到由激素参与的种子形态的调控和其他代谢物的积累[3]。从分子水平看,参与到种子形态外观调控、淀粉与储藏蛋白等初级代谢成分的合成调控、以及脂类与香味物质形成等次生代谢物合成调控的基因以及一些miroRNA都会对稻米品质的形成发挥着重要作用[3-4]。此外,一些环境因素如地域、高温和水肥等差异都对稻米品质有重要影响[5-9]。本文就近十年来控制稻米粒形、垩白、淀粉合成、储藏蛋白、香味、脂类和矿质元素等合成调控重要基因的克隆与功能研究进行综述。
1 稻米品质性状的构成
稻米品质表现为多样性,就优质食用而言,主要包括外观品质、碾磨品质、蒸煮与食味品质和营养品质4个方面[10]。这些品质性状直接决定了稻米的商品价值与营养价值和消费者的消费行为。
粒形与垩白是稻米外观品质的重要构成因素。粒形主要指籽粒的长度、宽度和长宽比;垩白是指稻米胚乳中白色不透明的部分,主要是由于其中的淀粉粒排列不紧密而导致存在着一些空腔进而造成的一种光学特性。粒长、长宽比、垩白粒率和垩白度是决定稻米商品价值的首要性状,少或无垩白以及长粒形稻米的商品价值高。糙米率、精米率和整精米率是碾磨品质的重要评价指标,其中整精米率最为重要,是指米粒长度达到完整精米粒平均长度3/4以上米粒的质量占总精米试样质量的百分率。
蒸煮与食味品质(eating and cooking quality,ECQ)是稻米品质构成中的最重要方面,由于胚乳是稻米的主要食用部分,而其中的淀粉又是其主要组分。因此,淀粉的组成与结构是决定稻米ECQ的最重要因素。尽管中国在2008年就已出台了国家标准《大米蒸煮食用品质感官评价方法》(GB/T15682-2008),然而通过人工品尝的方式无法精确鉴定。因此,通常采用一些理化指标作为参考,包括表观直链淀粉含量(apparent amylose content,AAC)、胶稠度(gel consistency,GC)和糊化温度(gelatinization temperature,GT)3个经典的理化指标[11];近年来又发展了一种快速鉴定ECQ的指标即稻米淀粉粘滞性谱(RVA谱)。AAC是影响ECQ的最主要因素,胶稠度通常与AAC呈负相关性,而糊化温度与稻米ECQ的关系较为密切[12]。稻米在蒸煮过程中的膨胀与伸长特性即出饭特性是ECQ的直接反映,可用米饭的膨胀与延伸特性表示。一般认为延伸性好的米粒不易粘结与断裂,具有较好的适口性。此外,其他一些组份如香味物质2-乙酰基-1-吡咯啉的含量也会在一定程度上影响蒸煮与食味品质。
营养品质主要是指稻米中的蛋白质含量及氨基酸组成。蛋白质是稻米的第二大成分,占糙米干重的8%—10%。一般认为精米中蛋白质含量对稻米的ECQ起负面效应,其含量越低,稻米的食口性越好[13-14]。从稻米营养角度来看,蛋白质含量高并且人体必需氨基酸含量高的稻米具有很好的营养品质表现[15]。精米中的蛋白质主要以谷蛋白和醇溶蛋白为主,其中,谷蛋白含量高且易被消化吸收,因此,其含量与氨基酸组成是稻米营养品质中最重要的影响因素[16]。从功能性稻米角度来看,低谷蛋白稻米非常适合Ⅰ型和Ⅱ型糖尿病患者食用。此外,稻米中的脂肪酸、维生素类和矿物质元素等微量储藏物质也是近年来受到广泛关注的营养成分。
2 外观品质相关基因的克隆与功能研究
粒形不仅与水稻产量形成有关,也是稻米品质表现的重要影响因素,尤其是对稻米的外观品质影响甚大。因此,粒形相关基因是稻米产量与品质改良中最受关注的一类基因。近年来,有关水稻粒形调控相关基因的克隆与功能研究已取得了长足的进展,从不同的水稻种质资源中至少已经分离出几十个粒形相关基因或等位基因。
2.1 粒形决定基因
根据粒形基因决定的表型特征,可将其分为3大类[17]。第一类包括、、、和等,这类基因突变会造成植株矮化从而间接造成小粒表型[18-20];第二类基因能够特异性调控籽粒形状,是通常所指粒形控制基因;第三类基因是指小圆粒基因(small and round seed),主要发现于粳稻亚种中[21-22]。
在育种实践中,对于稻米外观品质改良具有重要利用价值的主要是第二类基因(表1)。在控制粒长方面,是一个重要的主效QTL,其编码蛋白的OSR结构域是负向调节粒长的关键部位,突变后导致了长粒表型[23]。也是一个粒长负调控因子其编码一个含Kelch功能域的PPKL家族磷酸酶,通过调控细胞周期蛋白T1;3而控制籽粒大小[24]。位于同一基因位点,因2个串联重复片段存在而高表达,从而增加粒长[25-26]。在控制粒宽方面,是通过图位克隆获得的第一个宽粒基因其编码的锌指E3泛素连接酶能负向调控细胞分裂,突变后会导致细胞数目增加,从而表现出粒宽和粒重的增加[27]。与具有相似作用的/编码一种多聚泛素结合蛋白,该蛋白有功能时籽粒较窄而功能丧失后引起粒宽和粒重增加[28]。是粒宽的正调节因子,其高表达后会导致更大的籽粒,其不同的等位基因差异主要是启动区的序列变异造成的[29]。编码一个含GRAS基因家族成员的蛋白质,负调控水稻籽粒大小,突变的等位基因能够显著增加粒宽和粒重[30]。编码一个IAA葡萄糖水解酶活性蛋白,能控制IAA供应,功能丧失后通过对“源”器官的多效影响而增加籽粒重量[31]。编码一种类似SQUAMOSA启动子结合蛋白16,是细胞增殖的正调节因子,高表达的能增加粒宽和粒重[32]。编码一个细胞色素P450蛋白,高表达能够显著增加粒长和粒宽[33]。编码一个具有组蛋白乙酰转移酶活性的类GNAT蛋白,通过改变细胞数目增加粒长[34]。编码一个包含QLQ结构域和WRC结构域的GRF转录因子,因一个氨基酸的替换而产生大粒表型[35]。
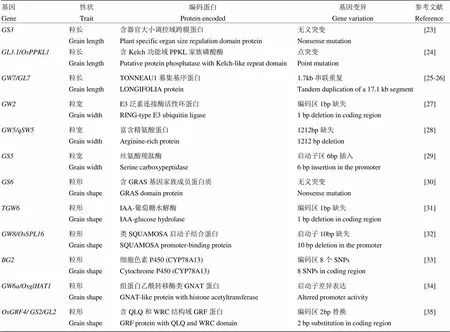
表1 具有育种利用价值的粒形基因
2.2 垩白相关QTL/基因
垩白不仅能够降低整精米率和稻米外观品质而且也能引起稻米的适口性变差[35-36]。经典遗传学和现代分子遗传学研究表明,稻米垩白性状受多基因控制并且还极易受环境因素的影响[36-39]。目前已有大量的垩白相关QTL和基因被鉴定出来,但只有少数几个已被精细定位和克隆。
在垩白QTL精细定位方面,Zhou等[40]利用染色体片段置换系将精细定位在44 kb的区段内;Guo等[41]利用染色体片段代换系将定位在140 kb的区段内。在基因克隆方面,垩白的极端表型—粉质突变体是垩白调控基因克隆的重要遗传材料,已通过对该类突变体的研究克隆了一批可能与垩白形成相关的基因。Kang等[42]首次克隆了粉质突变体基因,其编码一个丙酮酸磷酸激酶(pyruvate orthophosphate dikinase,PPDK),通过调节碳代谢而影响胚乳的灌浆。Wang等[39]进一步分析了高温条件下与垩白的关系,发现在高温条件下表达下调可能导致了垩白的增多。胚乳中储藏物质累积受阻往往也会导致垩白出现,Wang等[43]克隆的编码了一种细胞壁转化酶,其通过调控籽粒灌浆初期的碳源分流而影响储藏物质的累积速度,功能丧失会造成高垩白。She等[44]在一个化学诱变突变体中克隆了一个编码具有TPR结构域蛋白质的新基因,其主要通过调节胚乳中淀粉和蛋白质等储藏物的积累而引起垩白的出现。Wan等[45]克隆的胚乳粉质基因编码一个GTP酶(GTPase),其参与了胚乳细胞液泡中蛋白质的转运,该基因突变干扰了淀粉体的形成。Han等[46]克隆的编码了一个类二硫键异构酶PDIL1-1,该蛋白的缺失对胚乳内质网中淀粉体的合成造成了胁迫,从而造成淀粉的累积减少并表现出粉质突变。在另一粉质突变体中,Matsushima等[47]发现能够调控造粉体的发育,其突变后能够产生明显增大的淀粉粒并造成粉质表型。Li等[37]克隆的编码一个液泡H+-焦磷酸转移酶,该酶通过影响内膜转运系统的pH平衡而影响蛋白质体的合成,过量表达该基因可增加蛋白质体的量从而使淀粉粒无法紧密排列而造成垩白。
淀粉是胚乳中最主要的储藏物质,淀粉合成相关基因的表达受到影响很容易造成垩白表型。通过转录组分析,发现高垩白水稻中淀粉代谢类基因表达谱变化显著且通常表现为上调,而与淀粉代谢无关的一些糖类代谢基因趋向于下调表达;此外,参与胁迫反应和蛋白质降解的基因也会出现明显变化[48],这种变化趋势与高温胁迫所造成的垩白稻米中的基因表达变化趋势较为一致[49]。这说明垩白的形成是由多基因控制的复杂调控网络系统,而淀粉合成相关基因的表达失衡可能与稻米垩白的形成具有密切关系。
3 蒸煮与食味品质相关基因克隆与功能研究
淀粉是稻米胚乳的主要成分,稻米ECQ的评价指标大多属于淀粉的理化特性,因此,ECQ与稻米淀粉的组成和结构密切相关。从分子水平来看,水稻中参与胚乳淀粉合成与调控的基因都可能对稻米ECQ的形成发挥着重要作用[50-51]。参与水稻淀粉合成的酶类主要有ADP-葡萄糖焦磷酸化酶(AGPase)、颗粒结合淀粉合成酶(GBSSI)、可溶性淀粉合成酶(SSS)、淀粉分支酶(SBE)、淀粉去分支酶(DBE)、淀粉磷酸化酶(Pho)和淀粉异构酶(DPE)等,编码这些酶的基因可统称为SSRG(starch synthesis-related genes)[50-51]。此外,一些参与SSRG表达调控的转录因子也已被克隆。
3.1 淀粉合成相关基因
