Technology optimization of Medicago sativa leaf protein separation with foam fractionation
Liu Haibin, Zhang Wei, Chen Yuantao, Gao Zhongchao, Liu Long
(Department of Chemistry, Qinghai Normal University, Xining 810008, China)
Technology optimization of Medicago sativa leaf protein separation with foam fractionation
Liu Haibin, Zhang Wei※, Chen Yuantao, Gao Zhongchao, Liu Long
(Department of Chemistry, Qinghai Normal University, Xining 810008, China)
In order to purify Medicago sativa leaf protein effectively, foam fractionation which could increase both enrichment ratio and recovery of Medicago sativa leaf protein, was developed based on the research of the influences of initial pH value, the air flow rate, the loading liquid volume and the ratio of material to solvent. Then the best single factor was defined which was the loading liquid volume of 550 mL, pH value of 6.5, the ratio of material to solvent of 87.5 mg/L, the air flow rate of 250 mL/min. Based on the single factor experiment, Box-Behnken design was adopted to investigate the influence of four factors: pH value, the loading liquid volume, concentration and the air flow rate on recovery and enrichment ratio of leaf protein from Medicago sativa. Response surface and contour plots were finally graphed with the recovery and enrichment ratio as the response value. The foam fractionation of leaf protein from Medicago sativa was optimized by response surface methodology. The model P-value were both smaller than 0.0001 and the model were goodness-of-fit and the adequacy of the regression model. The optimum conditions for the foam fractionation of leaf protein from Medicago sativa were found to pH value of 6.92 , the ratio of material to solvent of 87.5 mg/L, the loading liquid volume of 600 mL, the air flow rate of 200 mL/min. Under the conditions, the recovery of Medicago sativa leaf protein was 92.2% and the enrichment ratio was 8.03. Considering actual process, The optimization processes were pH value of 7.0 , the ratio of material to solvent of 87.5 mg/L, the loading liquid volume of 600 mL, the air flow rate of 200 mL/min. The recovery of Medicago sativa leaf protein was 90.2% and the enrichment ratio was 7.64. respectively. The experimental results showed that foam fractionation was an effective method to separate leaf protein from Medicago sativa.
protein; technology; optimization; medicago sativa; foam fractionation
Biography:Liu Haibin, Extraction and separation of natural products. Xining Department of Chemistry, Qinghai Normal University, 810008, China. Email: lhb_86@163.com
※Corresponding author:Zhang Wei, professor. Extraction and separation of natural products Xining Department of Chemistry, Qinghai Normal University, 810008, China. Email: zhangwei@qhun.edu.cn
0 Introduction
Medicago sativa is a perennial herbaceous plant in leguminous clover. It is known for high yield and strong stress resistance. There are a lot of protein, vitamins(vitamin A, vitamin E), minerals, calcium, magnesium, iron and zinc, lutein and carotene in Medicago sativa. Especially protein, content of crude protein that is extracted from fresh Medicago sativa leaf is 50%-60%, even more 70%[1]. Amino acids that include 18 amino acids and 8 essential are as high as 5.6%-7.4%, which siuts for the acids standard recommended by united nations food agriculture organization[2]. The content of alanine and glycine, methionine, cystine, proline and threonine are higher than soybean protein. Utilizing and developing Medicago sativa leaf protein ease the demand for protein and provide protein-enriched products for special consumer groups and for particular consumers and for a wide range of markets.
So far, the extraction and purification of protein from Medicago sativa were principally performed by heating, acid (alkali), salting out, organic solvent and microbial fermentation methods[3]. Furthermore the problems of low yield, serious environmental pollution, low utilization, high cost and large investment were involved in these process. Therefore, it is very important to explore a new technology with high yield, low cost and investment and free of pollution, which is suitable for industrial production.
Foam fractionation technique, an ecological and economical method belonging to the adsorptive bubble separation techniques, is concerned with the recovery and concentration of surfactant molecules or other materials which could adsorb on the surface of the active agent. The feed solution containing the surfactant molecules is poured into a foam fractionation column, while the gas is sparged through the base of the column to create a dispersion of rising bubbles. Surface-active molecules adsorb to surfaces of the bubbles which are collected as foam. When this foam is broken into the so-called ‘‘foamate’’ stream, the surfactant in the solution is enriched since the foam has a relatively high surface area for a specific volume of interstitial liquid[4]. In recent years, this technique has been applied to the separation of biological macromolecules such as proteins[5]and enzymes[6-7]. So it has good potential applications in the field of biological and chemical separation due to its simple operation, high efficiency, low energy consumption and combining multiple factors characteristics.
In this paper, Medicago sativa was considered as the material. Factors were explored by pH value, the ratio of material to solvent, the air flow rate, the volume of liquid for the impact on purification of Medicago sativa leaf protein. Using response surface method optimizes the extractive technology of the Medicago sativa leaf protein in order to promote the application of the foam fractionation technology in leaf protein industry.
