磁共振弥散加权成像术在腹膜转移癌诊断中的应用价值
张换+童彤
摘 要 腹膜转移癌患者的预后较差,良好的影像学评估方法有助于其治疗方案的选择和治疗疗效的评估。腹膜转移癌在磁共振弥散加权成像术检查图像中表现为较之周围组织明显的高亮信号,与CT检查相比能明显提高腹膜癌的检出率,尤其是小转移癌灶以及膈下、肝包膜下、网膜囊、肠系膜根部和肠管浆膜表面的腹膜癌的检出率。
关键词 腹膜转移癌 磁共振成像术 弥散加权成像术
中图分类号:R735; R730.44 文献标识码:A 文章编号:1006-1533(2016)23-0015-03
The application of diffusion-weighted magnetic resonance imaging in the diagnosis of peritoneal carcinomatosis*
ZHANG Huan**, TONG Tong***
(Department of Radiology, Shanghai Cancer Center, Fudan University; Department of Oncology, Shanghai Medical School, Fudan University, Shanghai 200032, China)
ABSTRACT Peritoneal carcinomatosis is invariably associated with a poor prognosis. A good imaging evaluation method can benefit the options of individualized treatment and the assessment of curative effect. Peritoneal carcinomatosis is manifested as obvious highlight signal compared with the surrounding tissues on the magnitude diffusion-weighted images. Diffusionweighted magnetic resonance imaging, compared with CT, can significantly improve the accuracy in the detection of peritoneal cancer, especially for the small metastatic foci and the peritoneal cancers on some special anatomical sites under the diaphragm and liver capsule and of omental capsule, mesenteric root and intestinal serosal surface.
KEY WORDS peritoneal carcinomatosis; magnetic resonance imaging; diffusion-weighted imaging
腹膜转移癌是指恶性肿瘤直接种植于腹膜或经各种途径转移至腹膜腔形成的继发性腹膜肿瘤,最常见于卵巢癌、胃癌和结直肠癌患者,发生率约分别为71%、15%和10%[1]。腹膜转移癌患者的预后通常不良,诊出后的中位生存期仅约为6个月[2]。目前,对腹膜转移癌的最佳治疗策略是细胞减灭术(cytoreductive surgery, CRS)联合腹腔热灌注化疗(hyperthermic intraperitoneal chemotherapy, HIPEC),即通过手术切除可视癌灶,再用高浓度化疗药物溶液冲洗腹腔以杀灭残留癌细胞,能有效改善患者的生存期[3]。
腹膜癌指数(peritoneal cancer index, PCI)是一种通过评估腹腔13个分区中腹膜癌的分布和大小来量化腹膜癌负荷情况的评分系统(图1),为现最常用的腹膜转移癌分期系统[4],其评分被认为是腹膜转移癌患者的一个独立预后因素。此外,对接受CRS联合HIPEC治疗的腹膜转移癌患者,PCI评分也是能否达到完全性减瘤目标的一个重要影响因素[5-6]。因此,得知准确的术前PCI评分有助于适于CRS联合HIPEC治疗患者的选择并预测其预后。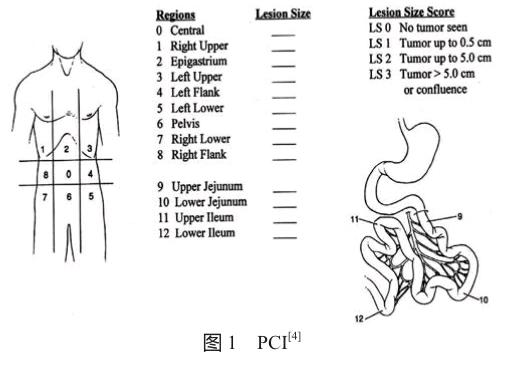
1 与CT检查相比,MRI检查用于腹膜转移癌诊断的优势
CT检查过去一直是最常用于腹膜转移癌的诊断、术前分期和随访的影像学评估方法,但其对软组织的分辨力有限,使得检出小腹膜转移癌灶的灵敏度较低[7-8]。一项研究显示,相较于术中发现,术前CT检查对直径<0.5 cm腹膜转移癌灶的检出率仅有11%[9]。此外,作为一种单纯形态学成像术,CT检查仅能从癌灶大小的形态学改变来评估治疗疗效,但不能反映癌灶的早期功能学改变,而对亚厘米级别的癌灶,很难以形态学改变来评估治疗疗效。