在植物中对SSRG的功能已有了比较清楚的认识,近年来主要是对这些基因的等位变异进行了较多的研究。在淀粉合成过程中第一个关键的酶是AGPase,其由4个大亚基基因和2个小亚基基因所编码[50,52]。AGPase的作用是将G-1-P中的葡萄糖残基转移到ATP上形成焦磷酸(PPi)和腺苷二磷酸葡萄糖(ADPG),是淀粉合成从“源”到“库”的关键一步反应。该酶有胞质型和淀粉体型2种类型,前者主要参与胚乳淀粉合成。该类基因突变后通常导致无法合成淀粉而出现籽粒干瘪的表型[53]。过量表达胞质型能够增强ADPG的供给而显著提高淀粉的合成量进而增加粒重[54-55]。在等位变异方面,作者的研究表明第1 633碱基处的变异对于直链淀粉含量和淀粉黏滞性谱起着微效的影响,而另一亚基基因的第511处碱基的变异对稻米胶稠度也存在微效的影响[11]。这说明选择合适的AGPase等位基因可以通过改善“源”的供给来修饰淀粉的理化特性而改良稻米品质。
GBSSI由水稻蜡质基因(,)编码,主要负责直链淀粉的合成,该基因的不同等位变异决定了稻米的直链淀粉含量[56],是控制ECQ的主效基因[11,57]。截止目前至少有8个已发表的等位基因,朱霁晖等[58]最近就此基因的等位变异进行了综述。在糯稻中,由于第2外显子23 bp的缺失造成了转录提前终止[59]。在非糯品种中,主要分化为Wx和Wx两种等位类型。其中,携带Wx的稻米直链淀粉含量都很高(25%以上),属于高直链淀粉类型。在Wx变异基础上,还存在一种第10外显子变异的等位基因Wx,尽管携带该等位基因的稻米AAC与Wx持平,但其糊化特性与胶稠度明显与携带Wx等位基因稻米不同[60-61],ZHANG等[62]的研究发现这种差异可能是由于直链淀粉的精细结构不同造成的。Wx主要分布在粳稻品种中,携带该基因稻米的直链淀粉含量属中等至较低水平(15%—18%)。与Wx相比,Wx的变异是由第一内含子剪接位点处G-T变异造成的,突变降低了前体mRNA的剪接效率从而减少了GBSSI的量进而导致了较低的直链淀粉含量[56]。此外,Mikami等[63]克隆了Wx等位基因,证明在第6外显子上发生的A-C变异使直链淀粉含量降至中等水平(18%—22%)。除了上述常规等位基因外,还有3个“软米基因”即Wx、Wx和Wx被克隆。与Wx相比,Wx在第4和第5外显子处存在两处突变,导致直链淀粉含量降到10%左右[64]。另一等位基因Wx是在此基础上在第5外显子发生了回复突变,携带该等位基因的稻米直链淀粉含量也在10%左右[65]。经测序分析,目前,中国的多数软米尤其是江苏的南粳系列软米都携带有该等位基因(未发表数据)。另一个软米基因为Wx(或者Wx),是由第4外显子的A-G突变造成的[63]。
研究表明一些具有相似直链淀粉含量的稻米,其品质表现会有很大不同。而支链淀粉精细结构的不同可能是导致这种差异的重要原因[66-67]。水稻支链淀粉主要由SSS催化合成,其主要有8种同工型[50]。SSSI是SSS的主要组分,占了总酶活的70%,主要负责支链淀粉短链(DP≤12)合成,其籼型等位基因编码的酶活性更强,能提高稻米淀粉粘滞性[50]。SSSII-3主要在胚乳中表达,对稻米品质影响最大。高振宇等[68-69]通过图位克隆方法分离了该基因(),其主要负责延伸支链淀粉的短支链(A+B1链),合成中等长度的分支链(B2+B3链)。该基因存在很多等位变异类型,但从编码的酶是否有活性来看,可以分为2类[50]。一类主要存在于籼稻中,表现为高活性,能够合成较多的中等长度的支链而表现为高糊化温度;而在一些粳稻品种中因该酶活性较低或丧失活性而表现为低糊化温度[50]。SSSIIIa主要在胚乳中发挥作用,负责长支链的延伸(DP≥30),突变后不仅能够明显增加垩白而且淀粉粘滞性也极低[70-71]。尽管SSSIV的2种同工型已鉴定出来,但水稻中关于其功能并不清楚,在拟南芥中的研究表明其过量表达后能够提高叶片瞬时淀粉的含量,据此推测其可能在水稻中具有增加淀粉含量的潜能[72]。
SBE是淀粉合成酶中唯一催化葡聚糖链产生分支的酶(催化α-1,6糖苷健形成)。水稻中有3种同工型,其中SBEI(又称为SBE1)优先催化直链淀粉短分支的形成,并参与支链淀粉较长链和中等长度链的合成,其突变后对稻米外观没有明显影响但能够降低稻米的糊化温度而可能具有较好的食味表现[73]。SBEIIa和SBEIIb(也称为SBE3和SBE4)同源性较高,主要负责支链淀粉短分支链的形成。SBEIIb的功能比较清楚,主要负责A链的合成(DP 8-12),且不与SBEIIa功能叠加,后者可能参与了维持淀粉分支酶复合体的结构并合成部分短链[50]。最近的一项研究表明,SBEIIa在体外表达时根据其突变位点不同可以合成不同长度的葡聚糖链,这暗示该基因可能以一种特殊的方式参与了支链的延伸并且其可能在培育高抗性淀粉作物中比较有价值[74]。ZHU等[75]通过同时下调SBEI和SBEIIb的表达而获得了AAC接近50%、抗性淀粉含量达到13%左右的转基因水稻材料,这种稻米是一种非常适用于糖尿病人食用的功能性产品。上述研究表明,通过合理的选择类基因的不同等位组合既可以达到改良ECQ的目的,也可以满足培育特殊功能性稻米的需要。
DBE的作用是水解α-1,6糖苷键,包括异淀粉酶(isoamylase,ISA1、ISA2和ISA3)和普鲁兰酶(pullulanase,PUL)2类。其中在水稻中仅有一个拷贝且与功能存在重叠,仅在胚乳中表达,功能丧失会导致短链(DP≤13)分支增加[76]。ISA1和ISA2是胚乳支链淀粉合成所必不可缺少的,突变后籽粒中无法形成淀粉,有关两者的功能目前仍有争议[50]。最新的研究表明ISA1可能与FLO6协同参与支链淀粉的合成[77]。ISA3主要参与瞬时淀粉的合成与降解,与储藏淀粉关系不大[78]。总体来看,DBE类基因对稻米品质的形成影响剧烈,突变或表达改变后会影响种子胚乳淀粉积累,从而造成糖质胚乳表型[79],因此,在稻米品质改良中可能需要筛选特定的等位基因加以利用。
Pho在水稻中有2种,分别是质体型(Pho1)和胞质型(Pho2),两者都能催化α-葡萄糖链非还原末端的葡萄糖延伸,研究发现Pho1是一种温度依赖型酶,主要在低温下参与淀粉合成调控[80]。DPE也存在质体型(DPE1)和胞质型(DPE2),都属于葡糖苷水解酶家族77(GH77),目前的研究表明DPE1能够通过转移麦芽糖到支链淀粉上参与淀粉结构修饰,其过量表达会造成较小的淀粉粒而抑制表达能够增加直链淀粉含量[81]。Hwang等[82]进一步发现Pho1能够与DPE1形成复合体参与低聚麦芽糖的合成,从而起始淀粉的合成,后者可能促进了其糖基转移酶活性。
尽管单一SSRG的功能已经研究得比较透彻,但ECQ是一个综合性状,是由众多SSRG共同控制的复杂网络。单个SSRG突变后会引起其他多个SSRG的变化,如ISA1被抑制后,能够改变和的表达模式[83]。在前期的研究中,Tian等[11]通过对不同等位基因单倍型关联分析和转基因验证试验,明确了这些SSRG控制ECQ的模式,发现和能够互作来影响AAC、GC和GT,其中对于AAC和GC是主效的,而对于GT是微效的。相反,对于GT表现为主效而对于AAC和GC为微效。此外,和作为微效基因同时影响GC和GT,、、和共同对AAC有微效的影响,而和分别对GC和GT起微效控制作用[11]。为排除对ECQ主效基因的影响,YAN等[84]对118份糯稻品种中17个SSRG不同单倍型与ECQ的另一指标RVA谱进行了关联分析,发现不同的SSRG之间也是通过互作来共同控制RVA谱特征值,且其中的对RVA谱特征值的影响最大。此外,通过染色体片段代换系对相关微效QTL进行了定位分析,表明一些微效QTL也参与了这些网络调控[85]。
3.2 参与淀粉合成相关转录因子
尽管已经非常明确SSRG在稻米ECQ形成中是以一个复杂的调控网络发挥作用的,但这个过程中各SSRG之间是怎样协调表达的并不是很清楚。因此,近年来有关SSRG的研究重点集中在对这些基因的表达调控方面。
不仅存在多个复等位变异,其表达调控方式也有多种。()编码了一种前体mRNA剪接因子,这些剪接因子以复合体的形式直接参与调控前体mRNA的剪接效率而影响成熟mRNA的量[86]。在其他调控方面,Zhu等[87]发现转录因子OsEBP89和OsEBP5能够以复合体的形式激活基因表达。Liu等[88]的研究证明的编码蛋白GBSSI能以寡聚体的形式发挥作用。此外,ZHANG等[89]最近的研究发现在高温条件下一些QTL能够稳定的剪接效率,从而使稻米具有高温钝感AAC的表现。
通过SSRG协同表达分析克隆的RSR1(rice starch regulator1)是参与SSRG表达调控的关键转录因子[90],能负向调控SSRG的表达。RSR1突变后能上调胚乳中多数SSRG的表达,其中15个基因上调表达最明显,这种多基因的上调表达可能破坏了淀粉合成的平衡而导致了直链淀粉的升高和支链淀粉的结构变化并造成了明显的垩白。另一转录因子OsbZIP58能够直接与、、、、和启动子结合,调控这些基因的表达,其突变后导致支链淀粉短链增加和中长链减少并造成了垩白[91]。后续的进一步研究发现,OsbZIP58与另一转录因子OsLOL1能够相互作用并且可能通过调控赤霉素的合成来影响籽粒淀粉的积累和种子的萌发[92]。
3.3 米粒延伸性相关QTL