1 Materials and methods
1.1 Materials
Medicago sativa leaf was Qingda No.1 and obtained from test field in Qinghai university of agriculture and animal husbandry. Coomassie brillant blue G-250.0 and Bovine serum albumin were obtained from Shanghai yuanye Bio-Technology Co.Ltd. Phosphoric Acid and ethanol of analytical grade were obtained from Sinopharm chemical reagent Co. Ltd.
TU-1901 double-beam ultraviolet-visible spectrophotometer was obtained from Beijing’s general instrument Co. Ltd. LZB Glass Rotameter (60–600.0 mL/min) was obtained from Nanjing laishun measurement and control equipment Co. Ltd. FB45/7 air compressor was obtained from shanghai jinbao air compressor Co. Ltd. pH apparatus, PHS-25 Research type, was obtained from Mettler Toledo instrument (Shanghai) Co. Ltd. BSA224S- CW analytical balance was obtained from sartorius scientific instruments (Beijing) Co. Ltd. HK-2A super constant temperature water bath was obtained from Nanjing University Institute of physics applied physics. GX-04 wanjiafu grinder was obtained from changfa instrument (zhejiang) Co. Ltd.
1.2 Column apparatus
The foam fractionation column which was marked scales was 800 mm high with an inner diameter of 35 mm and manufactured from glass. The air compressor and rotameter were used to control the flow of the compressed air which passed through quartz filter at the bottom of the column. Bubbles formed in the bulk liquid phase when the air passed through the distributor. The resultant foam then travelled up the foam phase of the column. In order to collect the foam, a U-tube was fitted at the top of the column and the flow of foam was directed to a collection vessel (Fig.1).
1.3 Extraction of Medicago sativa leaf protein
Medicago sativa leaf (Water content of 8.8%) was shattered by grinder and sized by 60 mush. 12.5 g of Medicago sativa powder, with 0.7% sodium hydroxide solution as the extraction agent, under the condition of temperature 60℃ and the ratio of material to solvent of 1:20 g/mL, in a stirring for 20 minutes, was extracted twice and combined filtrate to be used. Content of Medicago sativa leaf protein is 19.8%.
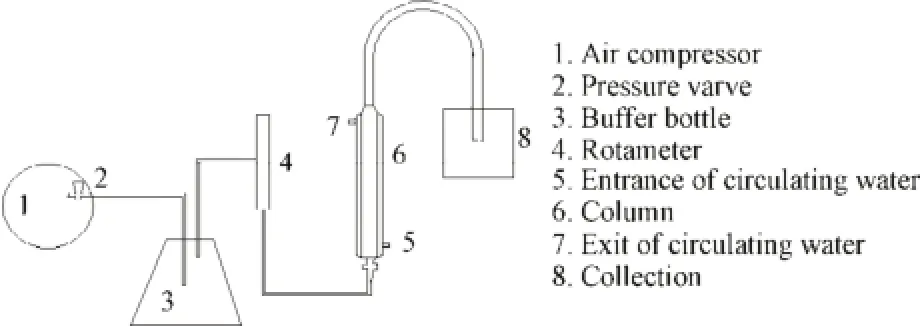
Fig.1 Schematic diagram of foam fractionation apparatus
1.4 Detection of bovine serum albumin concentration
Bovine serum albumin concentration is determined by bradford method[8]. Coomassie brilliant blue combines with protein in crystalline solutions and generats a kind of blue complex which is measured at 595 nm. Beer’s law is obeyed. In this paper, the relationship between bovine serum albumin concentration and absorbance was the linear regression equation, namely A=0.0394C+0.0226, correlation coefficient R2=0.9961, where C is the bovine serum albumin concentration (μg/mL) and A is the absorption value.
1.5 Determination of batch foam fractionation performances
Performance parameters of batch foam fractionation are enrichment ratio (E) and recovery percentage (R)[9].
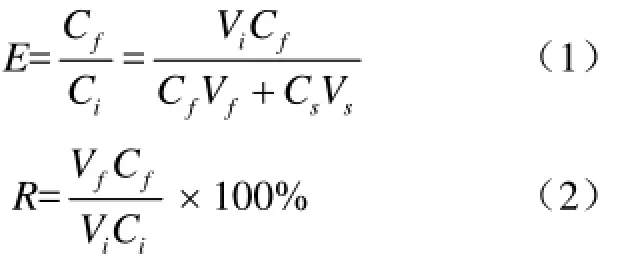
Where Ci(μg/mL), Cf(μg/mL) and Cs(μg/mL) are the ratio of material to solvent of the initial loading solution, the foamate and the residual solution, respectively. Vi(mL), Vf(mL), Vs(mL) are volumes of the initial loading solution, the foamate and the residual solution.
1.6 The single factor experiment
1.6.1 Effect of the ratio of material to solvent on the enrichment and recovery
The effects of the ratio of material to solvent on E and R were studied by using the column with the foam separation column under the conditions of initial the air flow rate of 300 mL/min, the liquid loading volume of 600 mL and pH value of 6.0. the ratio of material to solvent ranged from 70.0 to 157.0 mg/L.