MRI具有较高的软组织分辨力,可多层面、多角度成像,因此MRI检查可更准确地反映腹膜癌的分布和大小,更适宜用于腹膜转移癌的术前分期。一项研究显示,增强型MRI检查检出腹膜癌灶的灵敏度、特异性和准确度分别为84%、87%和86%,优于CT检查(分别为54%、91%和74%),尤其是对直径<1 cm癌灶的检出率大大提高(分别为75% ~ 80%和22% ~ 33%)[10]。近年来,随着弥散加权成像术(diffusion-weighted imaging, DWI)在腹部疾病影像学诊断中的广泛应用,临床上对腹膜转移癌的检出能力已得到明显提高[11-12]。
2 DWI的基本原理和参数以及腹膜转移癌在DWI检查图像中的表现
DWI的成像原理基于水分子布朗运动,即在机体组织中,由于细胞外微体系结构、主动运输机制和微血管循环等因素使得水分子弥散运动得以缓慢进行,这种水分子弥散运动的限制性合成效应反映了组织的微环境。DWI通过改变所施加的磁场梯度的幅度、持续时间和间隔时间等参数(即弥散敏感系数b值),能调节对水分子弥散运动的敏感性。b值较小时可得到较高信噪比的图像,但易受局部组织微循环灌注的影响,不能正确反映水分子的弥散运动。b值越高,越能较好地反映实际的水分子弥散运动[13]。检测到的水分子弥散程度可以表观扩散系数(apparent diffusion coefficient, ADC)来量化,其来源于至少两个b值的DWI信号强度的指数衰减[13]。腹膜转移癌在DWI检查图像上显示为高亮信号,但ADC值降低,表明水分子弥散受限,这可能与癌灶的多细胞无序排列、低血流灌注以及细胞分子水平的改变有关。
腹膜转移癌在DWI检查图像上表现为腹膜、网膜或肠系膜的结节样、团块样或条带样高亮信号,增强型MRI检查图像上还可见腹膜的增厚和异常强化(正常腹膜强化程度约等同于肌肉组织的强化程度)情况[14]。常规MRI联合DWI检查能提供准确的术前腹膜转移癌的形态、位置以及淋巴结肿大和腹水等情况[14]。
3 DWI检查在腹膜转移癌诊断中的应用
在DWI检查图像中,腹膜转移癌表现为较之周围组织明显的高亮信号,尤其是膈下、肝包膜下、网膜囊、肠系膜根部和肠管浆膜表面的腹膜癌的对比度明显更高,与CT检查相比能明显提高对腹膜转移癌的检出率[15-19]。Bozkurt等[16]的研究显示,联合高b值(800 s/mm2)DWI的腹部MRI检查对腹膜转移癌灶的检出率更高,灵敏度由常规MRI检查的58%提高到85%。Low等[20]的研究也证实,对腹膜转移癌患者,联合DWI的MRI检查所得的术前PCI评分与术中所得的PCI评分高度吻合,常规MRI、DWI及两者联合检查对腹膜转移癌灶的检出灵敏度分别为73%、71%和90%,特异性分别为90%、90%和91%,表明联合DWI的常规MRI检查可在很大程度上提高对腹膜转移癌的检出灵敏度。
动物模型研究提示,ADC值升高与死亡的肿瘤细胞密度正相关,ADC值的变化先于癌灶大小的变化,可用作治疗早期有效的指标[21-22]。一系列对不同肿瘤类型患者的临床研究也发现,早于癌灶可测体积缩小,治疗有效患者的ADC值明显升高,且这些患者在治疗前往往预后不良,表明治疗前的ADC值有希望用作治疗疗效预测指标[23-26]。但由于腹膜转移癌的解剖位置复杂且分布广泛,加之部分癌灶较小等,给对腹膜转移癌灶测量ADC值时感兴趣区域的选择带来了极大困难,目前尚无相关文献可以参考。
4 DWI检查用于腹膜转移癌诊断的相对局限性
目前,有关DWI检查的b值选择、后处理技术和数据分析方法尚无国内外统一标准。虽然DWI检查在腹膜转移癌评估中有明显的优势或潜力,但成本相对昂贵、图像采集时间较长且空间分辨率相对较低,图像质量又易受呼吸运动的影响或干扰,这些不利因素限制了其在临床上的广泛应用。
参考文献
[1] Koppe MJ, Boerman OC, Oyen WJ, et al. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies [J]. Ann Surg, 2006, 243(2): 212-222.
[2] Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer [J]. Cancer, 2008, 113(2): 315-325.
[3] Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial [J]. Ann Surg Oncol, 2011, 18(6): 1575-1581.
[4] Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis [J]. Cancer Treat Res, 1996, 82: 359-374.
[5] Yan TD, Sim J, Morris DL. Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy [J]. Ann Surg Oncol, 2007, 14(6): 1807-1817.