目前,已克隆了很多控制稻米AAC、GC、GT和RVA谱的基因,但是对于米粒延伸特性的遗传调控研究相对较慢,尚未有主效基因克隆,目前仅定位了几个微效QTL。早在1993年,Ahn等[93]对优质籼稻Basmati370中控制米粒延伸性的QTL进行了定位研究,在第8染色体上鉴定了一个QTL。随后,Amarawathi等[94]利用Basmati×Pusa构建的重组自交系群体在第11染色体上检测到一个QTL。何予卿等[95]通过QTL定位分析,认为第6染色体附近存在一多效性QTL,控制米粒延伸性。Ge等[96]利用珍汕97和明恢63创建的重组自交系群体在第2、6和11染色体上鉴定了3个QTL。Liu等[97]利用一个籼粳交群体在第2和5染色体上鉴定到了控制米粒延伸性的QTL。最近,Rathi等[98]利用关联分析在水稻第4染色体分子标记RM142附近鉴定了一个米粒延伸性QTL。综上可见,米粒延伸性的遗传较为复杂,是由多个微效基因控制的复杂性状,在基因克隆方面尚存在不少困难,在育种利用方面也只有少数QTL可以尝试利用。
4 营养品质相关基因克隆与功能研究
4.1 储藏蛋白与氨基酸合成和代谢相关基因
稻米储藏蛋白与稻米品质性状关系密切,其含量在籼粳稻间存在一定差异,在稻米不同层面也存在明显区别[14,99-100]。目前已在蛋白质含量QTL/基因定位和克隆方面开展了很多工作。有关储藏蛋白在水稻胚乳细胞内的定位已比较清楚,谷蛋白和球蛋白都定位于蛋白体Ⅱ中,醇溶蛋白定位于内质网中[101]。水稻中已鉴定了至少15个编码谷蛋白的结构基因,其编码产物需经高尔基体形成运输小泡运输至蛋白体Ⅱ中[102]。稻米中的谷蛋白含量直接与其编码基因有关,然而由于其编码基因拷贝数较多,单个结构基因突变后对总蛋白质含量影响较小[103],而参与储藏蛋白表达及转运调控的基因对种子储藏蛋白的累积影响较大。
研究发现,转录因子OsRISBZ1和OsRPBF能够协调控制储藏蛋白(主要为谷蛋白)基因的表达,两基因突变后种子储藏蛋白含量明显下降[104]。Wan等[45]在一个粉质突变体中克隆了一个能够调控谷蛋白运输的基因,其功能丧失后导致谷蛋白无法转运到蛋白体Ⅱ中;进一步的研究表明其编码蛋白OsRAB5A可能与OsVPS9A和OsGPA3 蛋白协同参与了谷蛋白前体从高尔基体向蛋白体Ⅱ的转运,并且鸟嘌呤核苷酸交换因子2(GEF2)在转运过程中也起重要作用[105]。Tian等[106]克隆了一个编码小GTPase的,发现3个同源基因能够同时调控谷蛋白和α-球蛋白从内质网向高尔基体的运输,其功能丧失后不仅出现粉质胚乳表型,籽粒充实度也变差。在控制蛋白质含量QTL研究方面,Peng等[107]首次克隆了一个主效QTL,该基因能够正向调控蛋白质含量,过量表达后能明显提高蛋白质合成相关基因的表达以及根部氨基酸的吸收速度进而提高蛋白质的合成量。
赖氨酸是稻米中的第一限制必须氨基酸。在高等植物中赖氨酸是通过天冬氨酸代谢通路合成[108]。在赖氨酸的合成途径中有2个受反馈抑制调节的关键酶,即天冬氨酸激酶(AK)和二氢吡啶羧酸合酶(DHPS)。AK被苏氨酸和赖氨酸反馈抑制,而且赖氨酸又是DHPS的反馈调节抑制因子。研究表明,植物体内赖氨酸合成的主要限速步骤是赖氨酸对DHPS的反馈抑制,同时,当赖氨酸含量提高后,会增加赖氨酸分解关键酶赖氨酸-酮戊二酸还原酶/酵母氨酸脱氢酶(LKR/SDH)的活性而加快赖氨酸的降解,致使赖氨酸在种子中不能得到有效的积累[108]。LONG等[15]通过突变体和转基因分析对水稻中赖氨酸代谢途径关键酶基因进行了功能分析,发现叶片中游离赖氨酸的积累主要受合成途径的调控,而在种子中主要受分解途径的调控。通过基因工程手段,过量表达水稻内源赖氨酸含量丰富的组蛋白 RLRH1和RLRH2,使稻米赖氨酸含量提高了35%[109]。而通过胚乳特异性表达富含赖氨酸的外源蛋白,也可以使种子赖氨酸含量提高30%[110]。最近,YANG等[111]通过同时表达反馈抑制不敏感的AK和DHPS以及抑制LKR/SDH表达,获得了游离赖氨酸含量提高25.3倍的水稻株系。
4.2 米香形成相关基因
香味是优质稻米的一个重要指标。稻米中的香味物质有很多种,其中最重要的是2-乙酰基-1-吡咯啉(2-acetyl-l-pyrroline, 2-AP)[112]。香味的遗传主要受1对隐性基因控制。Bradbury等[113]利用图位克隆法分离克隆了香味基因,其编码了甜菜碱乙醛脱氢酶2(betaine aldehyde dehydrogenase,BAD2)。Chen等[114]的研究发现其功能丧失的隐性等位基因和均能引起2-AP积累而导致香味产生。随后,Kovach等[115]深入分析了该香味基因的起源及进化关系,发现了 8种隐性等位基因,其中是香稻中普遍存在的优势等位基因,并且首先起源于粳稻,后来才导入到籼稻。最近的研究发现2-AP的积累与脯氨酸的含量呈正相关性[116],因此,Keyghobad等[117]通过过量表达合成脯氨酸的△1-吡咯啉-5-羧酸合成酶(△1-pyrroline-5-carboxylate synthetase)编码基因,使2-AP的含量提高了2倍。此外,除了遗传调控外,添加锌、镧金属离子和外源2-AP以及改善栽培条件如土壤和收获时间等环境因素都能不同程度地影响2-AP的含量[118-119]。因此,米香不仅是食味品质的重要指标,从代谢调控角度来看,其与氨基酸代谢关系密切。
4.3 脂肪酸合成与代谢相关基因
脂质是水稻中另一种重要的储藏物,主要包括磷脂和脂肪,在胚和糊粉层中最多,而在胚乳中主要以脂质-直链淀粉复合体的形式存在[120-121]。脂质不仅对种子活力(寿命)具有重要影响,稻米油更是一种具有高营养价值的食用油,因此,脂质是稻米营养品质决定的重要因素之一[121]。目前,有关脂类的代谢途径在植物中已有较多研究,水稻中已有很多相关QTL被鉴定出来,但克隆的基因数量较少[10,121]。从稻米营养品质来看,催化脂质氧化反应的脂肪酸氧化酶(LOX)是导致稻米陈化和营养成份下降的重要原因[122]。目前,在水稻中已经克隆了3个编码LOX的基因,分别为、、/[123-124],其中,和能负调控脂肪酸的降解,其表达下调或功能丧失后能延长稻米储藏时间而维持较好的适口性与营养成份。对于富含β-胡萝卜素的黄金稻米而言,下调表达后能够减少储藏过程中β-胡萝卜素的降解[123,125]。在脂质转运方面,目前的研究较少,但已经清楚的有两类转运蛋白,分别是非特异脂质转运蛋白和特异脂质转运蛋白[126]。在水稻中只有一个脂质转运蛋白编码基因被克隆,其表达下调后能够明显降低种子脂质含量,同时也对种子发育产生了不利影响[127]。因此,有关脂质代谢调控研究是未来脂肪组(lipidome)研究的重要方向。
4.4 其他营养成份合成与代谢相关基因
尽管维生素类和类黄酮代谢相关基因在其他模式植物如拟南芥中已比较清楚,但在水稻中只克隆了少量相关基因。
在维生素代谢方面,Chaudhary等[128]在水稻中鉴定了7个维生素E合成相关基因(、、、、和)。Wang等[129]通过对巨胚米突变体的分析发现(编码细胞色素氧化酶P450)突变后能明显提高维生素E合成相关基因的表达而提高维生素E的含量。Hwang等[130]研究表明过量表达()也能明显提高维生素E各成份的含量并且增加了稻米的耐储藏性。张桂云等[131]的研究显示在籼稻中γ-三烯生育酚的比例较粳稻高,而粳稻中α-生育酚比例较籼稻高。进一步,其通过过量表达拟南芥,使大部分的γ-三烯生育酚转化成了α-生育酚,提高了稻米中α-生育酚的比例[132]。Wang等[133]通过全基因组关联分析对控制水稻α-生育酚的QTL进行了分析,发现的不同等位基因及表达量与α-生育酚的含量关系密切。有关维生素C代谢基因目前研究的也比较清楚,但由于水稻中其含量极低,主要是通过引入外源基因来改良。ZHANG等[134]的研究表明,在水稻中过量表达维生素C合成途径后6步的拟南芥同源基因、、、、和能不同程度(1.4—2.5倍)提高维生素C的含量。
在类黄酮合成调控方面,已经明确红米中原花青素合成主要受正调控因子和调控,而在黑米中主要则由和2个位点参与花青素的合成[135]。随后的研究表明可能也在花青素合成中起着关键作用[136]。Oikawa等[137]最近克隆了,命名为其编码了一个bHLH转录因子,因启动子区的变异造成异位表达而激活花青素合成相关基因表达从而产生黑米表型。
稻米中的矿质元素也是稻米的营养成份之一,适量的有益矿质元素能够提高稻米的营养价值。在矿质元素转运与运输方面,目前,已鉴定了大量的金属离子转运相关基因,这些基因多参与了植物的非生物胁迫反应而直接与稻米矿质元素含量相关的基因只占少数[138]。Sperotto等[139]对水稻剑叶和种子中25个金属转运基因的表达及金属元素的积累进行了关联分析,发现其中9个基因(、、、、、、、和)可能正向调控稻米中铁和锌的含量。近年来的研究表明,过量表达水稻、、、、、、、、或等基因都能明显提高水稻种子金属离子尤其是铁离子的含量[140]。此外,过量表达拟南芥和、大麦和菜豆均能显著提高水稻铁和锌的含量[141-142]。而对于有害重金属镉而言,研究发现可以通过过量表达或者敲除来降低稻米中镉的积累[143]。
5 展望
水稻的品质表现是一个综合性状,就加工、外观以及优质食用和营养品质而言,粒形、淀粉和蛋白质组成是最重要的决定因素。目前,已经克隆了一大批粒形、ECQ和营养成份相关的调控基因,尽管很多基因的功能已比较清楚,但是多数基因往往都具有一因多效性,因此,真正在育种上被利用的基因还只是少数,并且这些少数基因中也只有特定的等位基因对稻米品质改善有利。