1.6.2 Effect of the pH value on the enrichment and recovery
The effect of pH value were studied under the condition the ratio of material to solvent of 105.0 mg/L, the loading liquid volume of 600 mL and the air flow rate of 300 mL/min. pH value ranged from 5.0 to 7.0.
1.6.3 Effect of the loading liquid volume on the enrichment and recovery
The effect of loading liquid volume on the performance of foam fractionation were investigated under the conditions that the ratio of material to solvent, pH value and the air flow rate fixed at 105.0 mg/L, 6.5 and 300 mL/min, respectively. On the basis of the column’s height, loading liquid volume ranged from 400 mL to 650 mL.
1.6.4 The effects of the air flow rate on the enrichment and recovery
The effects of the air flow rate were investigated under the conditions of the ratio of material to solvent of 105.0 mg/L, the loading liquid volume of 550 mL and pH value of 6.5, respectively. The air flow rate ranged from 100 mL/min to 400 mL/min.
1.7 Response surface design
The statistical software Design Expert 8.0.6 was used for design of experiments, regression and graphical analyses of data obtained and statistical analysis of the obtained models to evaluate the analysis of variance.
On Box-Behnken factorial design and regression analysis, Medicago sativa leaf protein was optimizated with four factors and three levels(Table 1). Center level were the pH value of 6.5, the ratio of material to solvent of 105.0 mg/L, the loading liquid volume of 550 mL, the air flow rate of 250 mL/min. Other levels were -1 and 1. A full factorial design was applied and a total of 29 runs that included five center points were performed in duplicates.

Table 1 Factors and levels used in response surface design
1.8 Verification of predictive model
Design-Expert software was used to provide the optimal conditions for the response surface analysis. Considering actual process, we modified date and wanted to verify the predictive model.
2 Results and Discussion
2.1 The enrichment and recovery of Medicago sativa leaf protein using foam fractionation
2.1.1 Effect of the ratio of material to solvent on the enrichment and recovery
As shown in Fig.2, the ratio of material to solvent had a significant effect on the separation efficiency. With increasing the ratio of material to solvent from 70.0 mg/L to 157.0 mg/L, the enrichment ratio decreased from 15.08 to 5.45 and the recovery percentage increased from 62.85% to 89.08%. With increasing the ratio of material to solvent from 70.0 mg/L to 105.0 mg/L, the recovery percentage rapidly increased, while the ratio of material to solvent was more than 105.0 mg/L, the recovery percentage tended to be constant. With the ratio of material to solvent increasing, the surface adsorption tended to saturation and adsorption quantity increased slowly. At the same time, with foam volume increasing, the content of water in the foam also increased, which led to the enrichment drop[9-11].
2.1.2 Effect of the pH value on the enrichment and recovery
pH value effects the performance of foam fractionation due to its effect on the net charge, the interfacial tension and the surface rigidity, especially for a protein. As shown in Fig.3, with the increasing of pH value from 5.0 to 7.0, the enrichment ratio of Medicago sativa protein decreased from 6.76 to 4.98 and then increased to 5.30. While the recovery percentage of protein increased from 41.67% to 81.31% and then decreased to77.71%. The recovery had a maximum peak at pH value of 6.5, which was its isoelectric point[12]. The net charge of the protein molecules was zero when pH value was located at its isoelectric point, resulting in impairing the double electrode layer around the protein molecules[13]. The decrease of net charge repulsion and the impairing double electrode layer decreased the thickness of foam film. Then bubble coalescence would be enhanced at certain degree. Foam would increase, when solution was its isoelectric point. So the recovery percentage of protein had a maximum peak. At the same time, rate of water content would increase and the enrichment ratio of Medicago sativa protein had a peak valley[14].