[6] Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer [J/OL]. Int Semin Surg Oncol, 2005, 2(1): 3 [2016-09-13]. http:// issoonline.biomedcentral.com/track/pdf/10.1186/1477-7800-2-3?site=issoonline.biomedcentral.com.
[7] Metser U, Jones C, Jacks LM, et al. Identification and quantification of peritoneal metastases in patients with ovarian cancer with multidetector computed tomography: correlation with surgery and surgical outcome [J]. Int J Gynecol Cancer, 2011, 21(8): 1391-1398.
[8] Coakley FV, Choi PH, Gougoutas CA, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer [J]. Radiology, 2002, 223(2): 495-499.
[9] Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis [J]. Ann Surg Oncol, 2009, 16(2): 327-333.
[10] Low RN, Barone RM, Lacey C, et al. Peritoneal tumor: MR imaging with dilute oral barium and intravenous gadoliniumcontaining contrast agents compared with unenhanced MR imaging and CT [J]. Radiology, 1997, 204(2): 513-520.
[11] Kyriazi S, Collins DJ, Morgan VA, et al. Diffusion-weighted imaging of peritoneal disease for noninvasive staging of advanced ovarian cancer [J]. Radiographics, 2010, 30(5): 1269-1285.
[12] Low RN, Gurney J. Diffusion-weighted MRI (DWI) in the oncology patient: value of breathhold DWI compared to unenhanced and gadolinium-enhanced MRI [J]. J Magn Reson Imaging, 2007, 25(4): 848-858.
[13] Kyriazi S, Kaye SB, deSouza NM. Imaging ovarian cancer and peritoneal metastases — current and emerging techniques[J]. Nat Rev Clin Oncol, 2010, 7(7): 381-393.
[14] Iafrate F, Ciolina M, Sammartino P, et al. Peritoneal carcinomatosis: imaging with 64-MDCT and 3T MRI with diffusion-weighted imaging [J]. Abdom Imaging, 2012, 37(4): 616-627.
[15] Low RN. MR imaging of the peritoneal spread of malignancy[J]. Abdom Imaging, 2007, 32(3): 267-283.
[16] Bozkurt M, Doganay S, Kantarci M, et al. Comparison of peritoneal tumor imaging using conventional MR imaging and diffusion-weighted MR imaging with different b values[J]. Eur J Radiol, 2011, 80(2): 224-228.
[17] Low RN, Sebrechts CP, Barone RM, et al. Diffusion-weighted MRI of peritoneal tumors: comparison with conventional MRI and surgical and histopathologic findings — a feasibility study [J]. AJR Am J Roentgenol, 2009, 193(2): 461-470.
[18] Soussan M, Des Guetz G, Barrau V, et al. Comparison of FDG-PET/CT and MR with diffusion-weighted imaging for assessing peritoneal carcinomatosis from gastrointestinal malignancy [J]. Eur Radiol, 2012, 22(7): 1479-1487.
[19] Michielsen K, Vergote I, Op de Beeck K, et al. Whole-body MRI with diffusion-weighted sequence for staging of patients with suspected ovarian cancer: a clinical feasibility study in comparison to CT and FDG-PET/CT [J]. Eur Radiol, 2014, 24(4): 889-901.
[20] Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures [J]. Ann Surg Oncol, 2015, 22(5): 1708-1715.
[21] Kim H, Morgan DE, Buchsbaum DJ, et al. Early therapy evaluation of combined anti-death receptor 5 antibody and gemcitabine in orthotopic pancreatic tumor xenografts by diffusion-weighted magnetic resonance imaging [J]. Cancer Res, 2008, 68(20): 8369-8376.
[22] Morse DL, Galons JP, Payne CM, et al. MRI-measured water mobility increases in response to chemotherapy via multiple cell-death mechanisms [J]. NMR Biomed, 2007, 20(6): 602-614.
[23] Niwa T, Ueno M, Ohkawa S, et al. Advanced pancreatic cancer: the use of the apparent diffusion coefficient to predict response to chemotherapy [J]. Br J Radiol, 2009, 82(973): 28-34.
[24] Sharma U, Danishad KK, Seenu V, et al. Longitudinal study of the assessment by MRI and diffusion-weighted imaging of tumor response in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy [J]. NMR Biomed, 2009, 22(1): 104-113.
[25] Koh DM, Scurr E, Collins D, et al. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients [J]. AJR Am J Roentgenol, 2007, 188(4): 1001-1008.
[26] Cui Y, Zhang XP, Sun YS, et al. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases [J]. Radiology, 2008, 248(3): 894-900.