在粒形方面,细长粒型稻米能够提高稻米的外观品质,但对产量可能有一定的负效应,整精米率也要低些[37,144]。宽粒水稻能够增加粒重和产量,但往往使垩白程度加重[27]。因此,在外观品质和产量需求方面可能需要作出一些取舍,一方面通过不同等位基因组合选育特定的粒形以达到增产的目的;另一方面需要协调粒形、粒重和稻米品质间的关系,选择最优组合以满足消费者的不同需求。尽管粒形在一定程度上能够减少稻米垩白,但垩白的遗传调控非常复杂且易受环境尤其是高温的影响非常明显[9,27]。由于全球气温的不断升高,在减少垩白方面除了选择环境耐受型新种质做为育种资源外,在分子标记辅助选择方面可以多开发一些关键基因或QTL的分子标记用于育种[144]。
基因调控网络的解析表明,控制ECQ的相关基因往往协同发挥作用,这暗示在稻米品质改良研究中一方面需要扩大种质资源范围,充分发掘一些关键优良等位基因和开发相关分子标记[145-146],另一方面也要从基因的互作与调控网络入手进行遗传调控。从目前消费者能够接受的角度看,通过分子标记辅助选择改良稻米品质是一种比较实用且有效的方法[146];而从改良速度和效率来看,基因工程技术则是一种快捷和高效的方式。随着越来越多重要品质基因的克隆尤其是不同物种间有利基因功能的阐明,转基因技术可加速对这些基因的利用,如富含维生素A的黄金大米和富含铁元素大米的创建就是很成功的例子[147-148]。目前,类似的研究结果已有很多,尽管在技术层面已经证明可以通过生物合成途径的调控来改良水稻品质和强化营养,但离商业化应用尚需时日。近几年发展起来的基因组编辑技术如CRISPR-Cas等[149]可能会在一定程度上加速包括品质改良在内的作物遗传改良。
References
[1] 程式华. 中国超级稻育种技术创新与应用. 中国农业科学, 2016, 49(2): 205-206.
Cheng S H. Breeding technique innovation and application of China’s super rice., 2016, 49(2): 205-206. (in Chinese)
[2] Rao Y C, Li Y Y, Qian Q. Recent progress on molecular breeding of rice in China.,2014, 33(4): 551-564.
[3] Zhou S R, Yin L L, Xue H W. Functional genomics based understanding of rice endosperm development., 2013, 16(2): 236-246.
[4] Peng T, Sun H Z, Du Y X, Zhang J, Li J Z, Liu Y X, Zhao Y F, Zhao Q Z. Characterization and expression patterns of micro RNAs involved in rice grain filling., 2013, 8(1): e54148.
[5] 王惠贞, 吴瑞芬, 李丹. 稻米品质形成和调控机理概述. 中国稻米,2016, 22(1): 10-13.
Wang H Z, Wu R F, Li D. Review on rice quality formation and its regulation mechanism., 2016, 22(1): 10-13. (in Chinese)
[6] 王娇, 王洁, 强爱玲, 官景得, 孙国才, 孙建昌, 齐国锋, 王兴盛, 韩龙植. 北方不同气候条件对稻米品质性状的影响. 中国稻米, 2015, 21(6): 13-18.
Wang J, Wang J, Qiang A L, Guan J D, Sun G C, Sun J C, Qi G F, Wang X S, Han L Z. The influence of different climatic ecological conditions on rice quality traits in northern China., 2015, 21(6): 13-18. (in Chinese)
[7] 陈帅君, 边嘉宾, 丁得亮, 崔晶. 不同有机肥处理对水稻品质和食味的影响. 中国稻米,2016,22(4): 42-45.
Chen S J, Bian J B, Ding D L, Cui J. Effects of organic fertilizers on quality and palatability of rice., 2016, 22(4): 42-45. (in Chinese)
[8] 高继平, 隋阳辉, 张文忠, 姚晨, 高明超, 赵明辉, 徐正进. 水稻灌浆期冠层温度对植株生理性状及稻米品质的影响. 中国水稻科学, 2015, 29(5): 501-510.
Gao J P, Sui Y H, Zhang W Z, Yao C, Gao M C, Zhao M H, Xu Z J. Effect of canopy temperature on physiological characteristic and grain quality at filling stage in rice., 2015, 29(5): 501-510. (in Chinese)
[9] Zhang C Q, Zhou L H, Zhu Z B, Lu H W, Zhou X Z, Qian Y T, Li Q F, Lu Y, Gu M H, Liu Q Q. Characterization of grain quality and starch fine structure of tworice () cultivars with good sensory properties at different temperatures during the filling stage., 2016, 64(20): 4048-4057.
[10] Bao j s. Genes and QTLs for rice grain quality improvement// Yan W G, Bao J S.(ISBN 978-953-51-1240-2). InTech Open Access publisher. 2014: 239-278.
[11] Tian Z X, Qian Q, Liu Q Q, Yan M X, Liu X F, Yan C J, Liu G F, Gao Z Y, Tang S Z, Zeng D L, Wang Y H, Yu J M, Gu M H, Li J Y. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities., 2009, 106(51): 21760-21765.
[12] Bao J S, Corke H, Sun M. Nucleotide diversity in starch synthase IIa and validation of single nucleotide polymorphisms in relation to starch gelatinization temperature and other physicochemical properties in rice (L.)., 2006, 113(7): 1171-1183.
[13] Ning H F, Qiao J F, Liu Z H, Lin Z M, Li G H, Wang Q S, Wang S H, Ding Y F. Distribution of proteins and amino acids in milled and brown rice as affected by nitrogen fertilization and genotype., 2010, 52(1): 90-95.
[14] 楠谷彰人. 中日水稻品种的食味比较. 北方水稻, 2007,5: 72-77.
Kusitani A. Comparison of palatability of rice varieties between China and Japan., 2007, 5: 72-77. (in Chinese)
[15] Long X H, Liu Q Q, Chan M L, Wang Q, Sun S S M. Metabolic engineering and profiling of rice with increased lysine., 2013, 11(4): 490-501.
[16] Ufaz S, Galili G. Improving the content of essential amino acids in crop plants: goals and opportunities., 2008, 147(3): 954-961.
[17] Huang R Y, Jiang L R, Zheng J S, Wang T S, Wang H C, Huang Y M, Hong Z L. Genetic bases of rice grain shape: so many genes, so little known., 2013, 18(4): 218-226.