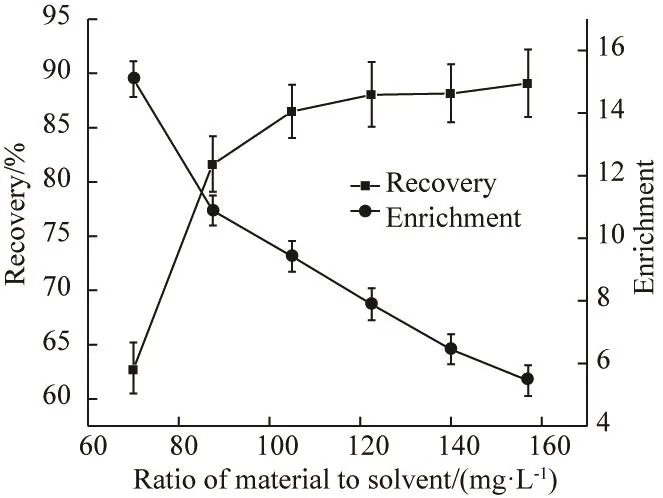
Fig.2 Effect of ratio of material to solvent on enrichment and recovery

Fig.3 Effect of pH value on enrichment and recovery
2.1.3 Effect of the loading liquid volume on the enrichment and recovery
When the height of the foam fractionation column was fixed, the variation of the loading liquid volume would make a change of the height of foam layer and bulk liquid layer, which further affected the protein adsorption on the gas-liquid interface, foam drainage and foam fractionation performances[15-16]. As shown in Fig.4, the protein enrichment ratio decreased from 11.15 to 4.34, while the recovery percentage increased from 61.34% to 87.63% with increasing the loading liquid volume from 400 mL to 650mL. With increasing the loading liquid volume, the resident time of the bubble in the liquid became shorter, which decreased the absorption time of protein on the interface. As a result, the total quantity of protein adsorbed on the gas-liquid interface was increased. Thus, the protein concentration in the residual solution at the condition of lower loading liquid volume was higher than that at higher loading volume[13,17]. Consequently, the low loading liquid volume made the recovery percentage lower. Furthermore, the height of the foam layer increased which made the resident time of the bubbles in the foam layer to shot, bubble coalescence and foam drainage happened. It led to higher water content and lower protein concentration in the foam. Thus, 550 mL was selected.
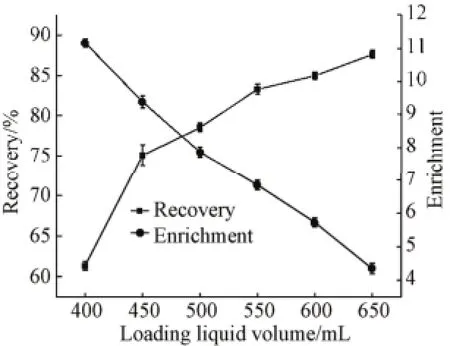
Fig.4 Effect of loading liquid volume on enrichment and recovery
2.1.4 The effects of the air flow rate on the enrichment and recovery
The air flow rate could significantly affect both interfacial adsorption and foam drainage. Statistical analysis revealed a important effect of the air flow rate on foam fractionation. As shown in Fig.5, the protein enrichment ratio decreased from 11.68 to 4.13 while the recovery percentage increased from 83.72% to 88.13% with increasing the air flow rate from 100 to 400 mL/min. The air flow rate influenced very much the wetness of the rising foam and therefore determined the liquid fraction of the foamate. The low air flow rates made the foam rise slowly inside the column, providing more time for processes like coalescence and drainage of superfluous liquid[18]. The former was based on the tendency of smaller gas bubbles to combine into one bigger gas bubble with a reduced surface compared to the aggregate surface of the small bubbles. Liberated water molecules could then drain off back to the feed while surface-active compounds stay adsorbed at the gas-liquid interface within the foam. Consequently the foamate was drier and concentration in surface-active molecules was higher[10,19].
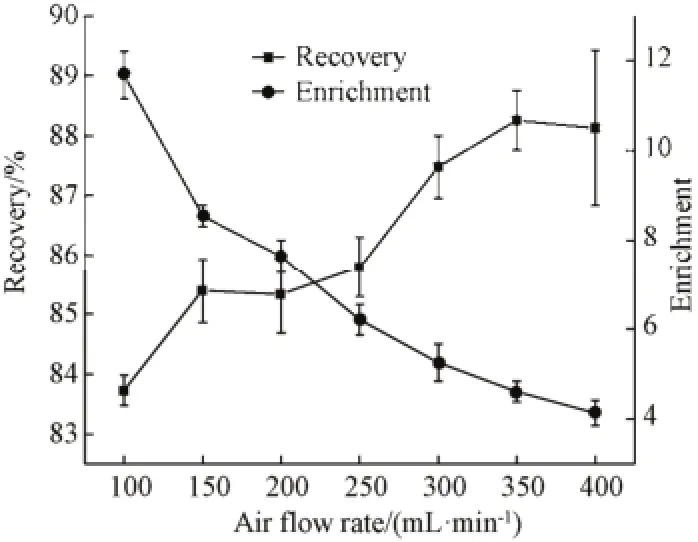
Fig.5 Effects of air flow rate on enrichment and recovery
2.2 Experimental design and statistical analysis.
2.2.1 Response surface design
A full factorial design was applied and a total of 29 runs were performed in duplicates. The four independent variables (A is the ratio of material to solvent; B is pH value; C is loading liquid volume and D is air flow rate) were investigated at three levels, as illustrated in Table 2.