[18] Ashikari M, Wu J, Yano M, Sasaki T, Yoshimura A. Rice gibberellin-insensitive dwarf mutant geneencodes the a-subunit of GTP-binding protein., 1999, 96(18): 10284-10289.
[19] Hong Z, Ueguchi-Tanaka M, Umemura K, Uozu S, Fujioka S, Takatsuto S, Yoshida S, Kitano H, Matsuoka M. A rice brassinosteroid-deficient mutant,(), is caused by a loss of function of a new member of cytochrome P450.,2003, 15(12): 2900-2910.
[20] Yamamuro C, hara Y, Wu X, Noguchi T, Fujioka S, Takatsuto S, Ashikari M, Kitano H, Matsuoka M. Loss of function of a rice brassinosteroid insensitive1 homolog prevents internode elongation and bending of the lamina joint.,2000, 12(9): 1591-1605.
[21] Abe Y, Mieda K, Ando T, Kono I, Yano M, Kitano H, Iwasaki Y. The(/) gene is involved in the regulation of seed size in rice., 2010, 85(5): 327-339.
[22] Kitagawa K, Kurinami S, Oki K, Abe Y, Ando T, Kono I, Yano M, Kitano H, Iwasaki Y. A novel kinesin 13 protein regulating rice seed length., 2010, 51(8): 1315-1329.
[23] Mao H L, Sun S Y, Yao J L, Wang C R, Yu S B, Xu C G, Li X H, Zhang Q F. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice.,2010, 107(45): 19579-19584.
[24] Zhang X J, Wang J F, Huang J, Lan H X, Wang C L, Yin C F, Wu Y Y, Tang H J, Qian Q, Li J Y, Zhang H S. Rare allele ofassociated with grain length causes extra-large grain and a significant yield increase in rice., 2012, 109(52): 21534-21539.
[25] Wang Y X, Xiong G S, Hu J, Jiang L, Yu H, Xu J, Fang Y, Zeng L J, Xu E B, Xu J, Ye W J, Meng X B, Liu R F, Chen H Q, Jing Y H, Wang Y H, Zhu X D, Li J Y, Qian Q. Copy number variation at thelocus contributes to grain size diversity in rice., 2015, 47(8): 944-948.
[26] Wang S K, Li S, Liu Q, Wu K, Zhang J, Wang S S, Wang Y, Chen X B, Zhang Y, Gao C X, Wang F, Huang H X, Fu X D. The-regulatory module determines grain shape and simultaneously improves rice yield and grain quality., 2015, 47(8): 949-954.
[27] Song X J, Huang W, Shi Min, Zhu M Z, Lin H X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase., 2007, 39(5): 623-630.
[28] Weng J F, Gu S H, Wan X Y, Gao H, Guo T, Su N, Lei C L, Zhang X, Cheng Z J, Guo X P, Wang J L, Jiang L, Zhai H Q, Wan J M. Isolation and initial characterization of, a major QTL associated with rice grain width and weight., 2008, 18(12): 1199-1209.
[29] Li Y B, Fan C C, Xing Y Z, Jiang Y H, Luo L J, Sun L, Shao D, Xu C J, Li X H, Xiao J H, He Y Q, Zhang Q F. Natural variation inplays an important role in regulating grain size and yield in rice., 2011, 43(12): 1266-1269.
[30] Sun L J, Li X J, Fu Y C, Zhu Z F, Tan L B, Liu F X, Sun X Y, Sun X W, Sun C Q., a member of the GRAS gene family, negatively regulates grain size in rice., 2013, 55(10): 1-12.
[31] Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, Kashiwagi T, Ujiie K, Shimizu B, Onishi A, Miyagawa H, Katoh E. Loss of function of the IAA-glucose hydrolase geneenhances rice grain weight and increases yield., 2013, 45(6): 707-711.
[32] Wang S K, Wu K, Yuan Q B, Liu X Y, Liu Z B, Lin X Y, Zeng R Z, Zhu H T, Dong G J, Qian Q, Zhang G Q, Fu X D. Control of grain size, shape and quality by, 2012, 44(8): 950-954.
[33] Xu F, Fang J, Ou S J, Gao S P, Zhang F, Du L, Xiao Y H, Wang H R, Sun X H, Chu J F, Wang G D, Chu C C. Variations incoding region influence grain size and yield in rice.,2015, 38(4): 800-811.
[34] Song X J, Kuroha T, Ayano M, Furuta T, Nagai K, Komeda N, Segami S, Miura K, Ogawa D, Kamura T, Suzuki T, Higashiyama T, Yamasaki M, Mori H, Inukai Y, Wu J, Kitano H, Sakakibara H, Jacobsen S E, Ashikari M. Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice., 2015, 112(1): 76-81.
[35] Che R H, Tong H N, Shi B H, Liu, Y Q, Fang S R, Liu D P, Xiao Y H, Hu B, Liu L C, Wang H R, Zhao M F, Chu C C. Control of grain size and rice yield by GL2-mediated brassinosteroid responses., 2016, 2(1): 15195.
[36] Bridgemohan P, Bridgemohan R S H. Crop nutrition studies on grain filling and chalkiness in rice., 2014, 6(10): 144-152.
[37] Li Y B, Fan C C, Xing Y Z, Yun P, Luo L J, Yan B, Peng B, Xie W B, Wang G W, Li X H, Xiao J H, Xu C G, He Y Q.encodes a vacuolar H+-translocating pyrophosphatase influencing grain chalkiness in rice., 2014, 46(4): 398-404.
[38] Qin Y, Kim S, Sohn J. Genetic analysis and QTL mapping for grain chalkiness characteristics of brown rice (L.)., 2009, 31(2): 155-164.
[39] Wang Z M, Li H X, Liu X F, He Y, Zeng H L. Reduction of pyruvate orthophosphate dikinase activity is associated with high temperature-induced chalkiness in rice grains., 2015, 89: 76-84.
[40] Zhou L J, Chen L M, Jiang L, Zhang W W, Liu L L, Liu X, Zhao Z G, Liu S J, Zhang L J, Wang J K, Wan J M. Fine mapping of the grain chalkiness QTLL.)., 2009, 118(3): 581-590.
[41] Guo T, Liu X L, Wan X Y, Weng J F, Liu S J, Liu X, Chen M J, Li J J, Su N, Wu F Q, Cheng Z J, Guo X P, Lei C L, Wang J L, Jiang L, Wan J M. Identification of a stable quantitative trait locus for percentage grains with white chalkiness in rice ()., 2011, 53(8): 598-607.
[42] Kang H, Park S, Matsuoka M, An G. White-core endospermfloury endosperm-4 in rice is generated by knockout mutations in the C-type pyruvate orthophosphate dikinase gene (OsPPDKB)., 2005, 42(6): 901-911.
[43] Wang E, Wang J J, Zhu X D, Hao W, Wang L Y, Li Q, Zhang L X, He W, Lu B R, Lin H X, Ma H, Zhang G Q, He Z H. Control of rice grain-filling and yield by a gene with a potential signature of domestication., 2008, 40(11): 1370-1374.
[44] She K, Kusano H, Koizumi K, Yamakawa H, Hakata M, Imamura T, Fukuda M, Naito N, Tsurumaki Y, Yaeshima M, Tsuge T, Matsumoto K, Kudoh M, Itoh E, Kikuchi S, Kishimoto N, Yazaki J, Ando T, Yano M, Aoyama T, Sasaki T, Satoh H, Shimada H. A novel factoris involved in regulation of rice grain size and starch quality., 2010, 22(10): 3280-3294.
[45] Wan Y H, Ren Y L, Liu X, Jiang L, Chen L M, Han X H, Jin M N, Liu S J, Liu F, Lv J, Zhou K N, Su N, Bao Y Q, Wan J M.regulates endomembrane organization and storage protein trafficking in rice endosperm cells., 2010, 64(5): 812-824.
[46] Han X H, Wang Y H, Liu X, Jiang L, Ren Y L, L F, Peng C, Li J J, Jin X M, Wu F Q, Wang J L, Guo X P, Zhang X, Cheng Z J, Wan J M. The failure to express a protein disulphide isomerase-like protein results in a floury endosperm and an endoplasmic reticulum stress response in rice., 2012, 63(1): 121-130.
[47] Matsushima R, Maekawa M, Kusano M, Kondo H, Fujita N, Kawagoe Y, Sakamoto W. Amyloplast-localized SUBSTANDARD STARCH GRAIN4 protein influences the size of starch grains in rice endosperm., 2014, 164(2): 623-636.
[48] Liu X L, Guo Tao, Wan X Y, Wang H Y, Zhu M Z, Li A L, Su N, Shen Y Y, Mao B G, Zhai H Q, Mao L, Wan J M. Transcriptome analysis of grain-filling caryopses reveals involvement of multiple regulatory pathways in chalky grain formation in rice., 2010, 11: 730.
[49] Yamakawa H, Hirose T, Kuroda M, Yamaguchi T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray.,2007, 144(1): 258-277.
[50] Jeon J S, Ryoo N, Hahn T R, Walia H, Nakamura Y. Starch biosynthesis in cereal endosperm, 2010, 48(6): 383-392.
[51] PFISTER B, ZEEMAN S C. Formation of starch in plant cells., 2016, 73(14): 2781-2807.
[52] Lee S K, Hwang S K, Han M, Eom J S, Kang H G, Han Y, Choi S B, Cho M H, Bhoo S H, An G, Hahn T R, Okita T W, Jeon J S. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (L.)., 2007, 65(4): 531-546.