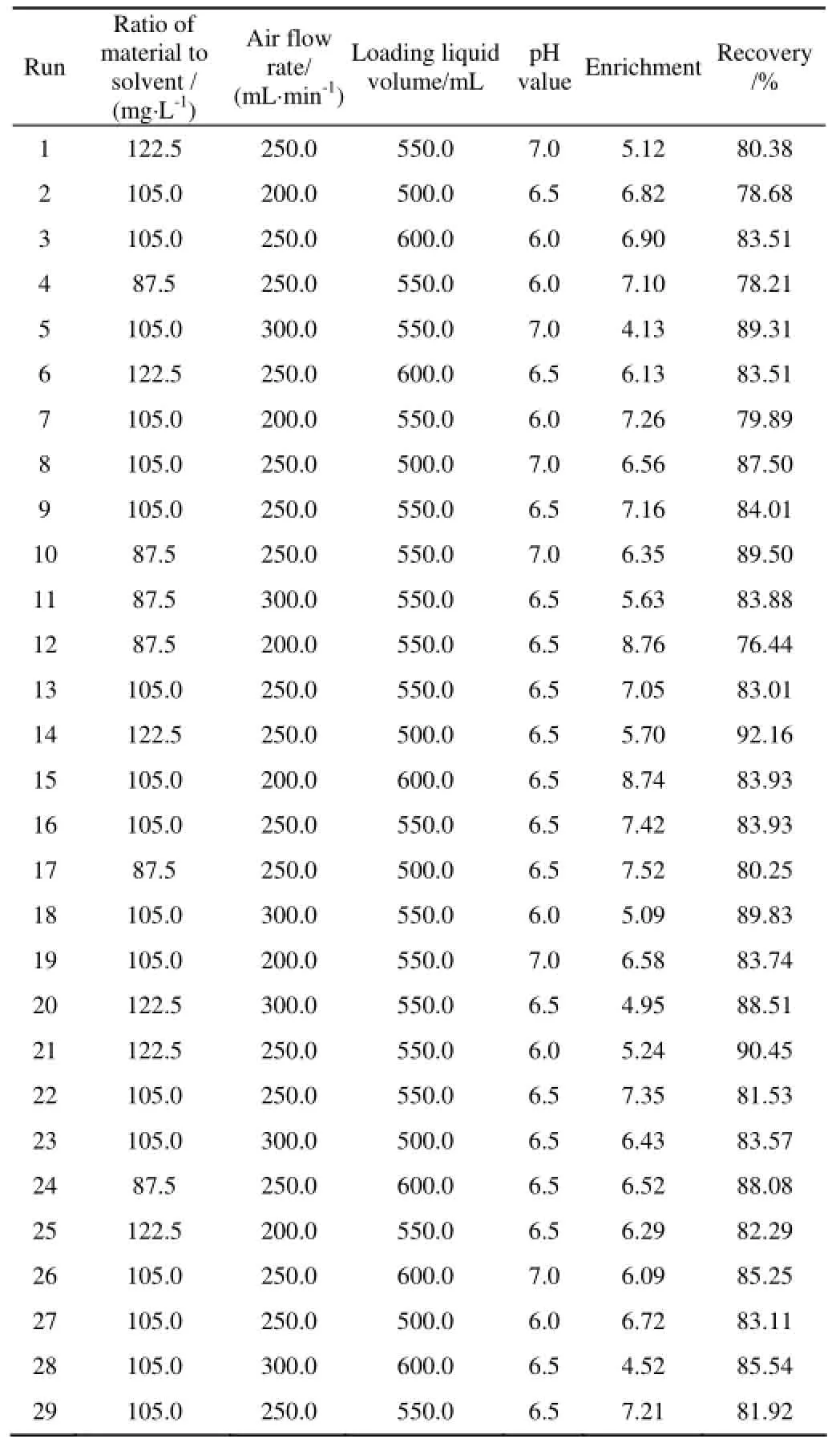
Table 2 Response surface design and experimental result
2.2.2 Statistical analysis and the model fitting
On analyzing the data by applying a multiple regression analysis approach, quadratic polynomial functions were fitted to the experimental data.
The response surface regression results from the central composite design are F values and P values along with the constant and coefficients (estimated using coded values) given in Table 3,4. The results of the analysis of variance (ANOVA) indicate that the quadratic model contributed significantly.