[53] Tuncel A, Kawaguchi J, Ihara Y, Matsusaka H, Nishi A, Nakamura T, Kuhara S, Hirakawa H, Nakamura Y, Cakir B, Nagamine A, Okita T W, Hwang S K, Satoh H.The rice endosperm ADP-glucose pyrophosphorylase large subunit is essential for optimal catalysis and allosteric regulation of the heterotetrameric enzyme.,2014, 55(6): 1169-1183.
[54] Tuncel A, Okita T W. Improving starch yield in cereals by over-expression of ADP glucose pyrophosphorylase: Expectations and unanticipated outcomes., 2013, 211: 52-60.
[55] Smidansky E D, Martin J M, Hannah L C, Fischer A M, Giroux M J. Seed yield and plant biomass increases in rice are conferred by deregulation of endosperm ADP-glucose pyrophosphorylase., 2003, 216(4): 656-664.
[56] Wang Z Y, Zheng F Q, Shen G Z, Gao J P, Snustad D P, Li M G, Zhang J L, Hong M M. The amylose content in rice endosperm is related to the post-transcriptional regulation of thegene., 1995, 7(4): 613-622.
[57] Gu M H, Liu Q Q, Yan C J, Tan s z. Grain quality of hybrid rice: Genetic variation and molecular improvement//Xie F M, Hardy B. editors.(ISBN 978-971-22- 0252-0). Los Baños (Philippines): International Rice Research Institute. 2009: 345-356.
[58] 朱霁晖, 张昌泉, 顾铭洪, 刘巧泉. 水稻基因的等位变异及育种利用研究进展. 中国水稻科学, 2015, 29(4): 431-438.
Zhu J H, Zhang C Q, Gu M H, Liu Q Q. Progress in the allelic variation ofgene and it’s application in rice breeding., 2015, 29(4): 431-438. (in Chinese)
[59] Wanchana S, Toojinda T, Tragoonrung S, Vanavichit A. Duplicated coding sequence in theallele of tropical glutinous rice (L.)., 2003, 165(6): 1193-1199.
[60] Tran N A, Daygon V D, Resurreccion A P, Cuevas R P, Corpuz H M, Fitzgerald M A. A single nucleotide polymorphism in thegene explains a significant component of gel consistency.,2011, 123(4): 519-525.
[61] Hoai T T T, Matsusaka H, Toyosawa Y, Suu T D, Satoh H, Kumamaru T. Influence of single-nucleotide polymorphisms in the gene encoding granule-bound starch synthase I on amylose content in Vietnamese rice cultivars., 2014, 64(2): 142-148.
[62] Zhang C Q, Zhu L J, Shao K, Gu M H, Liu Q Q. Toward underlying reasons for rice starches having low viscosity and high amylose: physiochemical and structural characteristics., 2013, 93(7): 1543-1551.
[63] Mikami I, Uwatoko N, Ikeda Y, Yamaguchi J, Hirano H Y, Suzuki Y, Sano Y. Allelic diversification at thelocus in landraces of Asian rice., 2008, 116(7): 979-989.
[64] Sato H, Suzuki Y, Okumo K, Hirano H, Imbe T. Genetic analysis of low-amylose content in a rice variety, ‘Milky Queen’., 2001, 3: 13-19.
[65] Yang J, Wang J, Fan F J, Zhu J Y, Chen T, Wang C L, Zheng T Q, Zhang J, Zhong W G, Xu J L. Development of AS-PCR marker based on a key mutation confirmed by resequencing ofin Milky Princess and its application insoft rice (L.) breeding., 2013, 132(6): 595-603.
[66] Han Y P, Xu M L, Liu X Y, Yan C J, Korban S S, Chen X L, Gu M H. Genes coding for starch branching enzymes are major contribution to starch viscosity characteristics in waxy rice (L.)., 2004, 166(2): 357-364.
[67] Zhu L J, Liu Q Q, Sang Y J, Gu M H, Shi Y C. Underlying reasons for waxy rice flours having different pasting properties., 2010, 120(1): 94-100.
[68] Gao Z Y, Zeng D L, Cheng F M, Tian Z X, Guo L B, Su Y, Yan M X, Jiang H, Dong G J, Huang Y C, BinHan, Li J Y, Qian Q., the key gene for gelatinization temperature is a modifier gene for gel consistency in rice., 2011, 53(9): 756-765.
[69] Zhang G Y, Cheng Z J, Zhang X, Guo X P, Su N, Jiang L, Mao L, Wan J M. Double repression of soluble starch synthase genesandin rice (L.) uncovers interactive effects on the physicochemical properties of starch., 2011, 54(6): 448-459.
[70] Fujita N, Yoshida M, Kondo T, Saito K, Utsumi Y, To-kunaga T, Nishi A, Satoh H, Park J H, Jane J L, Miyao A, Hirochika H, Nakamura Y. Characterization of-deficient mutants of rice: The function ofand pleiotropic effects bydeficiency in the rice endosperm., 2007, 144(4): 2009-2023.
[71] Fujita N, Satoh R, Hayashi A, Kodama M, Itoh R, AiharaS, Nakamura Y. Starch biosynthesis in rice endosperm requires the presence of either starch synthase I or IIIa., 2011, 62(14): 4819-4831.
[72] Gámez-Arjona F M, Li J, Raynaud S, Baroja- Fernández E, Muñoz F J, Ovecka M, Rage P, Bahaji A, Pozueta-Romero J, Mérida Á. Enhancing the expression of starch synthase class IV results in increased levels of both transitory and long-term storage starch., 2011, 9(9): 1049-1060.
[73] Satoh H, Nishi A, Yamashita K, Takemoto Y, Tanaka Y, Hosaka Y, Sakurai A, Fujita N, Nakamura Y. Starch- branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm., 2003, 133(3): 1111-1121.
[74] Li C, Wu A C, Go R M, Malouf J, Turner M S, Malde A K, Mark A E, Gilbert R G. The characterization of modified starch branching enzymes: toward the control of starch chain-length distributions., 2015, 10(4): e0125507.
[75] Zhu L J, Gu M H, Meng X L, Cheung S C K, Yu H X, Huang J, Sun Y, Shi Y C, Liu Q Q. High-amylose rice improves indices of animal health in normal and diabetic rats., 2012, 10(3): 353-362.
[76] Fujita N, Toyosawa Y, Utsumi Y, Utsumi Y, Higuchi T, Hanashiro I, Ikegami A, Akuzawa S, Yoshida M, Mori A, Inomata1 K, Itoh R, Miyao A, Hirochika H, Satoh H, Nakamura Y. Characterization of pillulanase (PUL)-deficient mutants of rice (L.) and the function of PUL on starch biosynthesis in the developing rice endosperm., 2008, 60(13): 1009-1023.
[77] Peng C, Wang Y H, Liu F, Ren Y L, Zhou K N, Lv J, Zheng M, Zhao S L, Zhang L, Wang C M, Jiang L, Zhang X, Guo X P, Bao Y, Wan J M.encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm.,2014, 77(6): 917-930.
[78] Silver D M, Kötting O, Moorhead G B G. Phosphoglucan phosphatase function sheds light on starch degradation., 2014, 19(7): 471-478.
[79] 赵华, 王俊敏, 张其芳, 赵倩, 梅淑芳, 刘向蕾, 程方民. 水稻糖质胚乳突变体籽粒灌浆过程的淀粉合成关键酶活性及其与淀粉理化特性关系. 中国水稻科学, 2015, 29(1): 73-81.
Zhao H, Wang J M, Zhang Q F, Zhao Q, Mei S F, Liu X L, Cheng F M. Activities of several starch synthesis enzymes in filling grains for rice sugary endosperm mutant () and it’s relation to starch quality., 2015, 29(1): 73-81. (in Chinese)
[80] Satoh H, Shibahara K, Tokunaga T, Nishi A, Tasaki M, Hwang S K, Okita T W, Kaneko N, Fujita N, Yoshida M, Hosaka Y, Sato A, Utsumi Y, Ohdan T, Nakamura Y. Mutation of the plastidial α-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm., 2008, 20(7): 1833-1849.
[81] DONG X B, ZHANG D, LIU J, LIU Q Q, LIU H L, TIAN L H, JIANG L, QU L Q. Plastidial disproportionating enzyme participates in starch synthesis in rice endosperm by transferring maltooligosyl groups from amylose and amylopectin to amylopectin., 2015, 169(4): 2496-2512.
[82] Hwang S K, Koper K, Satoh H, Okita T W. Rice endosperm starch phosphorylase (Pho1) assembles with disproportionating enzyme (Dpe1) to form a protein complex that enhances synthesis of malto-oligosaccharides., 2016, 291(38): 19994-20007.
[83] Sun W Q, Zhou Q L, Yao Y, Qiu X J, Xie K, Yu S B. Identification of genomic regions and the isoamylase gene for reduced grain chalkiness in rice.,2015, 10(3): e0122013.
[84] Yan C J, Tian Z X, Fang Y W, Yang Y C, Li J, Zeng S Y, Gu S L, Tang S Z, Gu M H. Genetic analysis of starch paste viscosity parameters in glutinous rice (L.)., 2011, 122(1): 63-76.
[85] 刘鑫燕, 朱孔志, 张昌泉, 洪燃, 孙鹏, 汤述翥, 顾铭洪, 刘巧泉. 利用9311来源的粳稻染色体片段代换系定位控制稻米糊化温度的微效QTL. 作物学报, 2014, 40(10): 1740-1747.