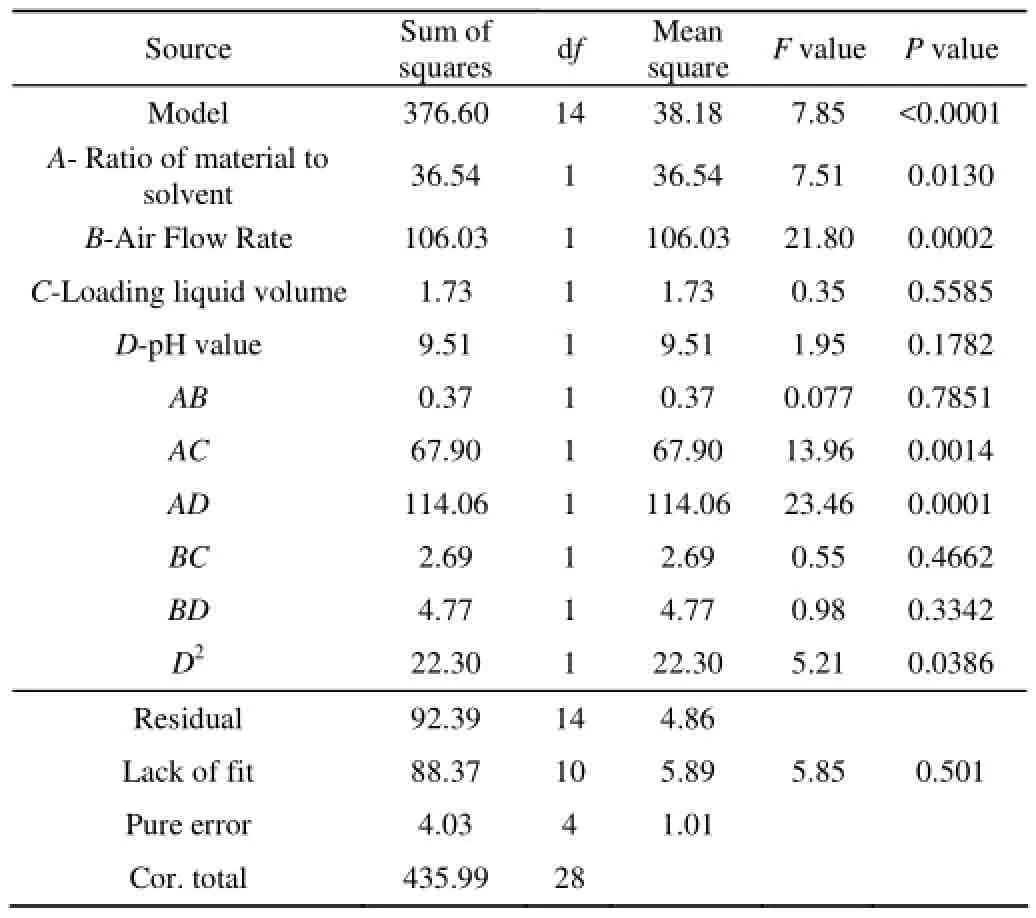
Table 3 ANOVA for response of recovery
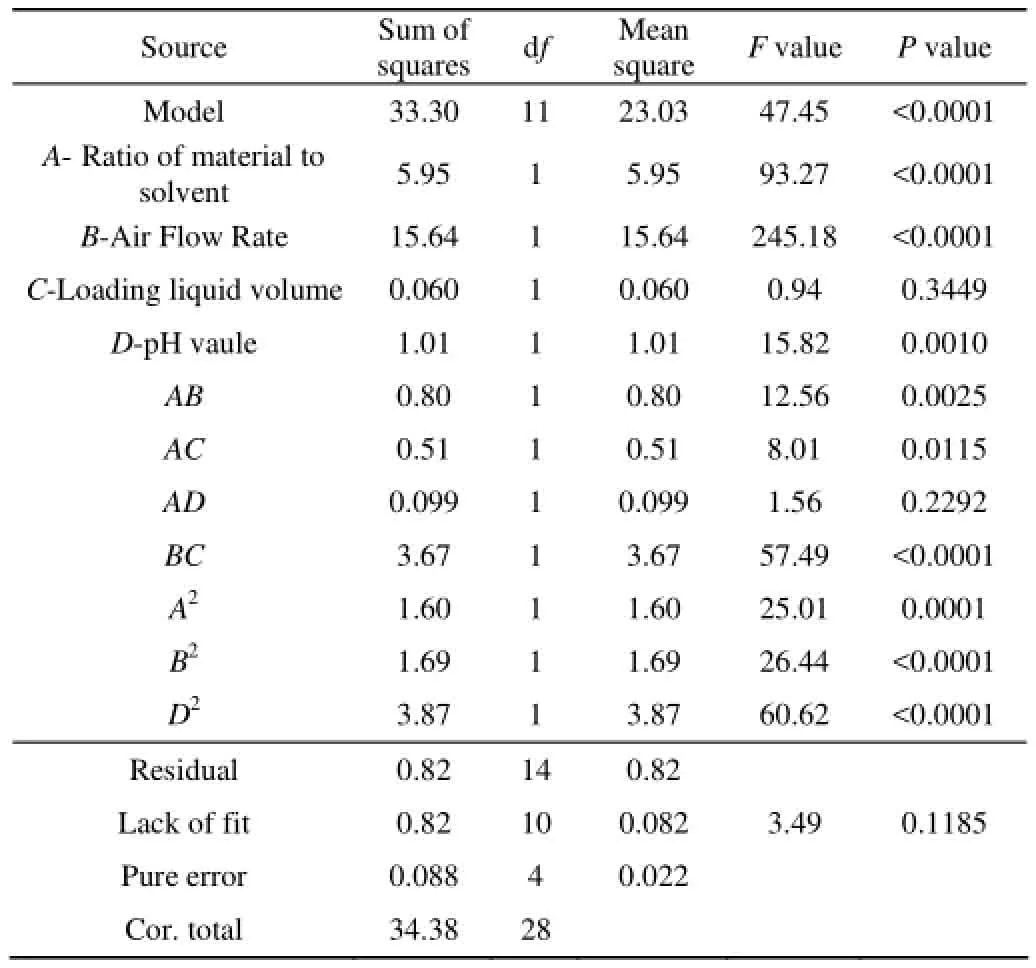
Table 4 ANOVA for response of enrichment
The analysis of variance, goodness-of-fit and the adequacy of the regression model were summarized in Table 3. The model p-value was smaller than 0.0001, meaning that the model was highly statistically significant[20]. The Model F-value of 44.45 and 7.85 implie that the model is significant. For the recovery, A, B, AC, AD and D2were significant model terms and The determination coefficient (R2=86.4%) indicated that only 13.6% of the total variation was not explained by the model. Which were caused by ionic strength, temperature, apertures of Quartz filter[21-22]and the tilt angle[23]. Order of factor: the air flow rate > the ratio of material to solven > pH value > the loading liquid volume. For enrichment ratio A, B, D, AB, AC, BC, A2, B2, D2were significant model terms and the determination coefficient (R2=96.86%) indicated that only 3.14% of the total variation was not included by the model.