Liu X Y, Zhu K Z, Zhang C Q, Hong R, Sun P, Tang S Z, Gu M H, Liu Q Q. Mapping of minor QTLs for rice gelatinization temperature using chromosome segment substitution lines from9311 in thebackground., 2014, 40(10): 1740-1747. (in Chinese)
[86] Kiswara G, Lee J H, Hur Y J, Cho J H, Lee J Y, Kim S Y, Sohn Y B, Song Y C, Nam M H, Yun B W, Kim K M. Genetic analysis and molecular mapping of low amylose gene(t) in rice (L.)., 2014, 127(1): 51-57.
[87] Zhu Y, Cai X L, Wang Z Y, Hong M M. An interaction between a MYC protein and an EREBP protein is involved in transcriptional regulation of the ricegene., 2003, 278(48): 47803-47811.
[88] Liu D R, Huang W X, Cai X L. Oligomerization of rice granule-bound starch synthase 1 modulates its activity regulation., 2013, 210: 141-150.
[89] Zhang H, Duan L, Dai J S, Zhang C Q, Li J, Gu M H, Liu Q Q, Zhu Y. Major QTLs reduce the deleterious effects of high temperature on rice amylose content by increasing splicing efficiency ofpre-mRNA., 2013, 127(2): 273-282.
[90] Fu F F, Xue H W. Coexpression analysis identifies rice starch regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator., 2010, 154(2): 927-938.
[91] Wang J C, Xu H, Zhu Y, Liu Q Q, Cai X L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm., 2013, 64(11): 3453-3466.
[92] Wu J H, Zhu C F, Pang J H, Zhang X R, Yang C L, Xia G X, Tian Y C, He C Z. OsLOL1, a C2C2-type zinc finger protein, interacts with OsbZIP58 to promote seed germination through the modulation of gibberellin biosynthesis in., 2014, 80(6): 1118-1130.
[93] Ahn S N, Bollich C N, McClung A M, Tanksley S D. RFLP analysis of genomic regions associated with cooked-kernel elongation in rice., 1993, 87(1/2): 27-32.
[94] Amarawathi Y, Singh R, Singh A K, Singh V P, Mohapatra T, Sharma T R, Singh N K. Mapping of quantitative trait loci for basmati quality traits in rice (L.)., 2008, 21(1): 49-65.
[95] 何予卿, 邢永忠, 葛小佳, 李香花, 徐才国. 水稻米饭延伸指数相关性状的基因定位研究. 分子植物育种, 2003, 1(5/6): 613-619.
He Y Q, Xing Y Z, Ge X J, Li X H, Xu C G. Gene mapping for elongation index related traits on cooked rice grain quality., 2003, 1(5/6): 613-619. (in Chinese)
[96] Ge X J, Xing Y Z, Xu C G, He Y Q. QTL analysis of cooked rice grain elongation, volume expansion, and water absorption using a recombinant inbred population., 2005, 124(2): 121-126.
[97] Liu L L, Yan X Y, Jiang L, Zhang W W, Wang M Q, Zhou S R, Shen Y, Shen Y Y, Liu S J, Chen L M, Wang J K, Wan J M. Identification of stably expressed quantitative trait loci for cooked rice elongation in non-Basmati varieties., 2008, 51(2): 104-112.
[98] Rathi S, Pathak K, Yadav R N S, Kumar B, Sarma R N. Association studies of dormancy and cooking quality traits in direct-seededrice., 2014, 93(1): 3-12.
[99] 周丽慧, 刘巧泉, 张昌泉, 徐勇, 汤述翥, 顾铭洪. 水稻种子蛋白质含量及组分在品种间的变异与分布. 作物学报, 2009, 35(5): 884-891.
Zhou L H, Liu Q Q, Zhang C Q, Xu Y, Tang S Z, Gu M H. Variation and distribution of seed storage protein content and composition among different rice varieties., 2009, 35(5): 884-891. (in Chinese)
[100] 周丽慧, 刘巧泉, 顾铭洪. 不同粒型稻米碾磨特性及蛋白质分布的比较. 作物学报, 2009, 35(2): 317-323.
Zhou L H, Liu Q Q, Gu M H. Milling characteristics and distribution of seed storage proteins in rice with various grain shapes., 2009, 35(2): 317-323. (in Chinese)
[101] Vitale A, Hinz G. Sorting of proteins to storage vacuoles: how many mechanisms?, 2005, 10(7): 316-323.
[102] Kawakatsu T, YAMAMOTO M P, Hirose S, YANO M, TAKAIWA F. Characterization of a new rice glutelin geneexpressed in the starchy endosperm., 2008, 59(15): 4233-4245.
[103] Liu F, Ren Y L, Wang Y H, Peng C, Zhou K N, Lv J, Guo X P, Zhang X, Zhong M S, Zhao S L, Jiang L, Wang H Y, Bao Y Q, Wan J M. OsVPS9A functions cooperatively with OsRAB5A to regulate post-Golgi dense vesicle-mediated storage protein trafficking to the protein storage vacuole in rice endosperm cells., 2013, 6(6): 1918-1932.
[104] Kawakatsu T, Yamamoto M P, Touno S M, Yasuda H, Takaiwa F. Compensation and interaction between RISBZ1 and RPBF during grain filling in rice., 2009, 59(6): 908-920.
[105] Wen L, Fukuda M, Sunada M, Ishino S, Ishino Y, Okita T W, Ogawa M, Ueda T, Kumamaruet T. Guanine nucleotide exchange factor 2 for rab5 proteins coordinated with glup6/gef regulates the intracellular transport of the proglutelin from the golgi apparatus to the protein storage vacuole in rice endosperm., 2015, 66(20): 6137-6147.
[106] Tian L H, Dai L L, Yin Z J, Fukuda M, Kumamaru T, Dong X B, Xu X P, Qu L Q. Small GTPase Sar1 is crucial for proglutelin and α-globulin export from the endoplasmic reticulum in rice endosperm., 2013, 64(10): 2831-2845.
[107] Peng B, Kong H L, Li Y B, Wang L Q, Zhong M, Sun L, GaoG J, Zhang Q L, Luo L J, Wang G W, Xie W B, Chen J X, Yao W, Peng Y, Lei L, Lian X M, Xiao J H, Xu C G, Li X H, He Y Q.functions as an important regulator of grain protein content and nutritional quality in rice., 2014, 5: 4847.
[108] Galili G, Amir R. Fortifying plants with the essential amino acids lysine and methionine to improve nutritional quality., 2013, 11(2): 211-222.
[109] Wong H W, Liu Q Q, Sun S S. Biofortification of rice with lysine using endogenous histones., 2015, 87(3): 235-248.
[110] Liu X, Zhang C C, Wang X R, Liu Q Q, Yuan D Y, Pan G, Sun S S, Tu J M. Development of high-lysine rice via endosperm-specific expression of a foreign lysine rich protein, gene., 2016, 16(1): 1-13.
[111] Yang Q Q, Zhang C Q, Chan M L, Zhao D S, Chen J Z, Wang Q, Li Q F, Yu H X, Gu M H, Sun S S, Liu Q Q. Biofortification of rice with the essential amino acid lysine: molecular characterization, nutritional evaluation, and field performance., 2016, 67(14): 4285-4296.
[112] Griglione A, Liberto E, Cordero C, Bressanello D, Cagliero C, Rubiolo P, Bicchi C, Sgorbini B. High-quality Italian rice cultivars: Chemical indices of ageing and aroma quality., 2015, 172: 305-313.
[113] Bradbury L, Fitzgerald T, Henry R, Jin Q, Waters D. The gene for fragrance in rice., 2005, 3(3): 363-370.
[114] Chen S H, Yang Y, Shi W W, Ji Q, He F, Zhang Z D, Cheng Z K, Liu X N, Xu M L., encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-Acetyl-1-Pyrroline, a major component in rice fragrance., 2008, 20(7): 1850-1861.
[115] Kovach M, Calingacion M, Fitzgerald M, McCouch S. The origin and evolution of fragrance in rice (L.)., 2009, 106(34): 14444-14449.
[116] Hinge V R, Patil H B, Nadaf A B. Aroma volatile analyses and 2AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (L.) cultivars., 2016, 9(1): 38.
[117] Keyghobad K, Kad T D, Zanan R L, Nadaf A B. 2-Acetyl-1-pyrroline augmentation in scented indica rice (L.) varieties through Δ1-pyrroline-5-carboxylate synthetase () gene transformation., 2015,177(7): 1466-1479.
[118] Mo Z W, Huang J X, Xiao D, Ashraf U, Duan M Y, Pan S G, Tian H, Xiao L Z, Zhong K Y, Tang X R. Supplementation of 2-AP, zn and la improves 2-acetyl-1-pyrroline concentrations in detached aromatic rice panicles in vitro., 2016, 11(2): e0149523.
[119] Wakte K, Zanan R, Hinge V, Khandagale K, Nadaf A, Henry R. Thirty-three years of 2-acetyl-1-pyrroline, a principal basmati aroma compound in scented rice (L.): a status review., 2016, doi: 10.1002/jsfa.7875.
[120] Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid., 2014, 2(2): 75-104.
[121] Lei L, Waters D L, Rose T J, Bao J S, King G J. Phospholipids in rice: Significance in grain quality and health benefits: a review., 2013, 139(1/4): 1133-1145.
[122] Long Q Z, Zhang W W, Wang P, Shen W B, Zhou T, Liu N N, Wang R, Jiang L, Huang J X, Wang Y H, Liu Y Q, Wan J M. Molecular genetic characterization of rice seed lipoxygenase 3 and assessment of its effects on seed longevity., 2013, 56(4): 232-242.
[123] Huang J X, Cai M H, Long Q Z, Liu L L, Lin Q Y, Jiang L, Chen S H, Wan J M. OsLOX2, a rice type I lipoxygenase, confers opposite effects on seed germination and longevity., 2014, 23(4): 643-655.