2.2.3 Optimization of the foam fractionation
Design-Expert software was used to provide the optimal conditions for the response surface analysis. The limits of pH vaule were 6.0-7.0, the limits of the ratio of material to solvent were 87.5-122.5 mg/L, the limits of loading liquid volume were 500- 600 mL, the limits of air flow rate of 200 -300 mL/min. The goals of the recovery and the enrichment ratio were max. The weights of the recovery and the enrichment ratio was 1:1. The optimization process were pH vaule of 6.92, the ratio of material to solvent of 87.5 mg/L, the loading liquid volume of 600 mL, the air flow rate of 200 mL/min. Under the conditions, the recovery of Medicago sativa leaf protein was 92.2% and the enrichment ratio was 8.03.
2.2.4 Verification of predictive model
Considering actual process, The optimization process were pH vaule of 7.0 , the ratio of material to solvent of 87.5 mg/L, the loading liquid volume of 600 mL, the air flow rate of 200 mL/min. The recovery of Medicago sativa leaf protein was 90.2% and the enrichment ratio was 7.64. Therefore, the model was suitable for the optimization of foam fractionation process.
3 Conclusion
Purification technology of Medicago sativa protein was optimized by response surface methodology. The optimum conditions for the foam fractionation of leaf protein from Medicago sativa were found to be pH value of 7.0, the ratio of material to solvent of 87.5mg/L, the loading liquid volume of 600 mL, the air flow rate of 200 mL/min. Under these conditions, the recovery and enrichment ratio reached to 90.2% and 7.64, respectively. Foam fractionation has the good separation effect for Medicago sativa protein and was a effective technology at low cost, which can provide a reference for Medicago sativa protein processing.
[Reference]
[1] Gao Haijuan. The nutritional value and utilization status of alfalfa leaf protein[J]. Feed Material Resource, 2014(6): 40-42. (in Chinese with English abstract)
[2] Li Jianmin, Hou Yuze. Physiological function and application of dietary fiber[J]. Journal of Henan University of Science and Technology: Agricultural Science, 2003, 23(2): 75-78. (in Chinese with English abstract)
[3] Dong Xinwei. Study on the Extraction Technology of Alfalfa Leaf Protein[D]. Chongqing: Southwestern University, 2006. (in Chinese with English abstract)
[4] Li Juan, Wu Zhaoling, Li Rui. Technology of streptomycin sulfate separation by two-stage foam separation[J]. Biotechnology Progress, 2012, 28(3): 733-739.
[5] Saleh Z S, Hossain M M. A study of the separation of proteins from multicomponent mixtures by a semi-batch foaming process[J]. Chemical Engineering Progress, 2001, 40(4): 371-378.
[6] Brown A K, Kaul A, Varley J, Continuous foaming for protein recovery: Part I. Recovery of β-casein[J]. Biotechnology and Bioengineering, 1999, 62(3): 278-290.
[7] Gerken B M, Nicolai A, Linke D, et al. Effective enrichment and recovery of laccase C using continuous foam fractionation[J]. Separation & Purification Technology, 2006, 49(3): 291-294.
[8] Bradford M M. A rapid and sensetitive method for the quantitation of microgram quantities utilizing the principle of protein dye binding[J]. Analytical Biochemistry, 1976, 72(1/2): 248-254.
[9] Zhang Qian, Zhang Zhe, Zhang Yuecheng, et al. Technology of foam fractionation coupled with crystallization for the enrichment and purification of folic acid[J]. Separation & Purification Technology, 2014, 133: 335-342.
[10] Firlbeck D, Faulstich M, Urmann C, et al. Central composite design for optimal technology of concentrating vanillic acid using foam fractionation[J]. Separation and Purification Technology, 2013, 119(46): 28-34.
[11] Boonyasuwat S, Chavadej, Malakul S P, et al. Anionic and cationic surfactant recovery from water using a multistage foam fractionator[J]. Chemical Engineering. 2003, 93(3): 241-252.
[12] Karakashev S I, Manev E D, Nguyen A V. Effect of double-layer repulsion on film drainage[J]. Colloids and Surfaces A-Physicochemical and Engineering Aspects, 2008, 319(1-3): 34-42.
[13] Liu Wei, Wu Zhaoliang, Wang Yanji. Recovery of isoflavones from the soy whey wastewater using two-stage batch foam fractionation[J]. Industrial & Engineering Chemistry Research, 2013, 52(38): 13761-13767.
[14] Xiao Zongyuan, Li Xiaorong, Shao Wenyao, et al. Purification and concentration of the total saikosaponins extracted from radix bupleuri using foam fractionation[J]. Separation Science & Technology, 2014, 49(3): 469-475.
[15] Qian Shaoyu, Wu Zhaoliang, Zheng Huijie, et al. Study on riboflavin recovery from wastewater by a batch foam separation process[J]. Separation Science & Technology, 2009, 44(11): 2681-2694.
[16] Bhattacharjee, Kumar S R, Gandhi K S. Prediction of separation factor in foam separation of proteins[J]. Chemical Engineering Science, 1997, 52(24): 4625-4636.
[17] Yan Jin, Wu Zhaoliang, Wang Weijun. Separation of Tea Saponin by Two-Stage Continuous Foam Fractionation[J]. Separation Science & Technology, 2012, 47(16): 2460-2466.
[18] Chan N Y, Hossain M M, Brooks M S. A preliminary study of protein recovery from mussel blanching water by a foaming Process[J]. Chemical Engineering Process, 2007, 46(5): 501-504.
[19] Sun Ruiping, Yin Hao, Lu Ke, et al. Technology of soy protein separation from wastewater by two-stage foam Fractionation[J]. Transactions of the Chinese Society of Agricultural Engineering, 2010, 22(11): 374-378. (in Chinese with English abstract)
[20] Chavan R S, Avhad D N, Rathod V K. Optimization of Aqueous Two-Phase Extraction of Protease Produced from Bacillus licheniformis NCIM 2042 Using Response Surface Methodology[J]. Separation Science & Technology. 2015, 50(1): 45-55.
[21] Hu Bin, Zhu Hailan, Wu Zhaoliang. The effect of the pore size of gas distributor on foam separation process[J]. Journal of Chemical Engineering of Chinese Universities, 2014, 28(2): 246-251. (in Chinese with English abstract)
[22] Rujirawanich V, Chuyingsakultip N, Triroj M, et al. Recovery of surfactant from an aqueous solution using continuous multistage foam fractionation: Influence of design parameters[J]. Chemical Engineering & Processing Process Intensification, 2012, 52(2): 41-46.
[23] Wang Yong, Wu Zhaoliang, Li Rui, et al. Enhancing foam drainage using inclined foam channels of different angles for recovering the protein from whey wastewater[J]. Colloids and Surfaces A: Physicochem. Eng. Aspects, 2013, 419: 28-36.
泡沫法分离苜蓿叶蛋白工艺优化
刘海彬,张 炜※,陈元涛,高中超,刘 龙
(青海师范大学化学系,西宁 810008)
为了研究泡沫分离苜蓿分离的条件,试验在单因素的基础上,采用响应面法对pH值、装液量、料液比、气体流速4个因素进行优化,以富集比和回收率为指标,最佳工艺条件:pH值为7.0、料液比为87.5 mg/L、装液量为600 mL、气体流速为600 mL/min。在此条件下叶蛋白的回收率为90.2%;富集比为7.64。泡沫分离法分离苜蓿蛋白是一种简单、有效的方法,该研究可为苜蓿叶蛋白的深加工提供参考。
蛋白;工艺;优化;苜蓿;泡沫分离
10.11975/j.issn.1002-6819.2016.09.038
O629.73 Document code: A Article ID: 1002-6819(2016)-09-00271-06
Liu Haibin, Zhang Wei, Chen Yuantao, Gao Zhongchao, Liu Long. Technology optimization of Medicago sativa leaf protein separation with foam fractionation[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2016, 32(9): 271-276. (in English with Chinese abstract)
10.11975/j.issn.1002-6819.2016.09.038 http://www.tcsae.org
刘海彬,张 炜,陈元涛,高中超,刘 龙. 泡沫法分离苜蓿叶蛋白工艺优化[J]. 农业工程学报,2016,32(9):271-276. doi:10.11975/j.issn.1002-6819.2016.09.038 http://www.tcsae.org
date:2015-10-26 Revised date:2016-03-14
Project supported by the Ministry of science and technology personnel service enterprise (2009GJG20017).