[124] Zhou G X, Ren N, Qi J F, Lu J, Xiang C Y, Ju H P, Cheng J A, Lou Y G. The 9-lipoxygenase osr9-lox1 interacts with the 13- lipoxygenase-mediated pathway to regulate resistance to chewing and piercing-sucking herbivores in rice., 2014, 152(1): 59-69.
[125] Gayen D, Ali N, Sarkar S N, Datta S K, Datta K. Down-regulation of lipoxygenase, gene reduces degradation of carotenoids of golden rice during storage., 2015, 242(1): 353-363.
[126] Misra B B. The black-box of plant apoplast lipidomes., 2016, 7: 323.
[127] Wang X, Zhou W, Lu Z, Ouyang Y, Chol S O, Yao J. A lipid transfer protein, OsLTPL36, is essential for seed development and seed quality in rice., 2015, 239: 200-208.
[128] Chaudhary N, Khurana P. Vitamin E biosynthesis genes in rice: molecular characterization, expression profiling and comparative phylogenetic analysis., 2009, 177(5): 479-491.
[129] Wang X, Song Y E, Li J Y. High expression of tocochromanol biosynthesis genes increases the vitamin E level in a new line of giant embryo rice., 2013, 61(24): 5860-5869.
[130] Hwang J E, Ahn J W, Kwon S J, Kim J B, Kim S H, Kang S Y, Kim D S. Selection and molecular characterization of a high tocopherol accumulation rice mutant line induced by gamma irradiation., 2014, 41(11): 7671-7681.
[131] 张桂云, 刘如如, 张鹏, 徐勇, 朱姜, 顾铭洪, 梁国华, 刘巧泉. 水稻籽粒维生素 E 及组分在品种间的变异与分布. 作物学报, 2012, 38(1): 55-61.
Zhang G Y, Liu R R, Zhang P, Xu Y, Zhu Ji, Gu M H, Liang G H, Liu Q Q. Variation and distribution of vitamin E and composition in the seeds among different rice varieties., 2012, 38(1): 55-61. (in Chinese)
[132] Zhang G Y, Liu R R, Zhang P, Li Y, Tang K X, Liang G H, Liu Q Q. Increased alpha-tocotrienol content in seeds of transgenic rice overexpressingγ-tocopherol methyltransferase., 2013, 22(1): 88-99.
[133] Wang X Q, Yoon M Y, Qiang H, Kim T S, Wei T, Choi B W, Lee Y S, Park Y J. Natural variations in, contribute to diversity of the α-tocopherol content in rice., 2015, 290(6): 2121-2135.
[134] Zhang G Y, Liu R R, Zhang C Q, Tang K X, Sun M F, Yan G H, Liu Q Q. Manipulation of the rice L-Galactose pathway: evaluation of the effects of transgene overexpression on ascorbate accumulation and abiotic stress tolerance., 2015, 10(5): e0125870.
[135] Furukawa T, Maekawa M, Oki T, Suda I, Iida S, Shimada H, Takamure I, Kadowaki K. Theandgenes are involved in proanthocyanidin synthesis in rice pericarp., 2007, 49(1): 91-102.
[136] Maeda H, Yamaguchi T, Omoteno M, Takarada T, Fujita K, Murata K, Iyama Y, Kojima Y, Morikawa M, Ozaki H, Mukaino N, Kidani Y, Ebitani T. Genetic dissection of black grain rice by the development of a near isogenic line., 2014, 64(2): 134-141.
[137] Oikawa T, Maeda H, Oguchi T, Yamaguchi T, Tanabe N, Ebana K, Yano M, Ebitani T, Izawa T. The birth of a black rice gene and its local spread by introgression., 2015, 27(9): 2401-2414.
[138] Hefferon K L. Nutritionally enhanced food crops; progress and perspectives., 2015, 16(2): 3895-3914.
[139] Sperotto R A, Boff T, Duarte G L, Santos L S, Grusak M A, Fett J P. Identification of putative target genes to manipulate Fe and Zn concentrations in rice grains., 2010, 167(17): 1500-1506.
[140] Wang M, Gruissem W, Bhullar N K. Nicotianamine synthase overexpression positively modulates iron homeostasis-related genes in high iron rice., 2013, 4: 156.
[141] Boonyaves K, Gruissem W, Bhullar N K. Nod, promoter-controlled, expression functions synergistically with, and, genes to increase iron in rice grains., 2015, 90(3): 1-9.
[142] Masuda H, Usuda K, Kobayashi T, Ishimaru Y, Kakei Y, Takahashi M, Higuchi K, Nakanishi H, Mori S, Nishizawa K N. Overexpression of the barley nicotianamine synthase geneincreases iron and zinc concentrations in rice grains., 2009, 2: 155-166.
[143] Yoneyama T, Ishikawa S, Shu F. Route and regulation of zinc, cadmium, and iron transport in rice plants (L.) during vegetative growth and grain filling: metal transporters, metal speciation, grain cd reduction and Zn and Fe biofortification., 2015, 16(8): 19111-19129.
[144] Sreenivasulu N, Jr B V, Misra G, Cuevas R P, Anacleto R, Kavi Kishor P B. Designing climate-resilient rice with ideal grain quality suited for high-temperature stress., 2015, 66(7): 1737-1748.
[145] Ao Y, Xu C W, Cui X F, Xu Y, Wang A, Qiao Z Y, Liu Q Q. A genetic diversity assessment of starch quality traits in rice landraces from the Taihu basin, China., 2016, 15(3): 493-501.
[146] Lau W C P, Latif M A, Rafii Y R, Ismail M R, Puteh A. Advances to improve the eating and cooking qualities of rice by marker-assisted breeding., 2016, 36(1): 1-12.
[147] Ye X D, Al-Babili S, Klöti A, Zhang J, Lucca P, Beyer P, Potrykus I. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm., 2002, 87(2): 303-305.
[148] Wirth J, Poletti S, Aeschlimann B, Yakandawala N, Drosse B, Osorio S, Tohge T, Fernie A R, Günther D, Gruissem W, Sautter C. Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin., 2009, 7(7): 631-644.
[149] Lau E. Breaking Mendelian inheritance with CRISPR-Cas.,2015, 16(5): 258-259.
(责任编辑 李莉)
Progresses in Research on Cloning and Functional Analysis of Key Genes Involving in Rice Grain Quality
Zhang Chang-quan, Zhao Dong-sheng, Li Qian-feng, Gu Ming-hong, Liu Qiao-quan
(Key Laboratory of Plant Functional Genomics of the Ministry of Education, College of Agriculture, Yangzhou University/ Co-Innovation Center for Modern Production Technology of Grain Crops, Yangzhou 225009, Jiangsu)
Rice (L.) is one of the most important cereal crops in worldwide and also a major stable food in China, thus it is very important to breed novel rice cultivars with high yield as well as good grain quality. Rice grain quality is a complex trait, and usually means rice or rice products meeting the demand of end-users. Therefore, the concept of rice grain quality covers multiple features revealed by the physical and chemical characteristics, including milled rice ratio, grain shape, appearance, cooking time, aroma and its retention after cooking, eating palatability, and nutrition. In general, rice grain quality includes as milling quality, apparent quality, eating and cooking quality (ECQ), and nutritional value. The grain shape is not only the factors associated with yield but also crucial aspects of grain quality. In the past decade, there were rapid and great achievements in the cloning and functional analyses of the genes involving in rice grain qualities. For grain size and shape, numerous QTLs and genes have been cloned and characterized. These cloned genes could be divided into three groups based on the phenotypes of the mutants. The first group is associated with not only grain shape but also plant phenotype, such as,,,and. The second group appears to specifically affect grain trait, including,,,,,,,,,,and, which are well valuable for improvement of grain yield and quality. The third group is called small and round seed, such as thegene. Chalkiness is associated with both grain appearance and milling property, and only few such QTLs have been finely mapped and cloned, including,,,,and. The starch comprises about 90% of the dry matter of rice endosperm, and thus the grain quality is greatly affected by starch composition and structure. Therefore, the starch biosynthesis plays a crucial role in the formation of rice quality, especially the eating and cooking quality. Recent studies had made deep understanding of the regulation network of starch biosynthesis related enzymes, and several transcriptional regulators had also been proven for involving in starch biosynthesis, such as Dull, OsEBP89, OsEBP5, OsRSR1 and OsbZIP58. For seed protein content, most of the genes for seed storage proteins have been well characterized, and some other genes, such as,,,,,,andhave also been identified associating with protein sorting and transporting. The aroma of cooked rice contributes to consumer sensory acceptance, and recent studies have confirmed that theandgenes are responsible for the synthesis of fragrance material 2-AP. As for the other nutritional factors, such as the contents of essential amino acid lysine, vitamins, anthocyanin and minerals, also many functional genes have been cloned or elucidated. Taken together, all of the above traits are known to be genetically controlled by multiple genes, and also interact with each other. In present review, the genetic networks involving in regulation of rice grain quality in the last decade were summarized and updated. It will give a better understanding of the genes that contribute to the overall grain quality as well as lay a foundation for development of new strategies for grain quality improvement with high yield in rice.
rice grain quality; gene cloning; quantitative trait locus (QTL); allelic variation; functional analysis
2016-08-12;接受日期:2016-09-18
国家转基因生物新品种培育重大专项(2016ZX08009003-004、2014ZX08009-024B)、国家自然科学基金(31561143008、31401354)、教育部博士点基金(20133250120001)
张昌泉,Tel:0514-87937537;E-mail:cqzhang@yzu.edu.cn。通信作者刘巧泉,Tel:0514-87979242;E-mail:qqliu@yzu.edu.cn

